Adiponectin as a Biomarker of Preeclampsia: A Systematic Review
Abstract
1. Introduction
1.1. Preeclampsia
1.1.1. Pathophysiology of Preeclampsia
1.1.2. Risk Factors
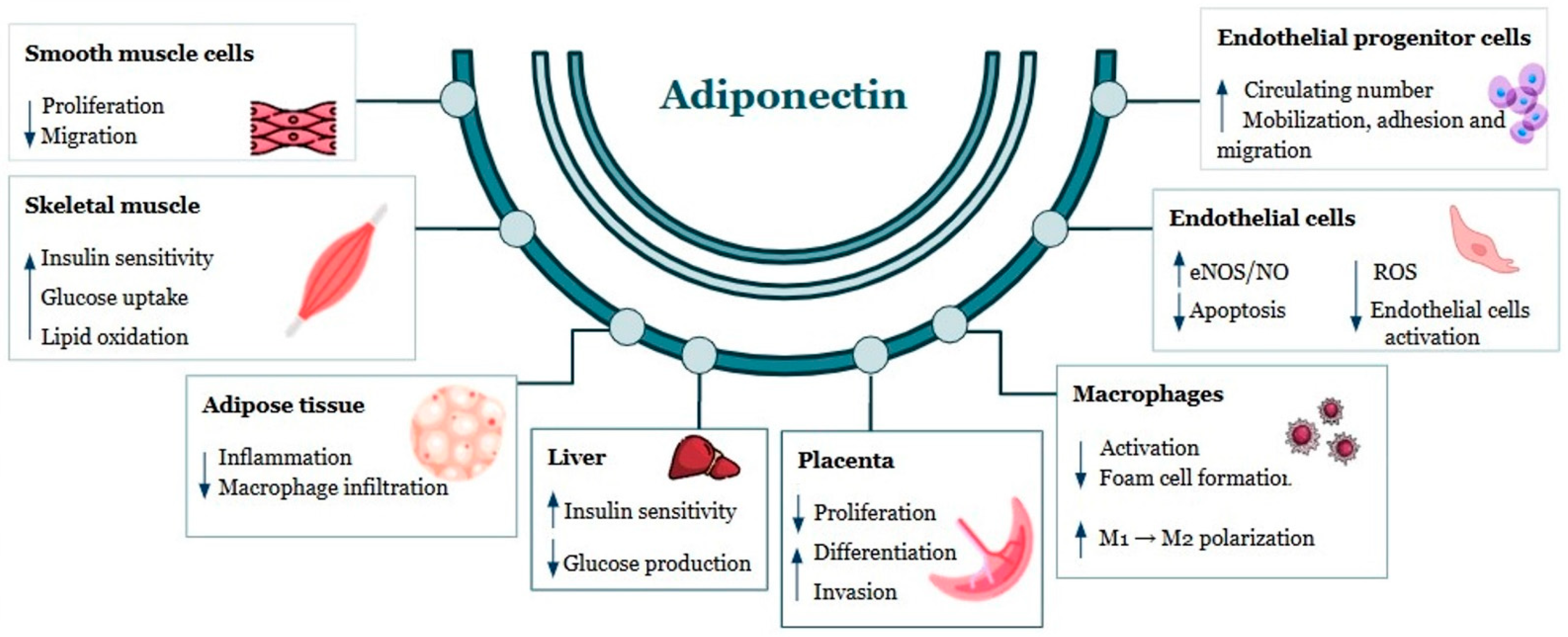
1.2. Adiponectin
Adiponectin Levels in Healthy Pregnancies
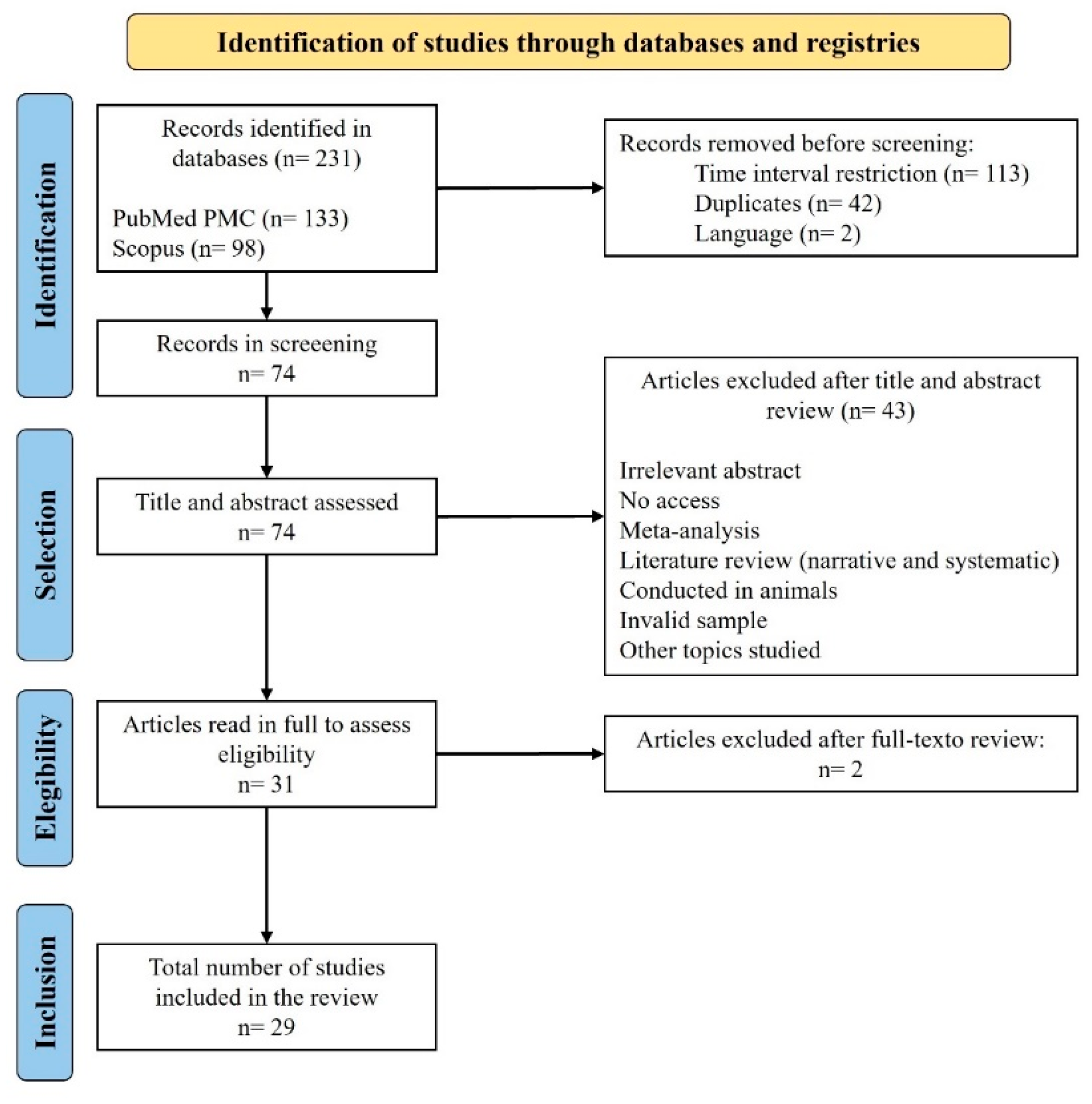
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
3. Results
Analysis of Evidence Levels in Included Studies
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β-hCG | Human chorionic gonadotrophic hormone |
| γGT | Gamma-glutamyl transferase |
| ADAM12 | Disintegrin and metalloprotease-12 |
| AdipoR1 | Adiponectin receptor 1 |
| AdipoR2 | Adiponectin receptor 2 |
| ADMA | Asymmetric dimethylarginine |
| AFP | Alpha-fetoprotein |
| AGEs | Advanced glycation end products |
| Apo | Apolipoprotein |
| APQ3 | Aquaporin 3 |
| BMI | Body mass index |
| CRP | C-reactive protein |
| DBP | Diastolic blood pressure |
| eNOS | Endothelial nitric oxide synthase |
| ESM-1 | Endothelial cell-specific molecule-1 |
| FRAP | Ferric reducing ability of plasma |
| FSTL3 | Follistatin like-3 |
| GMCSF | Granulocyte–macrophage colony-stimulating factor |
| HbA1c | Hemoglobin A1c |
| HDL-C | High-density lipoprotein cholesterol |
| IFN-γ | Gamma interferon |
| IL | Interleukin |
| LDL-C | Low-density lipoprotein cholesterol |
| MDA-TBARS | Malondialdehyde–thiobarbituric acid reactive substance |
| MMP | Matrix metalloproteinase |
| NO | Nitric oxide |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAPP-A | Pregnancy-associated plasma protein A |
| PE | Preeclampsia |
| PIGF | Placental growth factor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RBP4 | Retinol-binding protein 4 |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| sEng | Soluble endoglin |
| sFlt-1 | Soluble Fms-like tyrosine kinase 1 |
| SHBG | Sex-hormone-binding globulin |
| sOB-R | Soluble leptin receptor |
| sTNFR1 | Soluble tumor necrosis factor receptor 1 |
| TG | Triglycerides |
| TGF-β1 | Transforming growth factor beta 1 |
| TIMP | Tissue inhibitor of metalloproteinase |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| vWF | von Willebrand factor |
References
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee. ACOG Practice Bulletin No. 222: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Nova, A.; Sibai, B.M.; Barton, J.R.; Mercer, B.M.; Mitchell, M.D. Maternal plasma level of endothelin is increased in preeclampsia. Am. J. Obstet. Gynecol. 1991, 165, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; High Risk of Pre-Eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Dos Santos, E.; Pecquery, R.; de Mazancourt, P.; Dieudonne, M.N. Adiponectin and reproduction. Vitam. Horm. 2012, 90, 187–209. [Google Scholar] [CrossRef]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Dhariwal, N.K.; Lynde, G.C. Update in the Management of Patients with Preeclampsia. Anesthesiol. Clin. 2017, 35, 95–106. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Huppertz, B. The Critical Role of Abnormal Trophoblast Development in the Etiology of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.F. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan. J. Obstet. Gynecol. 2017, 56, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, N.; Shoemaker, A.; Wagner, S. Pathophysiology of hypertension in preeclampsia. Microvasc. Res. 2017, 109, 34–37. [Google Scholar] [CrossRef]
- Huang, Q.T.; Wang, S.S.; Zhang, M.; Huang, L.P.; Tian, J.W.; Yu, Y.H.; Wang, Z.J.; Zhong, M. Advanced oxidation protein products enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts: A possible link between oxidative stress and preeclampsia. Placenta 2013, 34, 949–952. [Google Scholar] [CrossRef]
- Mihu, D.; Razvan, C.; Malutan, A.; Mihaela, C. Evaluation of maternal systemic inflammatory response in preeclampsia. Taiwan. J. Obstet. Gynecol. 2015, 54, 160–166. [Google Scholar] [CrossRef]
- Sibley, C.P.; Pardi, G.; Cetin, I.; Todros, T.; Piccoli, E.; Kaufmann, P.; Huppertz, B.; Bulfamante, G.; Cribiu, F.M.; Ayuk, P.; et al. Pathogenesis of intrauterine growth restriction (IUGR)-conclusions derived from a European Union Biomed 2 Concerted Action project ‘Importance of Oxygen Supply in Intrauterine Growth Restricted Pregnancies’—A workshop report. Placenta 2002, 23 (Suppl. A), S75–S79. [Google Scholar] [CrossRef]
- Kawamura, T.; Kakogawa, J.; Takeuchi, Y.; Takani, S.; Kimura, S.; Nishiguchi, T.; Sugimura, M.; Sumimoto, K.; Kanayama, N. Measurement of placental oxygenation by transabdominal near-infrared spectroscopy. Am. J. Perinatol. 2007, 24, 161–166. [Google Scholar] [CrossRef]
- Kakogawa, J.; Sumimoto, K.; Kawamura, T.; Minoura, S.; Kanayama, N. Noninvasive monitoring of placental oxygenation by near-infrared spectroscopy. Am. J. Perinatol. 2010, 27, 463–468. [Google Scholar] [CrossRef]
- Zhou, C.C.; Zhang, Y.; Irani, R.A.; Zhang, H.; Mi, T.; Popek, E.J.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 2008, 14, 855–862. [Google Scholar] [CrossRef]
- Wang, Y.P.; Walsh, S.W.; Guo, J.D.; Zhang, J.Y. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am. J. Obstet. Gynecol. 1991, 165, 1695–1700. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Fondjo, L.A.; Owiredu, W.; Czika, A.; Nelson, W.; Lamptey, J.; Wang, Y.X.; Ding, Y.B. The role of adiponectin in placentation and preeclampsia. Cell Biochem. Funct. 2020, 38, 106–117. [Google Scholar] [CrossRef]
- Peres, G.M.; Mariana, M.; Cairrao, E. Pre-Eclampsia and Eclampsia: An Update on the Pharmacological Treatment Applied in Portugal. J. Cardiovasc. Dev. Dis. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Cheng, K.K.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Cross-talk between adipose tissue and vasculature: Role of adiponectin. Acta Physiol. 2011, 203, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Funahashi, T.; Shimomura, I. Pathophysiological significance of adiponectin. Med. Mol. Morphol. 2007, 40, 55–67. [Google Scholar] [CrossRef]
- Varady, K.A.; Allister, C.A.; Roohk, D.J.; Hellerstein, M.K. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J. Nutr. Biochem. 2010, 21, 188–195. [Google Scholar] [CrossRef]
- Fasshauer, M.; Paschke, R.; Stumvoll, M. Adiponectin, obesity, and cardiovascular disease. Biochimie 2004, 86, 779–784. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Abe, M.; Kusunoki, T.; Miki, T. Association of serum high molecular weight adiponectin and blood pressure among non-diabetic community-dwelling men. Clin. Exp. Hypertens. 2011, 33, 336–344. [Google Scholar] [CrossRef]
- Ji, L.; Brkic, J.; Liu, M.; Fu, G.; Peng, C.; Wang, Y.L. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol. Asp. Med. 2013, 34, 981–1023. [Google Scholar] [CrossRef]
- Nien, J.K.; Mazaki-Tovi, S.; Romero, R.; Erez, O.; Kusanovic, J.P.; Gotsch, F.; Pineles, B.L.; Gomez, R.; Edwin, S.; Mazor, M.; et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 2007, 35, 522–531. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tendean, H.M.M.; Kaeng, J.J.; Supandy, A. Adiponection Serum Levels in Severe Preeclampsia. Indones. J. Obstet. Gynecol. 2021, 9, 134–139. [Google Scholar] [CrossRef]
- Thagaard, I.N.; Hedley, P.L.; Holm, J.C.; Lange, T.; Larsen, T.; Krebs, L.; Christiansen, M. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019, 15, 78–83. [Google Scholar] [CrossRef] [PubMed]
- de Knegt, V.E.; Hedley, P.L.; Eltvedt, A.K.; Placing, S.; Wojdemann, K.; Shalmi, A.C.; Rode, L.; Kanters, J.K.; Sundberg, K.; Tabor, A.; et al. First-Trimester Maternal Serum Adiponectin/Leptin Ratio in Pre-Eclampsia and Fetal Growth. Life 2023, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Bawah, A.T.; Yeboah, F.A.; Nanga, S.; Alidu, H.; Ngala, R.A. Serum adipocytokines and adiposity as predictive indices of preeclampsia. Clin. Hypertens. 2020, 26, 19. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Zhang, Q.; Li, D.; Cai, L. Umbilical artery ultrasound haemodynamics combined with serum adiponectin levels can aid in predicting adverse pregnancy outcomes in patients with severe pre-eclampsia. J. Obstet. Gynaecol. 2023, 43, 2232656. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Barry, D.; Melhorn, S.; Easterling, T.; Gammill, H.; Schur, E. Evaluating Relationships between Visceral Fat Measures and Adipokines Concentrations among Women with a History of Preeclampsia. Am. J. Perinatol. 2020, 37, 1140–1145. [Google Scholar] [CrossRef]
- Dong, G.; Tian, Y.; Li, X. Adiponectin Participates in Preeclampsia by Regulating the Biological Function of Placental Trophoblasts through P38 MAPK-STAT5 Pathway. Iran. J. Public Health 2018, 47, 1838–1844. [Google Scholar]
- Vieira, M.C.; White, S.L.; Patel, N.; Seed, P.T.; Briley, A.L.; Sandall, J.; Welsh, P.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; et al. Prediction of uncomplicated pregnancies in obese women: A prospective multicentre study. BMC Med. 2017, 15, 194. [Google Scholar] [CrossRef]
- Lomakova, Y.D.; Chen, X.; Stein, T.P.; Steer, R.A. Decreased Adiponectin Levels in Early Pregnancy Are Associated with High Risk of Prematurity for African American Women. J. Clin. Med. 2022, 11, 3213. [Google Scholar] [CrossRef] [PubMed]
- Eleuterio, N.M.; Palei, A.C.; Rangel Machado, J.S.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Relationship between adiponectin and nitrite in healthy and preeclampsia pregnancies. Clin. Chim. Acta 2013, 423, 112–115. [Google Scholar] [CrossRef]
- Eleuterio, N.M.; Palei, A.C.; Rangel Machado, J.S.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Correlations between circulating levels of adipokines and anti-angiogenic factors in women with BMI < 30 and a late-onset preeclampsia. Hypertens. Pregnancy 2014, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Eleuterio, N.M.; Palei, A.C.; Rangel Machado, J.S.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens. 2015, 5, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.J.M.; Bobby, Z.; Chaturvedula, L.; Vinayagam, V.; Jacob, S.E.; Habeebullah, S. Maternal Adverse Outcomes in Hypertensive Disorders of Pregnancy and Their Association with Serum Adiponectin and Redox Markers. Fetal Pediatr. Pathol. 2022, 41, 1–17. [Google Scholar] [CrossRef]
- Noureldeen, A.; Qusti, S.Y.; Al-Seeni, M.N. Serum leptin, adiponectin, resistin, visfatin and inflammatory cytokines in normal weight and obese women with normal pregnancy and with preeclampsia. Life Sci. J. 2014, 11, 17–23. [Google Scholar]
- Salimi, S.; Farajian-Mashhadi, F.; Naghavi, A.; Mokhtari, M.; Shahrakipour, M.; Saravani, M.; Yaghmaei, M. Different profile of serum leptin between early onset and late onset preeclampsia. Dis. Markers 2014, 2014, 628476. [Google Scholar] [CrossRef]
- Weedon-Fekjaer, M.S.; Sheng, Y.; Sugulle, M.; Johnsen, G.M.; Herse, F.; Redman, C.W.; Lyle, R.; Dechend, R.; Staff, A.C. Placental miR-1301 is dysregulated in early-onset preeclampsia and inversely correlated with maternal circulating leptin. Placenta 2014, 35, 709–717. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Bai, J.; Li, Z.; Chen, Y. Altered Expressions of AQP3 and ADP Are Closely Related with the Risk of Preeclampsia Occurrence. Gynecol. Obstet. Investig. 2020, 85, 362–370. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Ahmadvand, H. Positive correlation between serum levels of adiponectin and homocysteine in pre-eclampsia. J. Obstet. Gynaecol. Res. 2013, 39, 641–646. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Ahmadvand, H. Leptin to adiponectin ratio in preeclampsia. Bangladesh Med. Res. Counc. Bull. 2013, 39, 18–21. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Qu, Y.; Wang, S.; Wang, X.; Liu, J. Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin. Chim. Acta 2016, 458, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Cui, J.; Chen, X.; Xiong, J.; Zhou, W. Circulating adipokine levels and preeclampsia: A bidirectional Mendelian randomization study. Front. Genet. 2022, 13, 935757. [Google Scholar] [CrossRef] [PubMed]
- Tobinaga, C.M.; Torloni, M.R.; Gueuvoghlanian-Silva, B.Y.; Pendeloski, K.P.; Akita, P.A.; Sass, N.; Daher, S. Angiogenic factors and uterine Doppler velocimetry in early- and late-onset preeclampsia. Acta Obstet. Gynecol. Scand. 2014, 93, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gungor, Z.B.; Ekmekci, H.; Tuten, A.; Toprak, S.; Ayaz, G.; Caliskan, O.; Sonmez, H.; Madazli, R.; Donma, O.; Kucur, M.; et al. Is there any relationship between adipocytokines and angiogenesis factors to address endothelial dysfunction and platelet aggregation in untreated patients with preeclampsia? Arch. Gynecol. Obstet. 2017, 296, 495–502. [Google Scholar] [CrossRef]
- Demir, B.C.; Atalay, M.A.; Ozerkan, K.; Doster, Y.; Ocakoglu, G.; Kucukkomurcu, S. Maternal adiponectin and visfatin concentrations in normal and complicated pregnancies. Clin. Exp. Obstet. Gynecol. 2013, 40, 261–267. [Google Scholar]
- Chandrasekaran, S.; Hunt, H.; Melhorn, S.; Gammill, H.S.; Schur, E.A. Adipokine profiles in preeclampsia. J. Matern. Fetal Neonatal Med. 2020, 33, 2812–2817. [Google Scholar] [CrossRef]
- Eleuterio, N.M.; Palei, A.C.; Machado, J.S.; Tanus-Santos, J.E.; Cavalli, R.C.; Sandrim, V.C. Role of adiponectin on antioxidant profile: Evaluation during healthy and hypertensive disorders of pregnancy. Blood Press. 2016, 25, 241–243. [Google Scholar] [CrossRef]
- Nevalainen, J.; Korpimaki, T.; Kouru, H.; Sairanen, M.; Ryynanen, M. Performance of first trimester biochemical markers and mean arterial pressure in prediction of early-onset pre-eclampsia. Metabolism 2017, 75, 6–15. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Garza-Veloz, I.; Carrillo-Sanchez, K.; Martinez-Gaytan, V.; Cortes-Flores, R.; Ochoa-Torres, M.A.; Guerrero, G.G.; Rodriguez-Sanchez, I.P.; Cancela-Murrieta, C.O.; Zamudio-Osuna, M.; et al. Expression levels of seven candidate genes in human peripheral blood mononuclear cells and their association with preeclampsia. Hypertens. Pregnancy 2014, 33, 191–203. [Google Scholar] [CrossRef]
- Rao, S.; Kumari, A.; Sharma, M.; Kabi, B.C. Predicting Maternal Serum Adiponectin and Leptin Level as Biomarkers of Pre-eclampsia: A Prospective Study. J. Obstet. Gynaecol. India 2021, 71, 58–65. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2; Oxford Centre for Evidence-Based Medicine: Oxford University, Oxford, UK, 2011. [Google Scholar]
- Marx, R.G.; Wilson, S.M.; Swiontkowski, M.F. Updating the assignment of levels of evidence. J. Bone Jt. Surg. Am. 2015, 97, 1–2. [Google Scholar] [CrossRef]
- Daskalakis, G.; Bellos, I.; Nikolakea, M.; Pergialiotis, V.; Papapanagiotou, A.; Loutradis, D. The role of serum adipokine levels in preeclampsia: A systematic review. Metabolism 2020, 106, 154172. [Google Scholar] [CrossRef]
- Salihu, H.M.; De La Cruz, C.; Rahman, S.; August, E.M. Does maternal obesity cause preeclampsia? A systematic review of the evidence. Minerva Ginecol. 2012, 64, 259–280. [Google Scholar]
- Abdi, F.; Aghaie, Z.; Rahnemaie, F.S.; Alimoradi, Z. A Systematic Review of First Trimester Biochemical and Molecular Predictive Tests for Preeclampsia. Curr. Hypertens. Rev. 2018, 14, 21–28. [Google Scholar] [CrossRef]
| Diagnostic Criteria for Preeclampsia | |
|---|---|
| Blood Pressure Elevation |
|
| |
| and | |
| Proteinuria |
|
| |
| or (in the absence of proteinuria at least one of the following criteria) | |
| Thrombocytopenia | Platelet count < 100,000/mm3 or other hematological complications such as hemolysis or disseminated intravascular coagulation. |
| Renal Insufficiency | Serum creatinine concentration > 1.1 mg/dL or doubling of serum creatinine levels in the absence of other renal diseases. |
| Impaired Liver Function | Elevated liver transaminases to twice the normal level, with or without upper right quadrant or epigastric abdominal pain. |
| Pulmonary Edema | - |
| Neurological Complications | New-onset headache unresponsive to medication and not attributable to another diagnosis; visual disturbances; altered mental status; eclampsia; stroke; or clonus. |
| Uteroplacental Dysfunction | Stillbirth, fetal growth restriction, or abnormal umbilical artery Doppler waveforms. |
| Moderate Risk Factors | High Risk Factors |
|---|---|
| Nulliparity | Chronic hypertension |
| BMI > 30 kg/m2, obesity | Chronic kidney disease |
| Maternal age | Pregestational and gestational diabetes |
| Medically assisted reproduction | Autoimmune diseases (anti-phospholipid antibody syndrome; systemic lupus erythematosus) |
| History of stillbirth | Thrombophilia |
| History of intrauterine growth restriction | Premature placental abruption |
| Interval between pregnancies > 5 years | Preeclampsia in previous pregnancy |
| Low schooling | Family history of preeclampsia |
| Obstructive sleep apnea syndrome | Multiple pregnancy |
| Authors and Year | Participants | Markers Analyzed | BMI Handling | Main Results |
|---|---|---|---|---|
| Tendean et al. [34] 2021 | 52 participants: - 26 pregnant women with severe preeclampsia - 26 pregnant women without preeclampsia | - Adiponectin | BMI reported but not adjusted | - Adiponectin levels are correlated with the development of severe preeclampsia, since pregnant women with preeclampsia showed significantly lower levels of adiponectin when compared to normotensive pregnant women. - No relationship between BMI and the incidence of preeclampsia. |
| Thagaard et al. [35] 2019 | 2503 participants stratified according to BMI (normal, moderate or severe obesity): - 93 pregnant women with hypertensive disorders of pregnancy (29 with hypertension and 64 with preeclampsia). | - Adiponectin - Leptin | Matched and adjusted for BMI | - Obese women have a lower concentration of adiponectin in preeclampsia, with a lower concentration in severe obesity. No association was found in normal-weight women. Leptin concentration had no association with the disease in normal-weight and moderately obese women; however, in women with severe obesity, a lower level of leptin was found - The adiponectin/leptin ratio was not associated with the development of preeclampsia in any of the groups. |
| de Knegt et al. [36] 2023 | 423 participants: - 126 pregnant women with preeclampsia (98- mild preeclampsia, 21- severe preeclampsia, 7- HELLP syndrome) - 297 pregnant women without preeclampsia | - Adiponectin - Leptin - Adiponectin/leptin ratio as a surrogate marker of insulin sensitivity - PAPP-A - β-hCG | Adjusted for BMI | - The adiponectin/leptin ratio was significantly lower in pregnancies with preeclampsia compared to controls. - Adiponectin and PAPP-A were negatively associated with preeclampsia, while leptin was positively associated, however the adiponectin/leptin ratio was a better predictor of the disease, although not clinically relevant as a single marker. - There was no association between the adiponectin/leptin ratio and clinical severity or time of onset of preeclampsia. - β-hCG concentrations were not significantly different between the two groups. |
| Bawah et al. [37] 2020 | 190 participants: - 90 pregnant women with preeclampsia - 100 pregnant women without preeclampsia | - Adiponectin - Leptin - Resistin - Visfatin - Lipids | Adjusted for BMI | - There was no significant difference in the lipid profile, with the exception of HDL-C, which was significantly lower in the preeclampsia group. BMI was significantly higher in pregnant women who developed preeclampsia. - Leptin, resistin and visfatin levels were significantly higher in pregnant women who developed preeclampsia. Adiponectin showed significantly lower levels in pregnant women who developed preeclampsia. - Resistin was considered the best predictor of the disease after controlling for BMI. However, adiponectin was considered the best predictor after controlling for BMI, age, parity and family history of diabetes and preeclampsia. |
| Zhang et al. [38] 2023 | 208 participants: - 118 pregnant women with severe preeclampsia - 90 pregnant women without preeclampsia | - Adiponectin | Restricted to normal BMI range (18.5–24.9 kg/m2) | - Adiponectin levels in patients with preeclampsia were significantly lower than in the healthy control group. - In the ultrasound evaluation of the umbilical artery, the pulsatility and resistance indices increased in the preeclampsia group compared to the control group. Furthermore, when correlated with serum adiponectin levels, it was shown that adiponectin levels were negatively related to pulsatility and resistance indices. |
| Chandrasekaran et al. [39] 2020 | 64 participants: - 16 pregnant women with non-severe preeclampsia - 30 pregnant women with severe preeclampsia - 18 pregnant women without preeclampsia | - Adiponectin - Leptin - Resistin | BMI reported but not adjusted or matched | - Plasma concentrations of adiponectin were significantly lower in patients with preeclampsia without severity characteristics compared to the other two groups (on average 1 to 2 years after delivery). Leptin and resistin concentrations did not differ between the groups. - After separating the participants according to body fat percentage, in the high-fat group (≥38%), leptin remained positively associated with visceral and subcutaneous fat areas, adiponectin correlated negatively with visceral fat area and resistin was positively associated with subcutaneous fat area. Among low-fat women (<38%), leptin was positively associated with the subcutaneous fat area, adiponectin showed a negative relationship for both areas, while resistin was not significantly associated with either fat compartment. |
| Dong et al. [40] 2018 | 82 participants: - 52 pregnant women with preeclampsia - 30 pregnant women without preeclampsia | - Adiponectin - Pathway P38 MAPK-STAT5 | Not reported | - p-38 with high expression and p-STAT5 with lower expression in placental trophoblasts of pregnant women with preeclampsia, when compared to pregnant women without the condition. - Adiponectin mRNA expression was found in both groups, but the level of expression was significantly lower in patients with preeclampsia. - The level of p-p38 expression was negatively correlated and the level of p-STAT5 expression was positively correlated with adiponectin expression levels. |
| Vieira et al. [41] 2017 | 1409 participants with a BMI ≥ 30 kg/m2: - 904 complicated pregnancies - 505 uncomplicated pregnancies | - Adiponectin; leptin - Haemoglobin A1c (HbA1c); fructosamine; insulin and C-peptide - IL-6; high-sensitivity C-reactive protein; t-PA antigen - Lipids (triglycerides (TG), total cholesterol, LDL-C (low-density lipoprotein cholesterol), HDL-C (high-density lipoprotein cholesterol)) - Liver-associated markers (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase (γGT), SHBG, and ferritin) - Vitamin D | Restricted to obese women (BMI ≥ 30 kg/m2) | - Higher concentrations of adiponectin were associated with a greater likelihood of uncomplicated pregnancy and labour. - Higher concentrations of HbA1c, insulin, SHBG and γGT were associated with a lower probability of pregnancy and uncomplicated labour. |
| Lomakova et al. [42] 2022 | 1776 participants: Pregnant, young and generally healthy - n = 646 African American women - n = 853 Hispanic women - n = 277 Caucasians | - Adiponectin - IL-6, IL-8, IL-10, TNF-α and GMCSF (granulocyte-macrophage colony-stimulating factor) - Resistin | Adjusted for BMI | - African American women had lower levels of adiponectin and higher levels of resistin and GMCSF compared to Hispanic or Caucasian women. Although African American pregnant women had higher levels of IL-8 and TNF-α, the trends for all cytokines (IL-8, TNF-α, IL-6, IL-10) were not statistically significant. - A higher number of African American women developed preeclampsia compared to the other ethnic groups. However, fewer African American women developed gestational diabetes. - Women with lower levels of adiponectin had a higher risk of developing preeclampsia when analysed with and without adjustment for pre-pregnancy BMI. |
| Eleuterio et al. [43] 2013 | 117 participants: - 47 pregnant women with preeclampsia - 70 pregnant women without preeclampsia Exclusion of pregnant women with BMI ≥ 30 kg/m2 | - Adiponectin - Nitrite - Endogenous NO inhibitor - Asymmetric dimethylarginine (ADMA) | Restricted to non-obese women (BMI ≤ 30 kg/m2) | - Increased concentration of adiponectin in preeclampsia. - Decreased nitrite concentration in preeclampsia. - Negative correlation between adiponectin and BMI in a healthy pregnancy; no correlation in preeclampsia. - Positive correlation between adiponectin and nitrite in a healthy pregnancy; no correlation in preeclampsia. - Strong positive correlation between adiponectin and nitrite levels when ADMA levels are reduced in pregnant women with preeclampsia, suggesting that high concentrations of this inhibitor may interfere with the physiological activation of endothelial nitric oxide synthase by adiponectin in preeclampsia. |
| Eleuterio et al. [44] 2014 | 63 participants: - 27 pregnant women with preeclampsia - 36 pregnant women without preeclampsia Exclusion of pregnant women with BMI ≥ 30 kg/m2 | - Adiponectin - Leptin - sFlt-1 - sEng | Restricted to non-obese women (BMI ≤ 30 kg/m2) | - Increased concentrations of adiponectin, leptin, sFlt-1 and sEng in pregnant women with preeclampsia compared to pregnant women without the condition. - Strong positive correlation between adiponectin and sFlt-1 and sEng in preeclampsia, but not in healthy pregnancies. - Significant positive correlation between leptin and sFlt-1 and sEng in preeclampsia, but not in healthy pregnancies. - Negative correlation between adiponectin and BMI and positive correlation between leptin and BMI in the group of pregnant women without preeclampsia. However, both correlations were not found in the group of pregnant women with preeclampsia. - Negative correlation between sFlt-1 and BMI in pregnant women with preeclampsia. |
| Eleuterio et al. [45] 2015 | 105 participants: - 59 pregnant women with preeclampsia - 46 pregnant women without preeclampsia Exclusion of pregnant women with BMI ≥ 30 kg/m2 | - Adiponectin - Leptin - Matrix metalloproteinase 2 and 9 (MMP 2 and 9) and tissue inhibitor of metalloproteinase 1 and 2 (TIMP 1 and 2) | Restricted to non-obese women (BMI ≤ 30 kg/m2) | - Levels of adiponectin, leptin, MMP2, TIMP1 and TIMP2 increased in pregnant women with preeclampsia. - There was a positive correlation between adiponectin and MMP2 and between adiponectin and TIMP2 in the group of pregnant women with preeclampsia. |
| Abraham et al. [46] 2020 | 248 participants: - 66 pregnant women with early preeclampsia - 68 pregnant women with late preeclampsia - 55 pregnant women with gestational hypertension - 59 healthy pregnant women | - Adiponectin - Leptin - Resistin - Redox markers malondialdehyde (MDA) and total antioxidant status (TAS) | Not reported | - Adiponectin levels were significantly higher in the preeclampsia group compared to the control and gestational hypertension groups. - Adiponectin levels in the gestational hypertension group were comparable to the control group. - Increased serum adiponectin, MDA and TAS were associated with adverse maternal outcomes in hypertensive diseases of pregnancy. - Leptin levels did not differ significantly between the groups. The leptin/adiponectin ratio and resistin levels were not significantly different between the groups. The correlation between MDA, leptin and adiponectin was not statistically significant. |
| Noureldeen [47] 2014 | 62 participants: 25 pregnant women with preeclampsia - 9 with BMI < 30 kg/m2 - 16 with BMI ≥ 30 kg/m2 37 normotensive pregnant women - 15 with BMI < 30 kg/m2 - 22 with BMI ≥ 30 kg/m2 | - Leptin - Adiponectin - Resistin - Visfatin - TNF-α - IL-6 | Matched by BMI | - Changes in adipocytokines were more pronounced in obese pregnant women with the disease than in normal-weight pregnant women complicated by preeclampsia. - When pregnant women with a BMI ≥ 30 kg/m2 the adipocytokines that showed significant changes included: - Leptin: decreased concentration in pregnant women with preeclampsia - Adiponectin: increased concentration in pregnant women with preeclampsia - Resistin: increased concentration in pregnant women with preeclampsia - TNF-α: increased concentration in pregnant women with preeclampsia |
| Salimi et al. [48] 2014 | 90 participants: - 45 pregnant women with preeclampsia - 45 pregnant women without preeclampsia | - Adiponectin - Leptin | Matched by BMI | - Maternal serum leptin and adiponectin showed significantly higher levels in pregnant women with preeclampsia. - In contrast to leptin, serum adiponectin did not differ between women with early onset and late onset preeclampsia. - Serum adiponectin was significantly higher in severe preeclampsia compared to pregnant women without preeclampsia. However, the difference between adiponectin levels in severe and mild preeclampsia was not significant. - A significant positive correlation was observed in the control group between: leptin and adiponectin; leptin and BMI. - A significant positive correlation was observed in the control group and in patients with preeclampsia between: adiponectin and BMI. - There were no significant differences in the leptin/adiponectin ratio between pregnant women with and without preeclampsia. |
| Weedon- Fekjaer et al. [49] 2014 | 72 participants: - 23 pregnant women with early onset preeclampsia - 26 pregnant women with late onset preeclampsia - 23 pregnant women without preeclampsia | - Adiponectin - Leptin - Resistin | Adjusted for BMI | - The concentration of adiponectin was significantly higher in the maternal circulation in both early and late onset preeclampsia compared to the control group. Placental expression of the adiponectin gene showed no significant difference between the groups. - Circulating leptin levels were significantly higher in both preeclampsia groups compared to the control group. Placental expression of the leptin gene was significantly higher in the early onset preeclampsia group compared to the late onset group. - There were no significant differences between the groups in terms of the plasma concentration of resistin and the expression of its gene in the placenta. |
| Zhou et al. [50] 2020 | 120 participants: - 60 with severe preeclampsia - 60 without preeclampsia | - Aquaporin 3 (APQ3) - Adiponectin - TG - LDL-C - HDL-C - Apolipoprotein (Apo) A and Apo B | Not reported | - APQ3 expression was lower in placental tissue and higher in foetal membranes in the case group compared to the control group. - Adiponectin levels in the umbilical cord blood of newborns in the case group were higher than in the control group. - TG and C- LDL levels were significantly higher in the case group. However, no significant differences were found for other parameters, including cholesterol, C- HDL, ApoA, ApoB and ApoB/ApoA. |
| Khosrowbeygi and Ahmadvand [51] 2013 | 60 participants: - 30 pregnant women with preeclampsia - 30 pregnant women without preeclampsia | - Adiponectin - Homocysteine | Matched by BMI | - When compared to pregnant women without preeclampsia: - Increased concentrations of total adiponectin and total homocysteine in mild and severe preeclampsia. Increased homocysteine-adiponectin ratio in severe preeclampsia. - No significant differences between mild and severe preeclampsia. |
| Khosrowbeygi and Ahmadvand [52] 2013 | 60 participants: - 30 pregnant women with preeclampsia - 30 pregnant women without preeclampsia | - Adiponectin - Leptin | Matched and adjusted for BMI | - Significant increase in adiponectin and leptin levels in pregnant women with mild and severe preeclampsia, compared to pregnant women without preeclampsia, before adjusting for BMI. No significant difference was found between mild and severe preeclampsia. - Significant increase in the leptin/adiponectin ratio in pregnant women with severe preeclampsia, compared to pregnant women without preeclampsia and with mild preeclampsia, before adjusting for BMI. No change when comparing pregnant women without preeclampsia and those with mild preeclampsia. - After adjustment for BMI: - Leptin values were slightly higher in the preeclampsia group - Adiponectin values were significantly lower in the preeclampsia group - The adjusted leptin/adiponectin ratio was significantly higher in the preeclampsia group |
| Song et al. [53] 2016 | 153 participants: - 74 pregnant women with preeclampsia - 79 pregnant women without preeclampsia | - Leptin - Adiponectin - Resistin | Adjusted for BMI | - There were no significant differences in serum adiponectin levels between the groups, even when stratifying between mild and severe preeclampsia. - BMI, serum leptin and resistin levels and the resistin/creatinine ratio were significantly higher in the preeclampsia group. - No correlation between serum adiponectin levels and BMI. No correlation between serum resistin levels and BMI. - Positive correlation between serum leptin levels and BMI, only in pregnant women without preeclampsia. |
| Chen et al. [54] 2022 | 141,068 participants of European descent: - 4743 cases of preeclampsia - 136,325 in the control group | - Adiponectin - Leptin - Resistin -sOB-R (soluble leptin receptor) - PAI-1 | Adjusted for BMI | - No correlation between levels of adipokines, particularly adiponectin, and preeclampsia. - PAI-1 could be a useful biomarker in the diagnosis and monitoring of preeclampsia therapy. |
| Tobinaga et al. [55] 2014 | 108 participants: - 54 pregnant women with preeclampsia - 54 pregnant women without pr-eclampsia | - Soluble fms-like tyrosine kinase 1 (sFlt-1) - Soluble Endoglin (sEng) - Adiponectin - Plasminogen activator inhibitor-1 (PAI-1) | Adjusted for BMI | - Adiponectin levels were similar in both groups, regardless of BMI and unrelated to uterine artery resistance. - PAI-1 levels were significantly higher in preeclampsia, but unrelated to uterine artery resistance. - Mean serum levels of sFlt-1, sEng and PAI-1 were significantly higher in patients with preeclampsia. - Serum levels of sFlt-1 and sEng were significantly higher in women with early onset preeclampsia compared to late onset preeclampsia, while concentrations of adiponectin and PAI-1 were similar between these subgroups. - Patients with preeclampsia had significantly higher mean uterine artery resistance. - Patients with preeclampsia and abnormal uterine artery doppler had higher serum sEng levels than those with normal doppler. |
| Güngör et al. [56] 2017 | 79 participants: - 30 pregnant women with early onset preeclampsia - 22 pregnant women with late onset preeclampsia - 27 pregnant women without preeclampsia Considering the division between early and late at 32 weeks’ gestation | - Plasma angiogenic factors (PlGF, VEGF) - Plasma anti-angiogenic factors (sFlt-1, endoglin) - Leptin, adiponectin and ghrelin - Endothelial dysfunction markers (vWF, NO) - Platelet function markers (ADP and collagen-induced platelet aggregation, P-selectin) | Matched by BMI | - Endoglin, leptin and vWF levels are increased in preeclampsia. - Levels of PIGF, collagen-induced platelet aggregation and P-selectin are decreased in preeclampsia. - No significant differences were found in adiponectin and ghrelin levels when comparing both groups. |
| Demir et al. [57] 2013 | 80 participants: - 52 pregnant women with preeclampsia - 28 pregnant women without preeclampsia | - Adiponectin - Visfatin | Not reported | - No significant changes in plasma concentrations of adiponectin and visfatin in pregnant women with preeclampsia compared to healthy pregnant women. - Preeclampsia severity was not shown to affect plasma values of adiponectin and visfatin. |
| Chandrasekaran et al. [58] 2020 | 117 participants stratified into subgroups: - Women with obesity - Normal weight women - 61 pregnant women with preeclampsia (n = 36 obese; n = 25 normal weight) - 56 pregnant women without preeclampsia (n = 29 obese; n = 27 normal weight) | - Visfatin and resistin (related to visceral fat) - Leptin and adiponectin (related to general adiposity) - Inflammatory cytokines: IFN-γ, IL-1β, IL-6, IL-2 | Adjusted for BMI | - Association between preeclampsia and significantly higher maternal concentrations of visfatin, resistin and inflammatory cytokines. - Preeclampsia was associated with increased levels of leptin, but not adiponectin. - There were no interactions between preeclampsia and obesity, suggesting that the increase in resistin, visfatin, inflammatory cytokines and leptin present in the condition did not differ according to the degree of obesity. - The concentrations of visfatin, resistin, adiponectin and leptin did not differ when pregnant women with preeclampsia were stratified according to degree of severity. Inflammatory cytokines, on the other hand, showed significantly higher concentrations in pregnant women with characteristics of disease severity. |
| Eleuterio et al. [59] 2016 | 108 participants: - 31 pregnant women with preeclampsia - 27 pregnant women with gestational hypertension - 50 healthy pregnant women with uncomplicated pregnancies | - Adiponectin - Leptin - Oxidative stress markers: malondialdehyde-thiobarbituric acid reactive substances (MDA-TBARS) - Plasma antioxidant activity [Ferric Reducing Ability of Plasma (FRAP)] | Not reported | - Plasma levels of adiponectin and leptin were similar in all the study groups. - FRAP showed gradual increases between the three groups, with a significant difference between the gestational hypertension and preeclampsia groups, the latter being higher. - MDA-TBARS plasma levels were similar between the groups. - There was a significant negative correlation between MDA-TBARS and adiponectin, suggesting a relationship between antioxidant levels and the glycoprotein in healthy pregnancies, which is altered in patients with gestational hypertension or preeclampsia. |
| Nevalainen et al. [60] 2017 | 864 participants distributed into training and test groups: - 71 pregnant women with early onset preeclampsia - 793 pregnant women without preeclampsia Training groups: - Controls (n = 652) - Early onset preeclampsia (n = 29) Test groups: - Controls (n = 141) - Early onset preeclampsia (n = 42) | - Alpha-fetoprotein (AFP); Placental growth factor (PIGF) - Soluble tumour necrosis factor receptor 1 (sTNFR1); Retinol binding protein 4 (RBP4); Disintegrin and metalloprotease-12 (ADAM12) - Soluble P-selectin - Follistatin like-3 (FSTL3) - Adiponectin; Angiopoietin-2 - Sex hormone binding globulin (SHBG) - PAPP-A (Pregnancy-associated plasma protein A); β-hCG (human chorionic gonadotrophic hormone) | Not reported | - The best individual maternal serum biomarkers in the first trimester of pregnancy for predicting the development of early preeclampsia were: AFP, PIGF, RBP4 and sTNFR1. - The best screening combination in the test set was achieved by adding sTNFR1, mean arterial pressure and AFP, PlGF or RBP4 to the combined first trimester screening information of maternal characteristics, PAPP-A and β-hCG. - When assessing adiponectin, SHBG and FSTL3 levels, the results were contradictory: adiponectin and SHBG values increased in the test set and decreased in the training sample set; FSTL3 values decreased in the test set and increased in the training sample set. |
| Martinez- Fierro et al. [61] 2014 | 177 participants: - 108 pregnant women with preeclampsia - 60 with mild preeclampsia - 48 with severe preeclampsia - 69 normotensive pregnant women | Gene expression: -Hemeoxygenase 1 - Superoxide dismutase - Vascular endothelial growth factor A - Transforming growth factor beta 1 (TGF-β1) - IL-6, IL-15 - Adiponectin | Not reported | - The onset and severity of preeclampsia was reflected in the imbalance in the expression of the Vascular Endothelial Growth Factor A and TGF-β1 genes, with their under-expression being a constant in most cases. - IL-6, IL-15 and adiponectin showed low or no expression in the peripheral blood mononuclear cell samples evaluated. Therefore, the association between IL6, IL-15 and adiponectin expression and preeclampsia could not be assessed. |
| Rao et al. [62] 2021 | 120 participants: - 60 pregnant women with preeclampsia - 60 pregnant women without preeclampsia | - Adiponectin - Leptin | BMI reported but not adjusted | - Adiponectin levels were higher in the study group than in the control group. However, the difference was not statistically significant. - Leptin levels in the study group were significantly higher than in the control group. - The adiponectin/leptin ratio was significantly lower in the study group. However, it was significantly higher in pregnant women with severe preeclampsia compared to pregnant women with mild preeclampsia. - Severe preeclampsia is associated with significantly higher adiponectin levels compared to mild preeclampsia. |
| BMI Considered | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin in PE | Inclusion of Only Obese Pregnant Women | Exclusion of Underweight and/or Overweight and/or Obese Pregnant Women | No BMI Restriction in the Exclusion Criteria | No BMI Restriction in the Exclusion Criteria, but Result Observed Only in Obese Pregnant Women | ||||||||
| Increase | 1 | 2 | 3 | 2 | 1 | |||||||
| No change | 1 | 1 | 1 | 3 | 2 | |||||||
| Decreased | 1 | 1 | 2 | 3 | 3 | 1 | ||||||
| It was not possible to determine | 1 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrilho, I.; Mariana, M.; Cairrao, E. Adiponectin as a Biomarker of Preeclampsia: A Systematic Review. Reprod. Med. 2025, 6, 29. https://doi.org/10.3390/reprodmed6040029
Carrilho I, Mariana M, Cairrao E. Adiponectin as a Biomarker of Preeclampsia: A Systematic Review. Reproductive Medicine. 2025; 6(4):29. https://doi.org/10.3390/reprodmed6040029
Chicago/Turabian StyleCarrilho, Inês, Melissa Mariana, and Elisa Cairrao. 2025. "Adiponectin as a Biomarker of Preeclampsia: A Systematic Review" Reproductive Medicine 6, no. 4: 29. https://doi.org/10.3390/reprodmed6040029
APA StyleCarrilho, I., Mariana, M., & Cairrao, E. (2025). Adiponectin as a Biomarker of Preeclampsia: A Systematic Review. Reproductive Medicine, 6(4), 29. https://doi.org/10.3390/reprodmed6040029







