Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
- AC below the 3rd centile or estimated fetal weight (EFW) below the 3rd centile or absent end diastolic flow in the umbilical artery
- 2.
- Both of the following
- -
- EFW or AC circumference below the 10th centile and
- -
- Pulsatility index (PI) of the uterine artery above the 95th centile or pulsatility index in the umbilical artery (UA PI) above the 95th centile.
- AC below the 3rd centile, or EFW below the 3rd centile
- 2.
- At least two of the following:
- -
- AC below the 10th centile or EFW below the 10th centile
- -
- AC or EFW crossing centiles >2 quartiles
- -
- Cerebro–placental ratio (CPR) below the 5th centile or UA PI above the 95th centile.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition for placental fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.A.; Da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound. Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound. Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Stampalija, T.; Thornton, J.; Marlow, N.; Napolitano, R.; Bhide, A.; Pickles, T.; Bilardo, C.M.; Gordijn, S.J.; Gyselaers, W.; Valensise, H.; et al. Fetal cerebral Doppler changes and outcome in late preterm fetal growth restriction: Prospective cohort study. Ultrasound. Obstet. Gynecol. 2020, 56, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, B.; Novelli, G.P.; Gagliardi, G.; Farsetti, D.; Valensise, H. Pregnancy complications in chronic hypertensive patients are linked to pre-pregnancy maternal cardiac function and structure. Am. J. Obstet. Gynecol. 2020, 223, 425.e1–425.e13. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Farsetti, D.; Pisani, I.; Tiralongo, G.M.; Gagliardi, G.; Lo Presti, D.; Novelli, G.P.; Vasapollo, B. Hemodynamic maladaptation and left ventricular dysfunction in chronic hypertensive patients at the beginning of gestation and pregnancy complications: A case control study. J. Matern. Fetal Neonatal. Med. 2020, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Tiralongo, G.M.; Pisani, I.; Farsetti, D.; Lo Presti, D.; Gagliardi, G.; Basile, M.R.; Novelli, G.P.; Vasapollo, B. Maternal hemodynamics early in labor: A possible link with obstetric risk? Ultrasound. Obstet. Gynecol. 2018, 51, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Farsetti, D.; Lo Presti, D.; Pisani, I.; Tiralongo, G.M.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P. Preterm delivery and elevated maternal total vascular resistance: Signs of suboptimal cardiovascular adaptation to pregnancy? Ultrasound. Obstet. Gynecol. 2016, 48, 491–495. [Google Scholar] [CrossRef]
- Masini, G.; Tay, J.; McEniery, C.M.; Wilkinson, I.B.; Valensise, H.; Tiralongo, G.M.; Farsetti, D.; Gyselaers, W.; Vonck, S.; Lees, C.C. Maternal cardiovascular dysfunction is associated with hypoxic cerebral and umbilical Doppler changes. J. Clin. Med. 2020, 9, 2891. [Google Scholar] [CrossRef]
- Pisani, I.; Tiralongo, G.M.; Lo Presti, D.; Gagliardi, G.; Farsetti, D.; Vasapollo, B.; Novelli, G.P.; Andreoli, A.; Valensise, H. Correlation between maternal body composition and haemodynamic changes in pregnancy: Different profiles for different hypertensive disorders. Pregnancy Hypertens 2017, 131–134. [Google Scholar] [CrossRef]
- Gagliardi, G.; Tiralongo, G.M.; LoPresti, D.; Pisani, I.; Farsetti, D.; Vasapollo, B.; Novelli, G.P.; Andreoli, A.; Valensise, H. Screening for pre-eclampsia in the first trimester: Role of maternal hemodynamics and bioimpedance in non-obese patients. Ultrasound. Obstet. Gynecol. 2017, 50, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, B.; Lo Presti, D.; Gagliardi, G.; Farsetti, D.; Tiralongo, G.M.; Pisani, I.; Novelli, G.P.; Valensise, H. Restricted physical activity in pregnancy reduces maternal vascular resistance and improves fetal growth. Ultrasound. Obstet. Gynecol. 2018, 51, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Parra-Saavedra, M.; Crovetto, F.; Triunfo, S.; Savchev, S.; Parra, G.; Sanz, M.; Gratacos, E.; Figueras, F. Added value of umbilical vein flow as a predictor of perinatal outcome in term small-for-gestational-age fetuses. Ultrasound. Obstet. Gynecol. 2013, 42, 189–195. [Google Scholar] [CrossRef]
- Fernandez, S.; Figueras, F.; Gomez, O.; Martinez, J.M.; Eixarch, E.; Comas, M.; Puerto, B.; Gratacos, E. Intra- and interobserver reliability of umbilical vein blood flow. Prenat. Diagn. 2008, 28, 999–1003. [Google Scholar] [CrossRef]
- Parra-Saavedra, M.; Crovetto, F.; Triunfo, S.; Savchev, S.; Peguero, A.; Nadal, A.; Parra, G.; Gratacos, E.; Figueras, F. Placental findings in late-onset SGA births without Doppler signs of placental insufficiency. Placenta 2013, 34, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Deter, R.L.; Harrist, R.B.; Park, S.K. Estimating fetal age: Computer-assisted analysis of multiple fetal growth parameters. Radiology 1984, 152, 497–5011. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- Ciobanu, A.; Wright, A.; Syngelaki, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound. Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef]
- Bhide, A.; Acharya, G.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, L.; Kiserud, T.; Lee, W.; et al. ISUOG practice guidelines: Use of Doppler ultrasonography in obstetrics. Ultrasound. Obstet. Gynecol. 2013, 41, 233–239. [Google Scholar] [CrossRef]
- Flo, F.; Wilsgaard, T.; Acharya, G. Longitudinal reference ranges for umbilical vein blood flow at a free loop of the umbilical cord. Ultrasound. Obstet. Gynecol. 2010, 36, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Rigano, S.; Bozzo, M.; Ferrazzi, E.; Bellotti, M.; Battaglia, F.C.; Galan, H.L. Early and persistent reduction in umbilical vein blood flow in the growth-restricted fetus: A longitudinal study. Am. J. Obstet. Gynecol. 2001, 185, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Mappa, I.; Bitsadze, V.; Słodki, M.; Khizroeva, J.; Makatsariya, A.; D’antonio, F. Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: Prospective cohort study. Ultrasound. Obstet. Gynecol. 2020, 55, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Dantas, A.P.; Miranda, J.; Crovetto, F.; Eixarch, E.; Rodriguez-Sureda, V.; Dominguez, C.; Casu, G.; Rovira, C.; Nadal, A.; et al. Premature placental aging in term small-for-gestational-age and growth-restricted fetuses. Ultrasound. Obstet. Gynecol. 2019, 53, 615–622. [Google Scholar] [CrossRef]
- Galan, H.L.; Jozwik, M.; Rigano, S.; Regnault, T.R.; Hobbins, J.C.; Battaglia, F.C.; Ferrazzi, E. Umbilical vein blood flow determination in the ovine fetus: Comparison of Doppler ultrasonographic and steady-state diffusion techniques. Am. J. Obstet. Gynecol. 1999, 181, 1149–1153. [Google Scholar] [CrossRef]
| Early FGR (n = 9) | Late FGR (n = 22) | SGA (n = 34) | p-Value * | ||
|---|---|---|---|---|---|
| Age (years) | 33.00 (33.00 to 34.00) | 31.50 (25.25 to 37.00) | 32.00 (29.00 to 35.5) | 0.29 | |
| BMI (kg/m2) | 24.50 (23.30 to 28.70) | 25.89 (24.30 to 27.00) | 23.50 (22.25 to 26.54) | 0.19 | |
| Nulliparous | 6 (67%) | 17 (77%) | 24 (71%) | 0.79 | |
| Gestational age (days) | 203 (176 to 209) | 244 (234 to 250) | 228 (217 to 239) | <0.00001 | § † ‡ |
| AC (mm) | 210 (193 to 229) | 271 (260 to 284) | 262 (248 to 277) | ||
| AC centile | 1.00 (1.00 to 2.00) | 2.00 (1.00 to 2.00) | 5.00 (4.00 to 8.75) | <0.000001 | † ‡ |
| EFW (g) | 912 (660 to 1080) | 1896 (1555 to 1994) | 1668 (1414 to 1942) | ||
| EFW centile | 2.00 (1.00 to 3.00) | 2.00 (1.00 to 3,75) | 6.50 (5.00 to 8.75) | <0.001 | † ‡ |
| UA PI | 1.30 (1.10 to 1.40) | 0.96 (0.83 to 1.14) | 1.02 (0.86 to 1.10) | ||
| UA PI centile | 88.00 (64.00 to 97.00) | 66.00 (20.25 to 90.00) | 63.00 (29.25 to 73.75) | 0.14 | |
| UA PI > 95th centile | 3 (33%) | 4 (18%) | 0 (0%) | 0.01 | † ‡ |
| MCA PI | 1.81 (1.70 to 1.98) | 1.73 (1.60 to 1.98) | 1.95 (1.83 to 2.09) | ||
| MCA PI centile | 52.00 (49.00 to 81.00) | 38.00 (18.75 to 65.00) | 61.50 (52.50 to 76.00) | 0.06 | |
| MCA PI < 5th centile | 1 (11%) | 3 (14%) | 0 (0%) | 0.09 | |
| CPR | 1.52 (1.29 to 1.61) | 1.79 (1.55 to 2.26) | 1.93 (1.75 to 2.32) | ||
| CPR centile | 38.00 (15.00 to 42.00) | 30.50 (13.00 to 74.00) | 52.00 (34.75 to 77.75) | 0.12 | |
| CPR < 5th centile | 2 (22%) | 3 (14%) | 0 (0%) | 0.04 | † ‡ |
| Early FGR (n = 9) | Late FGR (n = 22) | SGA (n = 34) | p-Value * | ||
|---|---|---|---|---|---|
| UV diameter (cm) | 0.52 (0.46 to 0.56) | 0.63 (0.56 to 0.67) | 0.68 (0.65 to 0.73) | ||
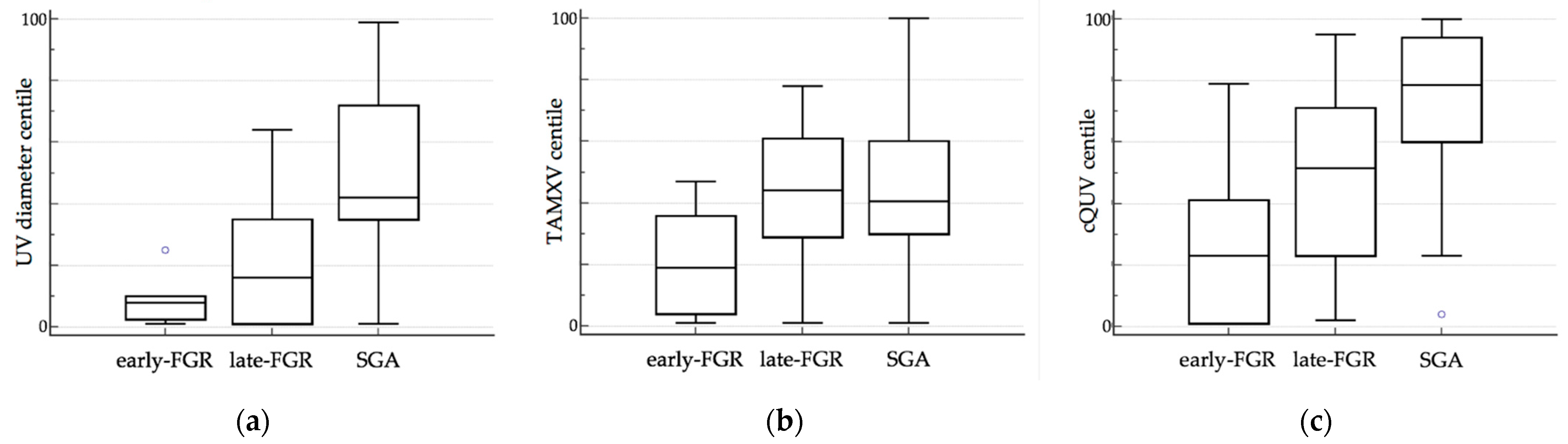
| UV diameter centile | 8.00 (3.00 to 10.00) | 16.00 (1.50 to 33.75) | 42.00 (35.00 to 71.75) | <0.00001 | † ‡ |
| TAMXV (cm/s) | 13.00 (11.54 to 13.66) | 16.30 (15.05 to 17.11) | 15.35 (14.28 to 17.30) | ||
| TAMXV centile | 18.90 (5.00 to 34.04) | 44.44 (29.02 to 59.49) | 40.82 (30.07 to 60.13) | 0.04 | § † |
| QUV (mL/min) | 67 (63 to 96) | 152 (106 to 173) | 177 (153 to 197) | ||
| QUV centile | 2.00 (1.00 to 5.00) | 11.52 (1.00 to 24.63) | 47.52 (28.27 to 66.01) | <0.000001 | † ‡ |
| cQUV (mL/min/kg) | 81 (60 to 89) | 81 (69 to 92) | 105 (93 to 131) | ||
| cQUV centile | 23.12 (1.00 to 40.30) | 51.68 (24.58 to 69.44) | 78.82 (60.28 to 93.72) | <0.001 | § † ‡ |
| cQUV < 10th centile | 3 (33%) | 5 (23%) | 1 (3%) | 0.02 | † ‡ |
| Cut Off | AUC (CI 95%) | p-Value | Sensibility | Specificity | |
|---|---|---|---|---|---|
| <32 weeks | |||||
| cQUV centile | >45 | 0.93 (0.75-0.99) | <0.0001 | 93.75 | 87.50 |
| ≥32 weeks | |||||
| cQUV centile | >50 | 0.72 (0.56-0.85) | <0.01 | 94.40 | 50.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farsetti, D.; Pometti, F.; Tiralongo, G.M.; Lo Presti, D.; Pisani, I.; Gagliardi, G.; Vasapollo, B.; Novelli, G.P.; Valensise, H. Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction. Reprod. Med. 2021, 2, 50-56. https://doi.org/10.3390/reprodmed2010006
Farsetti D, Pometti F, Tiralongo GM, Lo Presti D, Pisani I, Gagliardi G, Vasapollo B, Novelli GP, Valensise H. Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction. Reproductive Medicine. 2021; 2(1):50-56. https://doi.org/10.3390/reprodmed2010006
Chicago/Turabian StyleFarsetti, Daniele, Francesca Pometti, Grazia Maria Tiralongo, Damiano Lo Presti, Ilaria Pisani, Giulia Gagliardi, Barbara Vasapollo, Gian Paolo Novelli, and Herbert Valensise. 2021. "Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction" Reproductive Medicine 2, no. 1: 50-56. https://doi.org/10.3390/reprodmed2010006
APA StyleFarsetti, D., Pometti, F., Tiralongo, G. M., Lo Presti, D., Pisani, I., Gagliardi, G., Vasapollo, B., Novelli, G. P., & Valensise, H. (2021). Fetal Umbilical Vein Flow in the Classification of Fetuses with Growth Restriction. Reproductive Medicine, 2(1), 50-56. https://doi.org/10.3390/reprodmed2010006





