Can We Safely Decrease Early-Term Delivery and Cesarean Section Rate in Pregnancies Complicated by Fetal Transposition of Great Arteries?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Data Collection
2.3. Perinatal Management
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population and Participant Characteristics
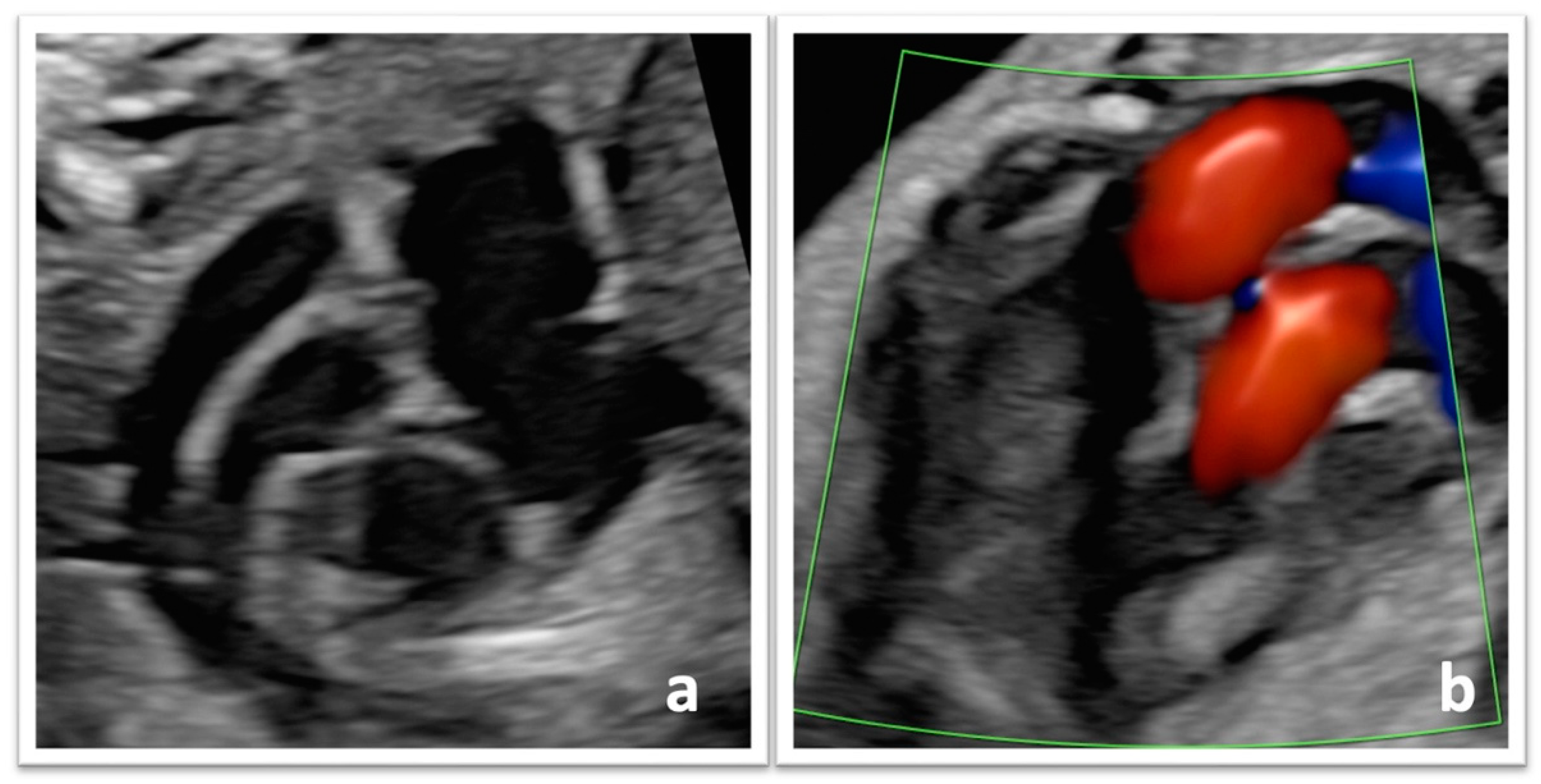
3.2. Diagnostic Features and Follow-Up
3.3. Obstetric Outcomes
3.4. Initial Management and Surgical Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marek, J.; Tomek, V.; Škovránek, J.; Povýšilová, V.; Šamánek, M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: A 21-year experience. Heart 2011, 97, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Valenzuela, N.J.; Peixoto, A.B.; Júnior, E.A. Prenatal diagnosis of transposition of the great arteries: An updated review. Ultrasonography 2020, 39, 331–339. [Google Scholar] [CrossRef]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch Gynecol Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef]
- Jouannic, J.M.; Gavard, L.; Fermont, L.; Le Bidois, J.; Parat, S.; Vouhé, P.R.; Dumez, Y.; Sidi, D.; Bonnet, D. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation 2004, 110, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Coltri, A.; Butera, G.; Fermont, L.; Le Bidois, J.; Kachaner, J.; Sidi, D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999, 99, 916–918. [Google Scholar] [CrossRef]
- Blyth, M.; Howe, D.; Gnanapragasam, J.; Wellesley, D. The hidden mortality of transposition of the great arteries and survival advantage provided by prenatal diagnosis. BJOG 2008, 115, 1096–1100. [Google Scholar] [CrossRef]
- van Velzen, C.L.; Haak, M.C.; Reijnders, G.; Rijlaarsdam, M.E.B.; Bax, C.J.; Pajkrt, E.; Hruda, J.; Galindo-Garre, F.; Bilardo, C.M.; de Groot, C.J.M.; et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet. Gynecol. 2015, 45, 320–325. [Google Scholar] [CrossRef]
- Hellström-Westas, L.; Hanséus, K.; Jögi, P.; Lundström, N.-R.; Svenningsen, N. Long-distance transports of newborn infants with congenital heart disease. Pediatr. Cardiol. 2001, 22, 380–384. [Google Scholar] [CrossRef]
- Sarris, G.E.; Balmer, C.; Bonou, P.; Comas, J.V.; da Cruz, E.; Di Chiara, L.; Di Donato, R.M.; Fragata, J.; Jokinen, T.E.; Kirvassilis, G.; et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. Eur. J. Cardio-Thoracic Surg. 2017, 51, e1–e32. [Google Scholar] [CrossRef]
- Lundström, N.; Berggren, H.; Björkhem, G.; Jögi, P.; Sunnegårdh, J. Centralization of pediatric heart surgery in Sweden. Pediatr. Cardiol. 2000, 21, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tworetzky, W.; McElhinney, D.B.; Reddy, V.M.; Brook, M.M.; Hanley, F.L.; Silverman, N.H. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001, 103, 1269–1273. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Wypij, D.; Bellinger, D.C.; Rappaport, L.A.; Heffner, L.J.; Jonas, R.A.; Newburger, J.W. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics 2004, 113, e335–e340. [Google Scholar] [CrossRef]
- Levey, A.; Glickstein, J.S.; Kleinman, C.S.; Levasseur, S.M.; Chen, J.; Gersony, W.M.; Williams, I.A. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr. Cardiol. 2010, 31, 587–597. [Google Scholar] [CrossRef]
- ACOG. Committee Opinion No. 764: Medically Indicated Late-Preterm and Early-Term Deliveries. Obstet. Gynecol. 2019, 133, e151–e155. [Google Scholar] [CrossRef]
- Clark, S.L.; Miller, D.D.; Belfort, M.A.; Dildy, G.A.; Frye, D.K.; Meyers, J.A. Neonatal and maternal outcomes associated with elective term delivery. Am. J. Obstet. Gynecol. 2009, 200, 156.e1–156.e4. [Google Scholar] [CrossRef]
- Laas, E.; on behalf of the EPICARD study group; Lelong, N.; Ancel, P.-Y.; Bonnet, D.; Houyel, L.; Magny, J.-F.; Andrieu, T.; Goffinet, F.; Khoshnood, B. Impact of preterm birth on infant mortality for newborns with congenital heart defects: The EPICARD population-based cohort study. BMC Pediatr. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- McLaren, R., Jr.; London, V.; Stein, J.L.; Minkoff, H. Adverse outcomes in early term versus full-term deliveries among higher-order cesarean births. J. Matern. Fetal. Neonatal. Med. 2021, 35, 5464–5469. [Google Scholar] [CrossRef]
- Costello, J.M.; Pasquali, S.K.; Jacobs, J.P.; He, X.; Hill, K.D.; Cooper, D.S.; Backer, C.L.; Jacobs, M.L. Gestational age at birth and outcomes after neonatal cardiac surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation 2014, 17, 2511–2517. [Google Scholar] [CrossRef]
- Hickey, E.J.; Nosikova, Y.; Zhang, H.; Caldarone, C.A.; Benson, L.; Redington, A.; Van Arsdell, G.S. Very low-birth-weight infants with congenital cardiac lesions: Is there merit in delaying intervention to permit growth and maturation? J. Thorac. Cardiovasc. Surg. 2012, 143, 126–136.e1. [Google Scholar] [CrossRef][Green Version]
- Donofrio, M.T.; Levy, R.J.; Schuette, J.J.; Skurow-Todd, K.; Sten, M.-B.; Stallings, C.; Pike, J.I.; Krishnan, A.; Ratnayaka, K.; Sinha, P.; et al. Specialized delivery room planning for fetuses with critical congenital heart disease. Am. J. Cardiol. 2013, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Craigo, S.D. Indicated preterm birth for fetal anomalies. Semin. Perinatol. 2011, 35, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Curzon, C.L.; Milford-Beland, S.; Li, J.S.; O’Brien, S.M.; Jacobs, J.P.; Jacobs, M.L.; Welke, K.F.; Lodge, A.J.; Peterson, E.D.; Jaggers, J. Cardiac Surgery in Infants with Low Birth Weight is Associated with Increased Mortality: Analysis of the Society of Thoracic Surgeons Congenital Heart Database. J. Thorac. Cardiovasc. Surg. 2008, 135, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kansy, A.; Tobota, Z.; Maruszewski, P.; Maruszewski, B. Analysis of 14,843 Neonatal Congenital Heart Surgical Procedures in the European Association for Cardiothoracic Surgery Congenital Database. Ann. Thorac. Surg. 2010, 89, 1255–1259. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists ACOG. Practice Bulletin No. 106: Intrapartum Fetal Heart Rate Monitoring: Nomenclature, Interpretation, and General Management Principles. Obstet. Gynecol. 2009, 114, 192–202. [Google Scholar] [CrossRef]
- Hraska, V.; Podnar, T.; Kunovsky, P.; Kovacikova, L.; Kaldararova, M.; Horvathova, E.; Masura, J.; Mayer, J. Is a learning curve for arterial switch operation in small countries still acceptable? Model for cooperation in Europe. Eur. J. Cardio-Thoracic Surg. 2003, 24, 352–357. [Google Scholar] [CrossRef]
- Cnota, J.F.; Gupta, R.; Michelfelder, E.C.; Ittenbach, R.F. Congenital heart disease infant death rates decrease as gestational age advances from 34 to 40 weeks. J. Pediatr. 2011, 159, 761–765. [Google Scholar] [CrossRef]
- Costello, J.M.; Polito, A.; Brown, D.W.; McElrath, T.F.; Graham, D.A.; Thiagarajan, R.R.; Bacha, E.A.; Allan, C.K.; Cohen, J.N.; Laussen, P.C. Birth before 39 weeks’ gestation is associated with worse outcomes in neonates with heart disease. Pediatrics 2010, 126, 277–284. [Google Scholar] [CrossRef]
- Słodki, M.; Axt-Fliedner, R.; Zych-Krekora, K.; Wolter, A.; Kawecki, A.; Enzensberge, C.; Gulczyńska, E.; Respondek-Liberska, M. The international prenatal cardiology collaboration group new method to predict need for Rashkind procedure in fetuses with dextro-transposition of the great arteries. Ultrasound Obstet. Gynecol. 2018, 51, 531–536. [Google Scholar] [CrossRef]
- Escobar-Diaz, M.C.; Freud, L.R.; Bueno, A.; Brown, D.W.; Friedman, K.G.; Schidlow, D.; Emani, S.; Del Nido, P.J.; Tworetzky, W. Prenatal diagnosis of transposition of the great arteries over a 20-year period: Improved but imperfect. Ultrasound Obstet. Gynecol. 2015, 45, 678–682. [Google Scholar] [CrossRef]
- Sanapo, L.; Moon-Grady, A.J.; Donofrio, M.T. Perinatal and Delivery Management of Infants with Congenital Heart Disease. Clin. Perinatol. 2016, 43, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Y.; Hogan, W.J.; Conturie, C.; Sunderji, S.; Duffy, J.Y.; Peyvandi, S.; Boe, N.M.; Melber, D.; Fajardo, V.M.; Tandel, M.D.; et al. Multi-Institutional Practice-Patterns in Fetal Congenital Heart Disease Following Implementation of a Standardized Clinical Assessment and Management Plan. J. Am. Heart Assoc. 2021, 10, e021598. [Google Scholar] [CrossRef] [PubMed]
- Quaegebeur, J.M.; Rohmer, J.; Ottenkamp, J.; Buis, T.; Kirklin, J.W.; Blackstone, E.H.; Brom, A.G. The arterial switch operation. An eight-year experience. J. Thorac. Cardiovasc. Surg 1986, 92, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Kadour-Peero, E.; Bleicher, I.; Vitner, D.; Sloma, R.; Bahous, R.; Levy, E.; Sagi, S.; Gonen, R. When should repeat cesarean delivery be scheduled, after two or more previous cesarean deliveries? J. Matern. Fetal. Neonatal Med. 2018, 31, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Giorgione, V.; Fesslova, V.; Boveri, S.; Candiani, M.; Khalil, A.; Cavoretto, P. Adverse perinatal outcome and placental abnormalities in pregnancies with major fetal congenital heart defects: A retrospective case-control study. Prenat. Diagn. 2020, 40, 1390–1397. [Google Scholar] [CrossRef]
- Inversetti, A.; Fesslova, V.; Deprest, J.; Candiani, M.; Giorgione, V.; Cavoretto, P. Prenatal Growth in Fetuses with Isolated Cyanotic and Non-Cyanotic Congenital Heart Defects. Fetal. Diagn. Ther. 2020, 47, 411–419. [Google Scholar] [CrossRef]
| All TGA (n = 17) | |
|---|---|
| Maternal age (years), median (range) | 35 (19–39) |
| BMI, kg/m2, median (range) | 23.2 (16.4–31.7) |
| BMI ≥ 30 kg/m2, n (%) | 1 (5.9%) |
| Smoking, n (%) | 2 (11.8%) |
| Gravidity, median (range) | 2 (1–3) |
| Previous pregnancy, n (%) | 9 (52.9%) |
| Previous cesarean section, n (%) | 2 (11.8%) |
| Twin pregnancy, n (%) | 0 |
| CHD in previous pregnancy, n (%) | 1 (5.9%) |
| Type of CHD | Pulmonary stenosis |
| All TGA (n = 17) | |
|---|---|
| GA at first consultation in our department (weeks), median (range) | 20.5 (14.1–32.6) |
| Prenatal diagnosis | |
| Isolated TGA, n (%) | 11 (64.7%) |
| TGA + VSD, n (%) | 5 (29.4%) |
| Complex TGA, n (%) | 1 (5.9%) |
| Invasive procedure of prenatal diagnosis, n (%) | 12 (70.6%) |
| Abnormal, n (%) | 1 (5.9%) |
| GA at last visit (weeks), median (range) | 37.6 (34.6–41.0) |
| EFW at last visit (grams), median (range) | 3251 (2239–3502) |
| EFW centile, median (range) | 56 (24–85) |
| RCIU, n (%) | 0 |
| All TGA (n = 17) | |
|---|---|
| Gender (female), n (%) | 7 (41.2%) |
| Elective cesarean section, n (%) | 1 (5.9%) |
| Vaginal birth, n (%) | 14 (82.4%) |
| Operative birth, n (%) | 4 (23.5%) |
| Vacuum extraction, n (%) | 4 (100%) |
| Urgent cesarean section, n (%) | 2 (11.8%) |
| Gestational age at delivery (weeks), median (range) | 39.2 (37.2–41.0) |
| Gestational age < 39 weeks, n (%) | 2 (11.8%) |
| Gestational age < 40 weeks, n (%) | 12 (70.6%) |
| Gestational age < 41 weeks, n (%) | 16 (94.1%) |
| Hour at delivery, median (range) | 5 p.m. (2 a.m.–10 p.m.) |
| Saturday to Sunday admission and birth, n (%) | 3 (17.6%) |
| Birthweight (grams), median (range) | 3300 (2399–3891) |
| 5-min APGAR score, median (range) | 9 (4–10) |
| 5-min APGAR score < 7, n (%) | 2 (11.8%) |
| Umbilical cord arterial pH, median (range) | 7.28 (7.18–7.44) |
| Umbilical cord arterial pH < 7.1, n (%) | 0 |
| Mortality before surgery, n (%) | 1 (5.8%) |
| All TGA (n = 17) | |
|---|---|
| Postnatal diagnosis | |
| Isolated TGA, n (%) | 10 (58.8%) |
| TGA + VSD, n (%) | 3 (17.7%) |
| Complex TGA, n (%) | 4 (23.5%) |
| Use of prostaglandin E2, n (%) | 17 (100%) |
| BAS (Rashkind), n (%) | 16 (94.1%) |
| Age at BAS (Rashkind) (hours), median (range) | 5 (2–408) |
| Surgery (switch), n (%) | 14 (82.4%) |
| Age at surgery (days), median (range) | 8 (3–30) |
| Intubation or mechanical ventilation, n (%) | 4 (23.5%) |
| Metabolic acidosis, n (%) | 1 (5.9%) |
| Length of hospital stays (days), median (range) | 29 (11–71) |
| Post-surgery complications, n (%) | 6 (46.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimenea, A.; García-Díaz, L.; Méndez, A.; Antiñolo, G. Can We Safely Decrease Early-Term Delivery and Cesarean Section Rate in Pregnancies Complicated by Fetal Transposition of Great Arteries? Reprod. Med. 2023, 4, 233-241. https://doi.org/10.3390/reprodmed4030021
Chimenea A, García-Díaz L, Méndez A, Antiñolo G. Can We Safely Decrease Early-Term Delivery and Cesarean Section Rate in Pregnancies Complicated by Fetal Transposition of Great Arteries? Reproductive Medicine. 2023; 4(3):233-241. https://doi.org/10.3390/reprodmed4030021
Chicago/Turabian StyleChimenea, Angel, Lutgardo García-Díaz, Ana Méndez, and Guillermo Antiñolo. 2023. "Can We Safely Decrease Early-Term Delivery and Cesarean Section Rate in Pregnancies Complicated by Fetal Transposition of Great Arteries?" Reproductive Medicine 4, no. 3: 233-241. https://doi.org/10.3390/reprodmed4030021
APA StyleChimenea, A., García-Díaz, L., Méndez, A., & Antiñolo, G. (2023). Can We Safely Decrease Early-Term Delivery and Cesarean Section Rate in Pregnancies Complicated by Fetal Transposition of Great Arteries? Reproductive Medicine, 4(3), 233-241. https://doi.org/10.3390/reprodmed4030021







