Expression of Wilm’s Tumor Gene (WT1) in Endometrium with Potential Link to Gestational Vascular Transformation
Abstract
1. Introduction
2. Material and Methods
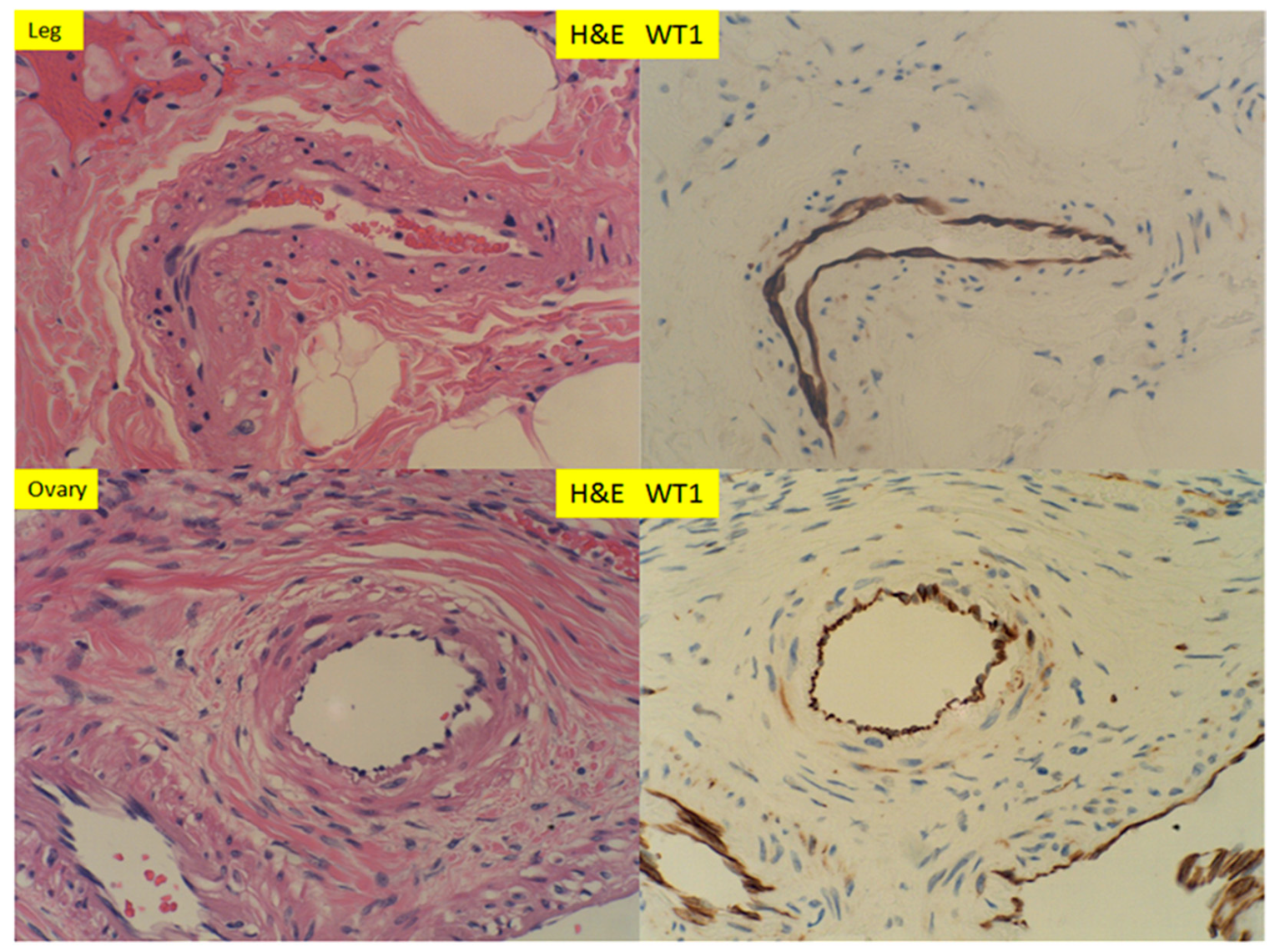
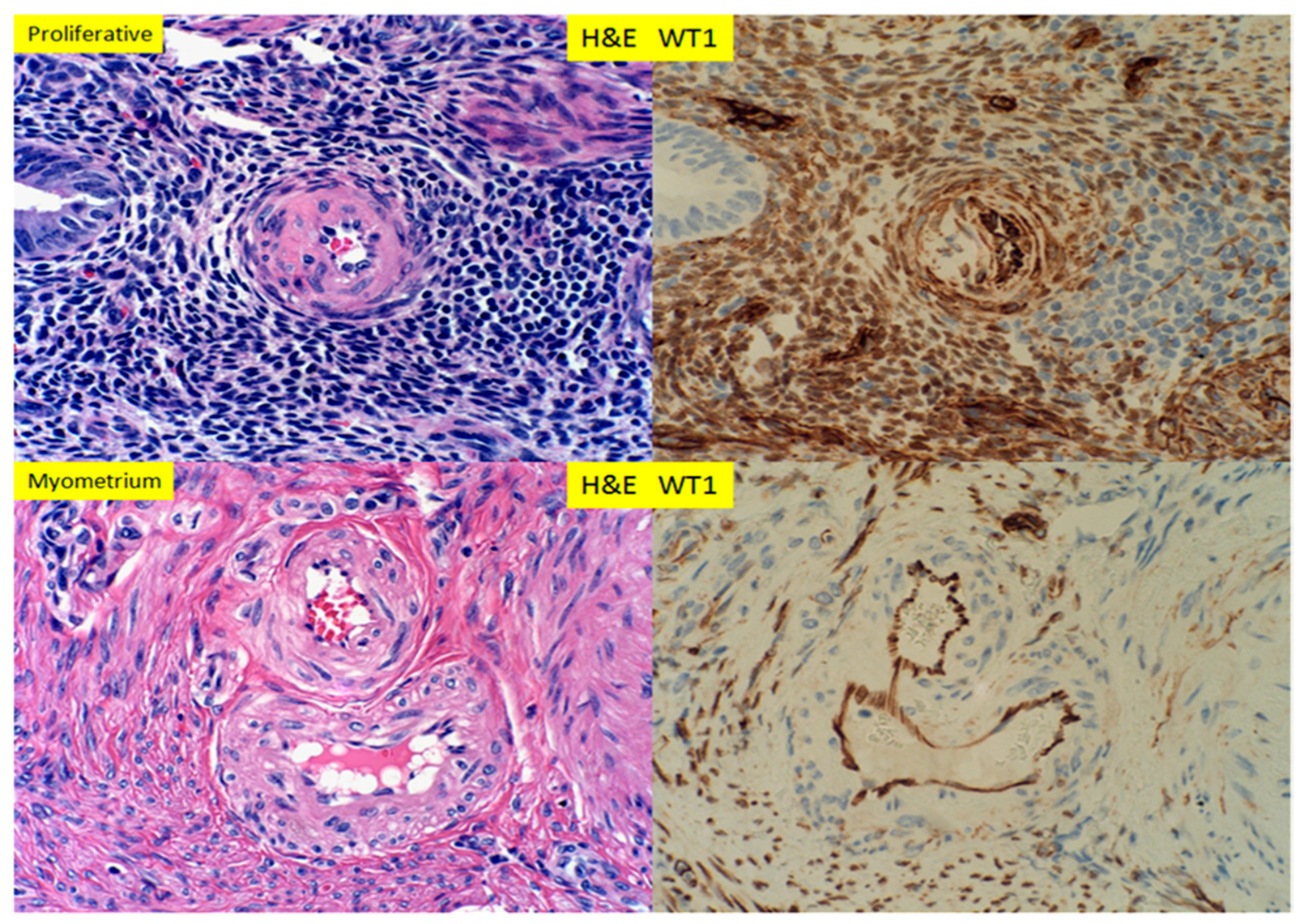
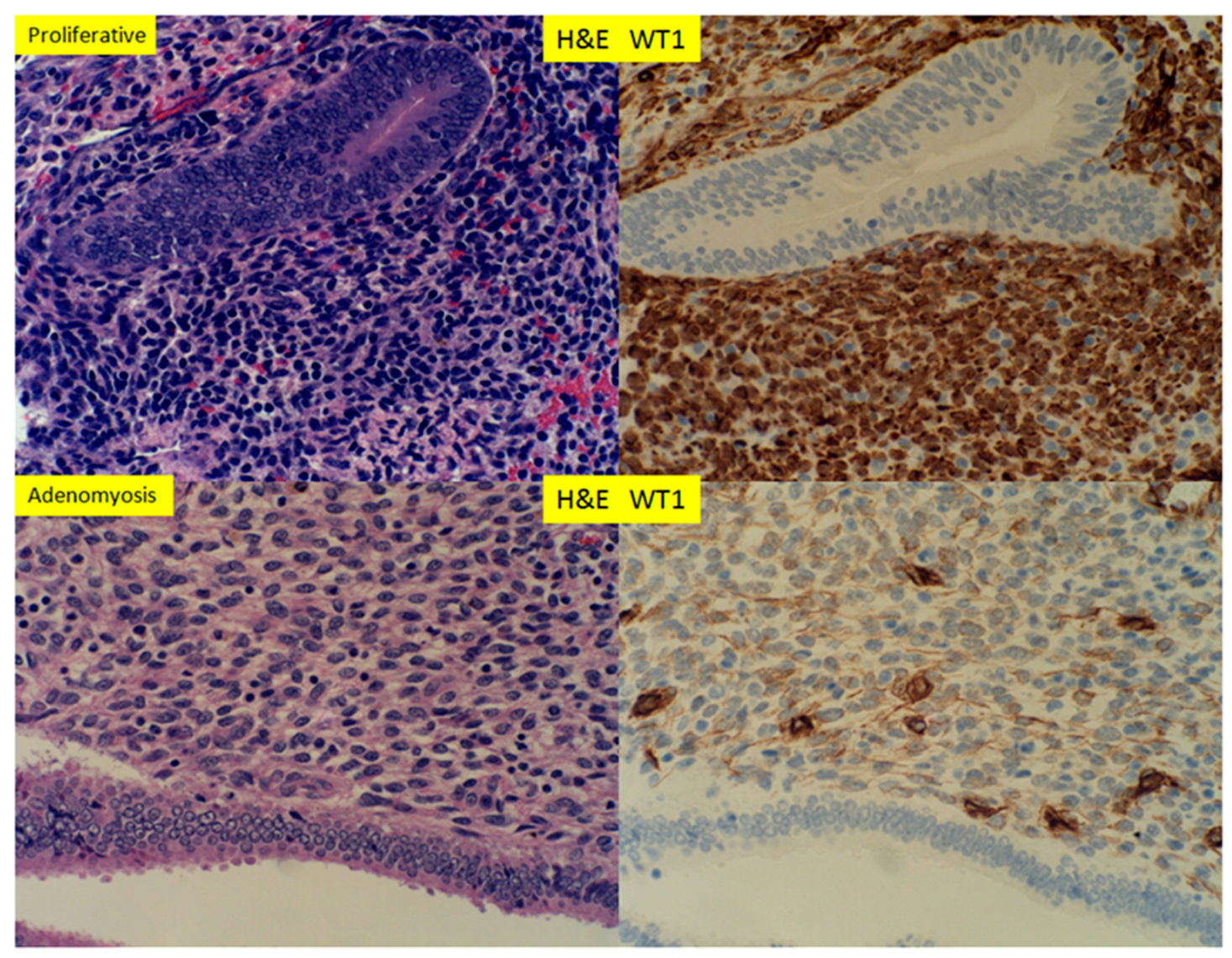
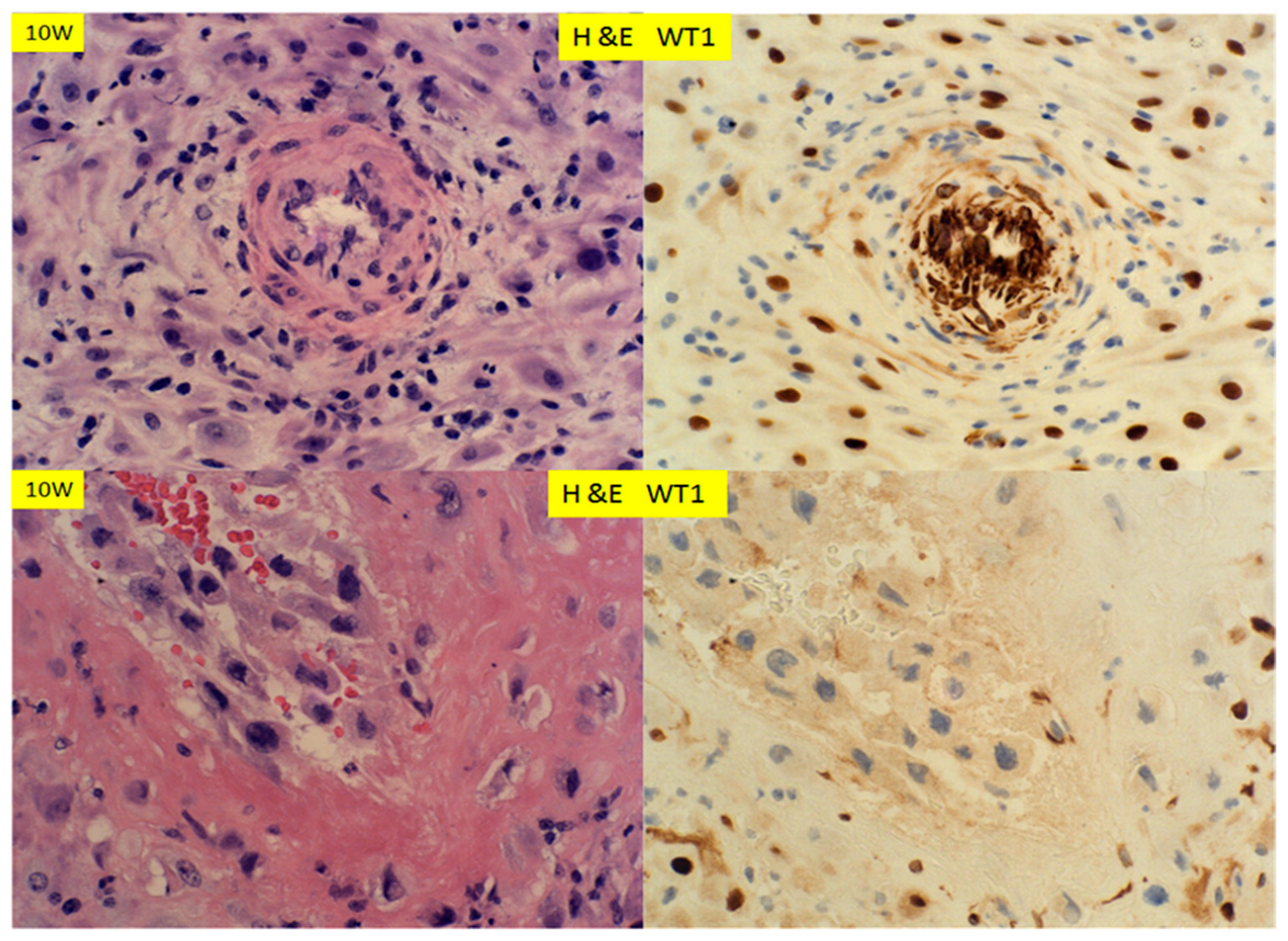
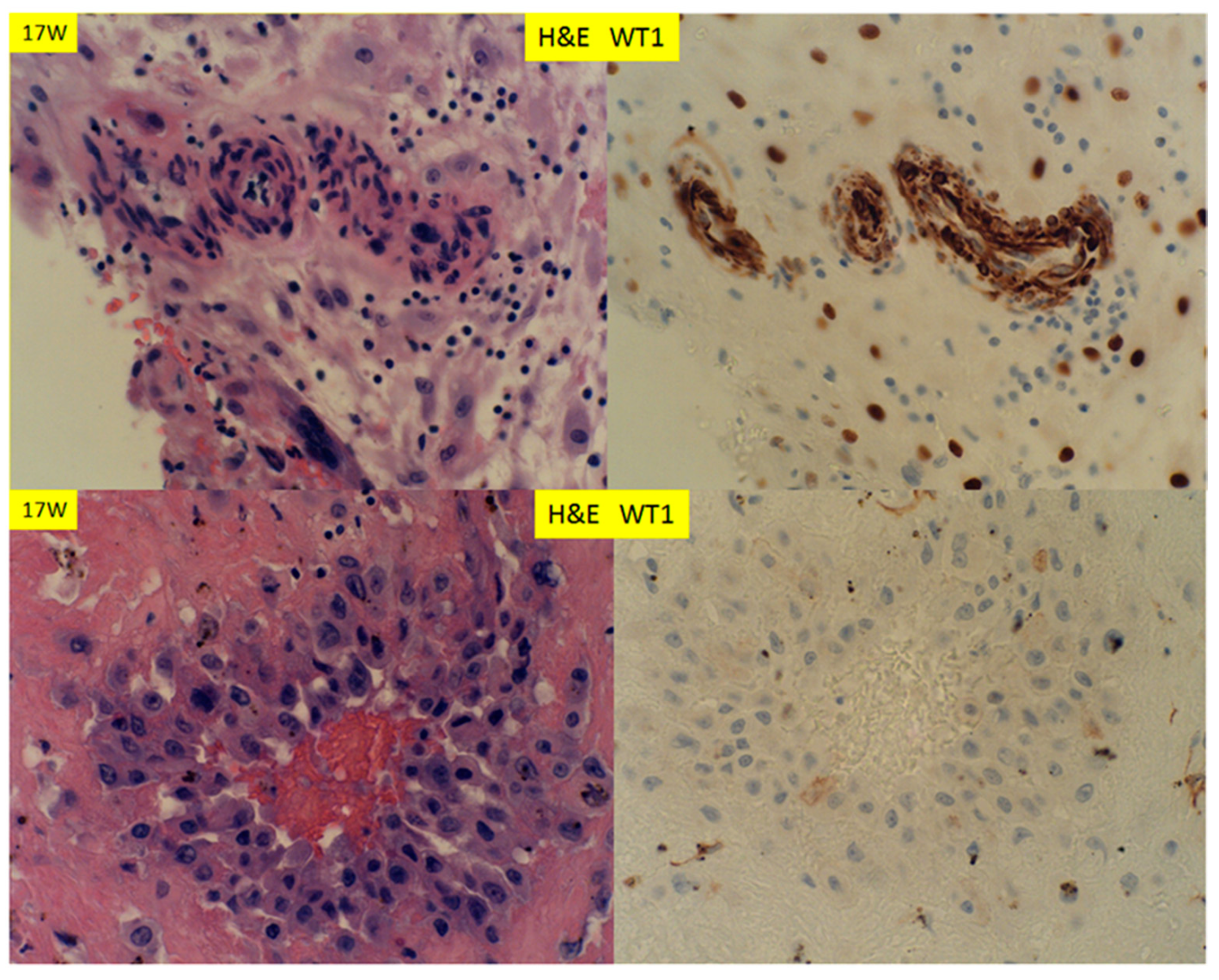
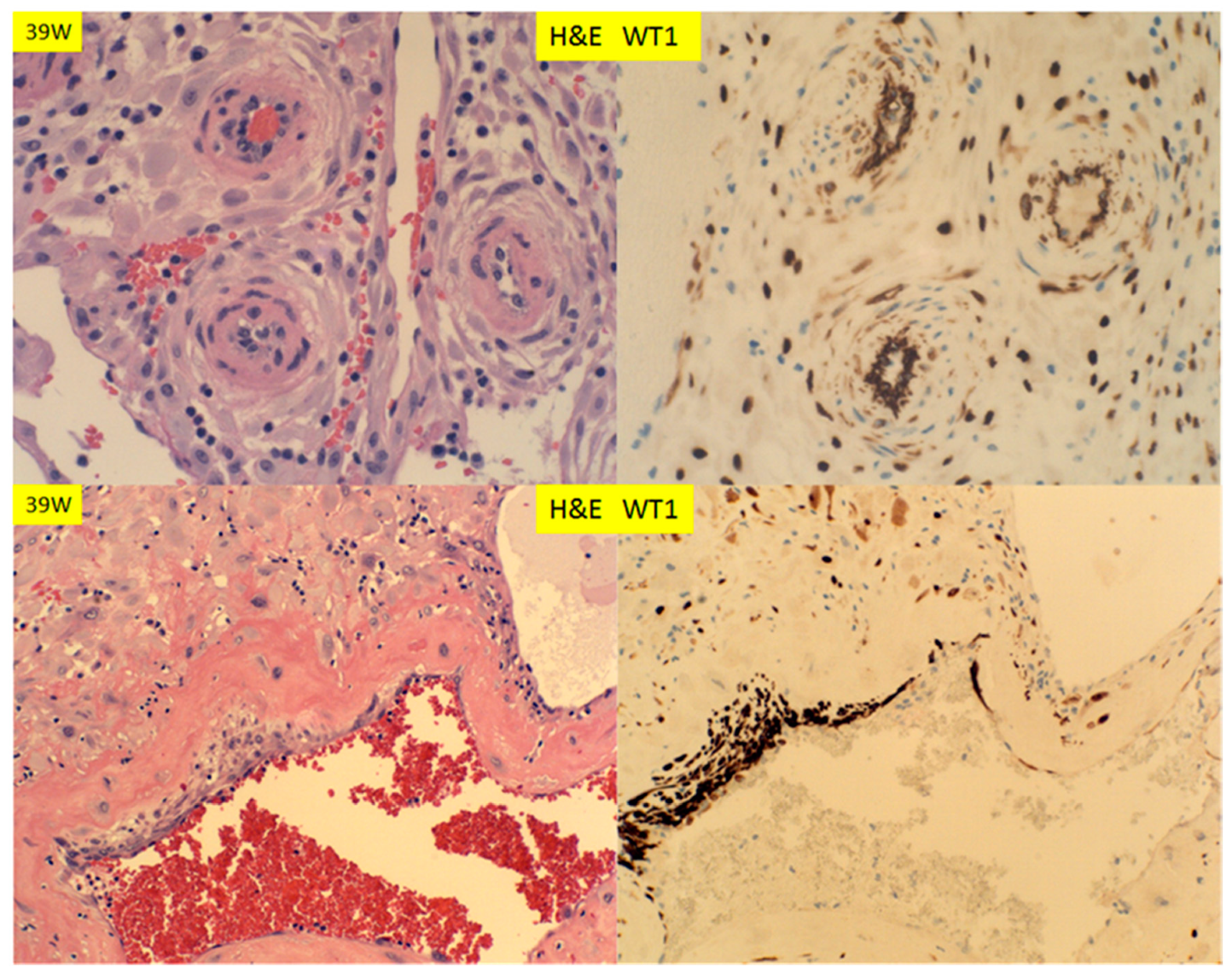
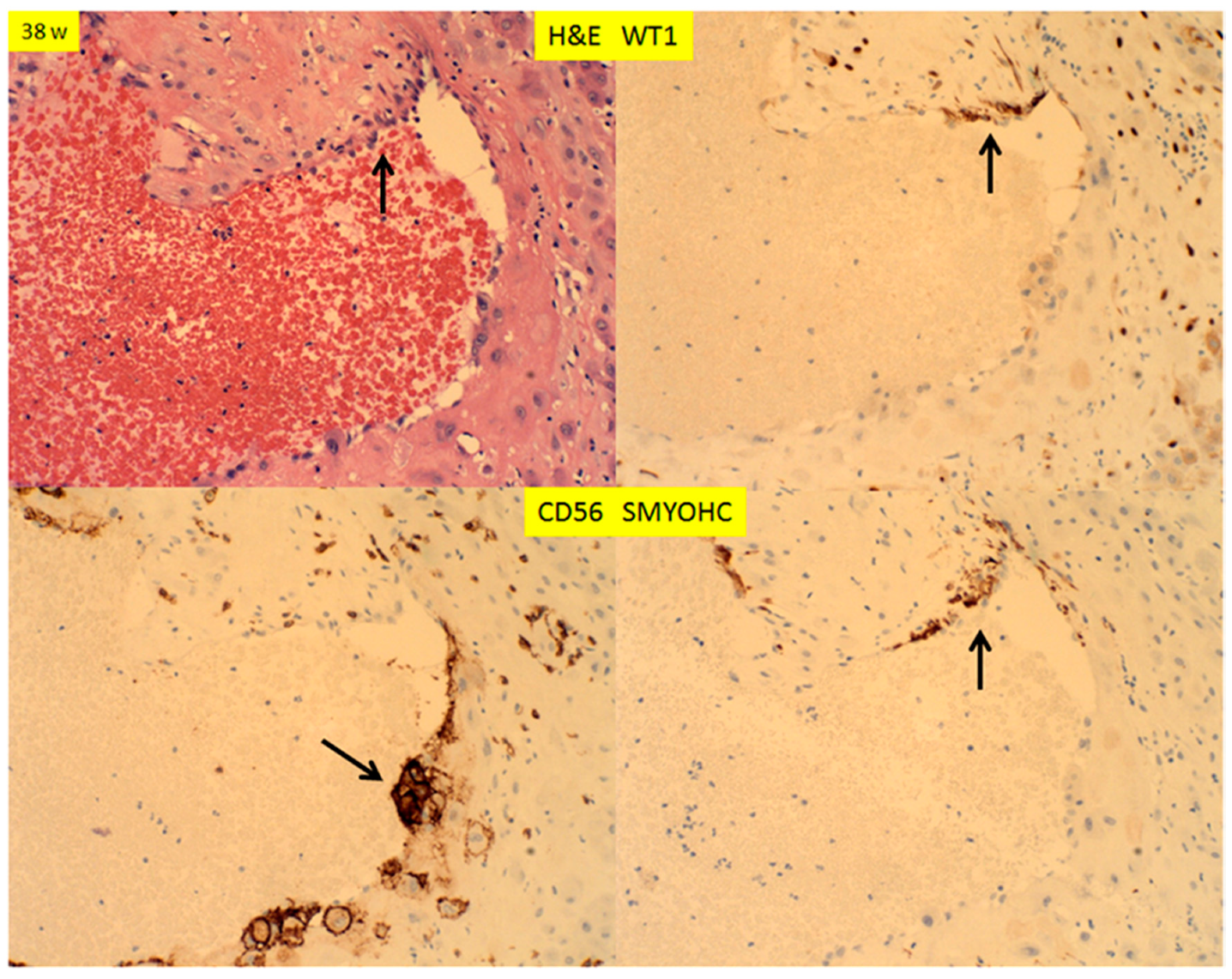
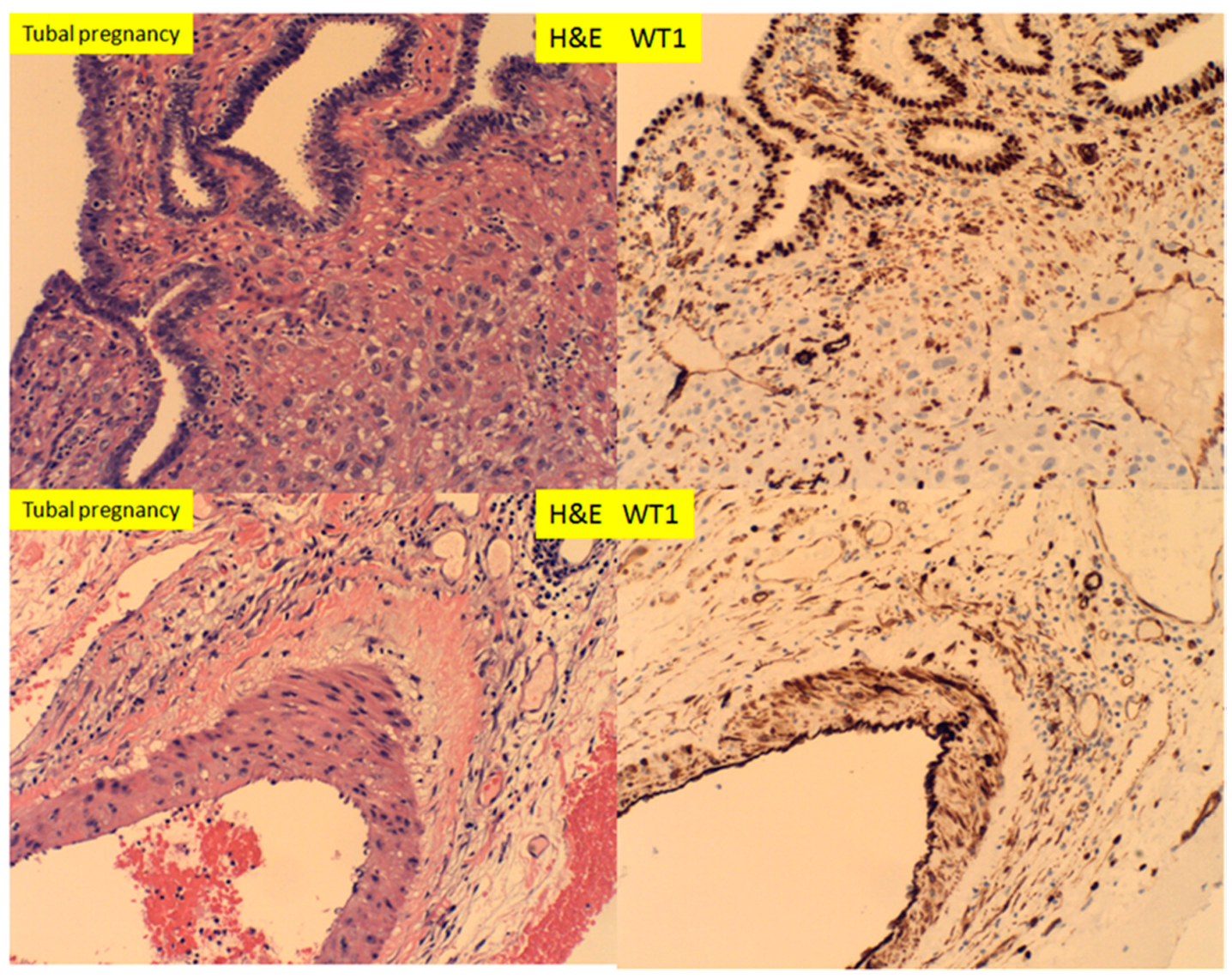
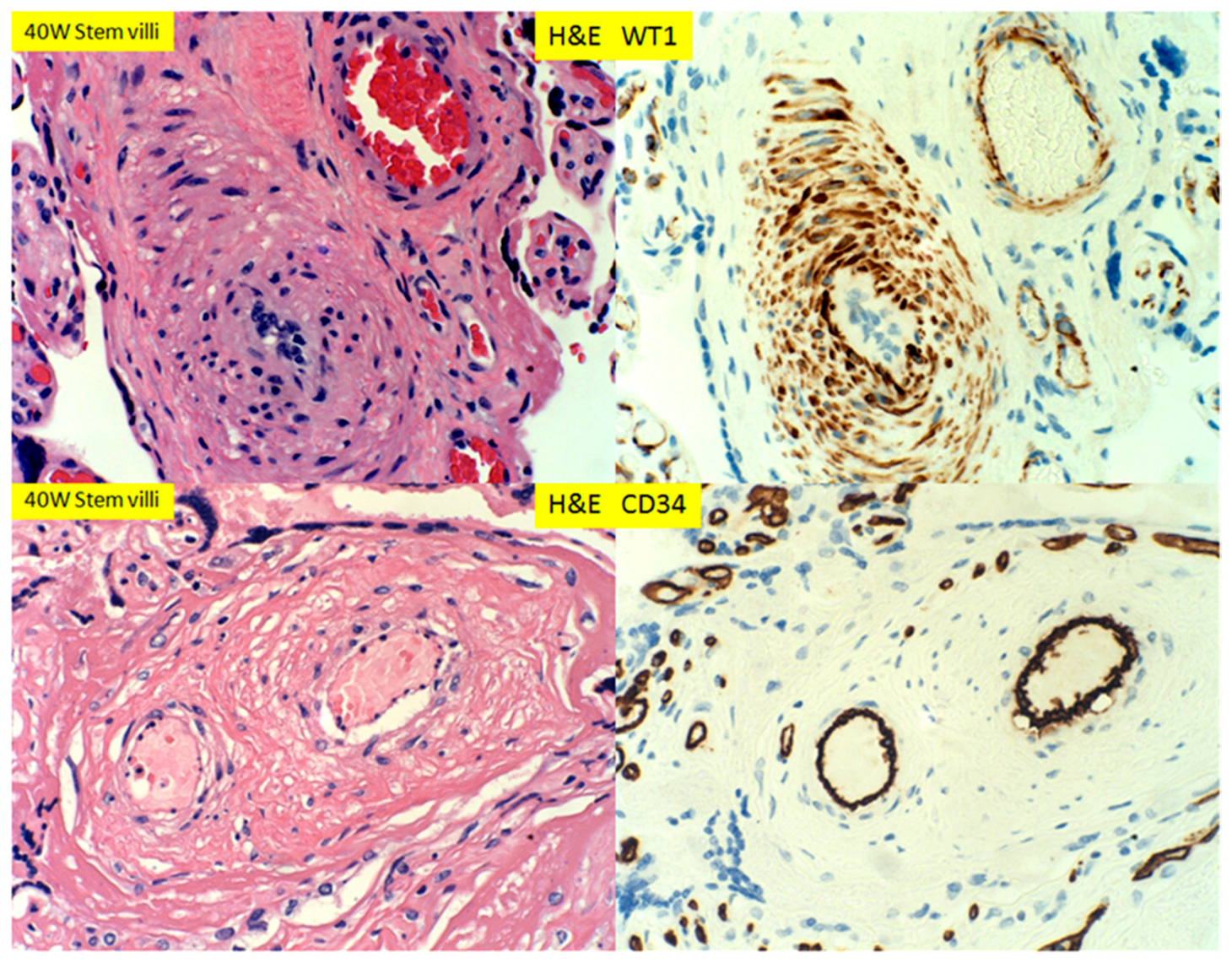
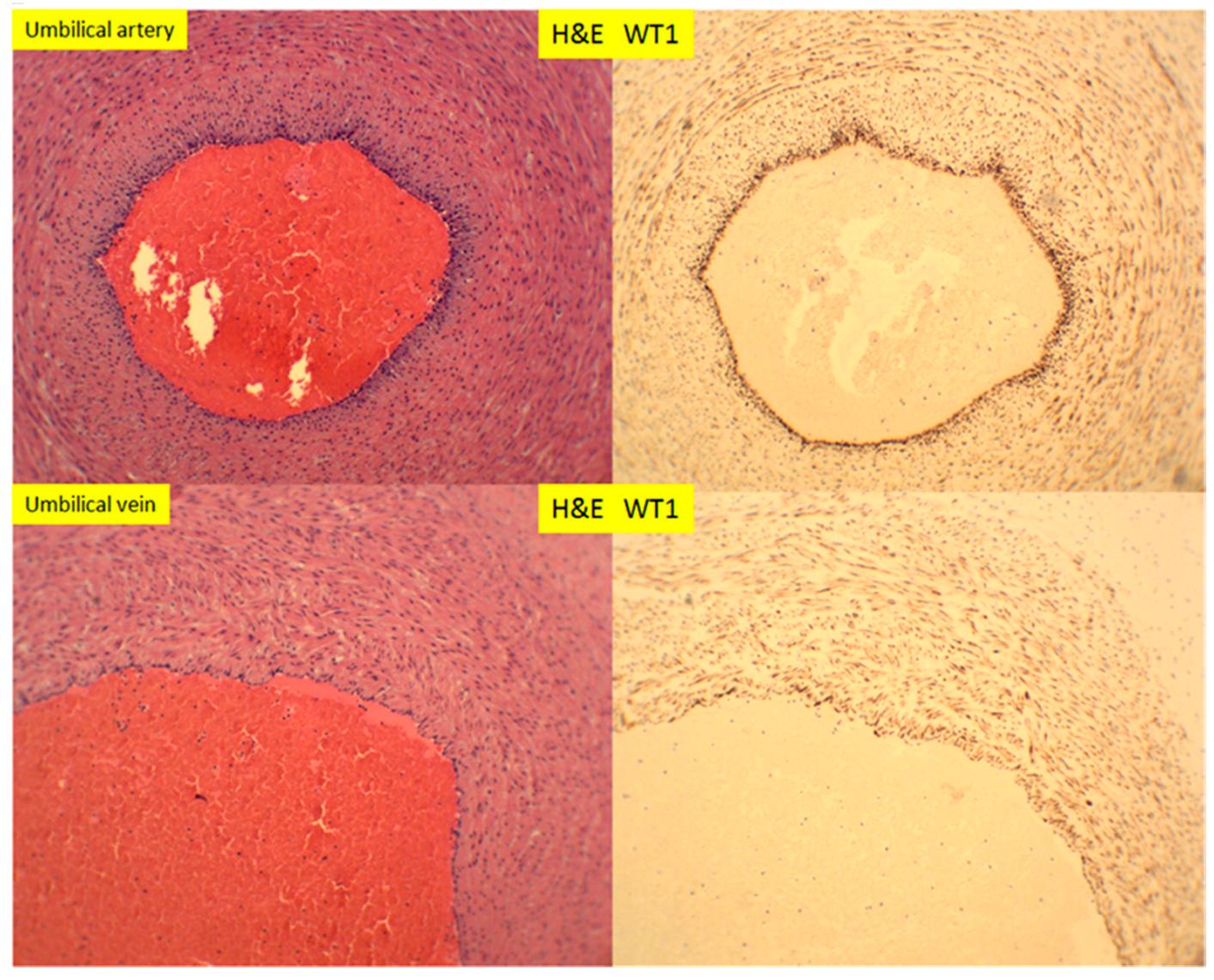
3. Results
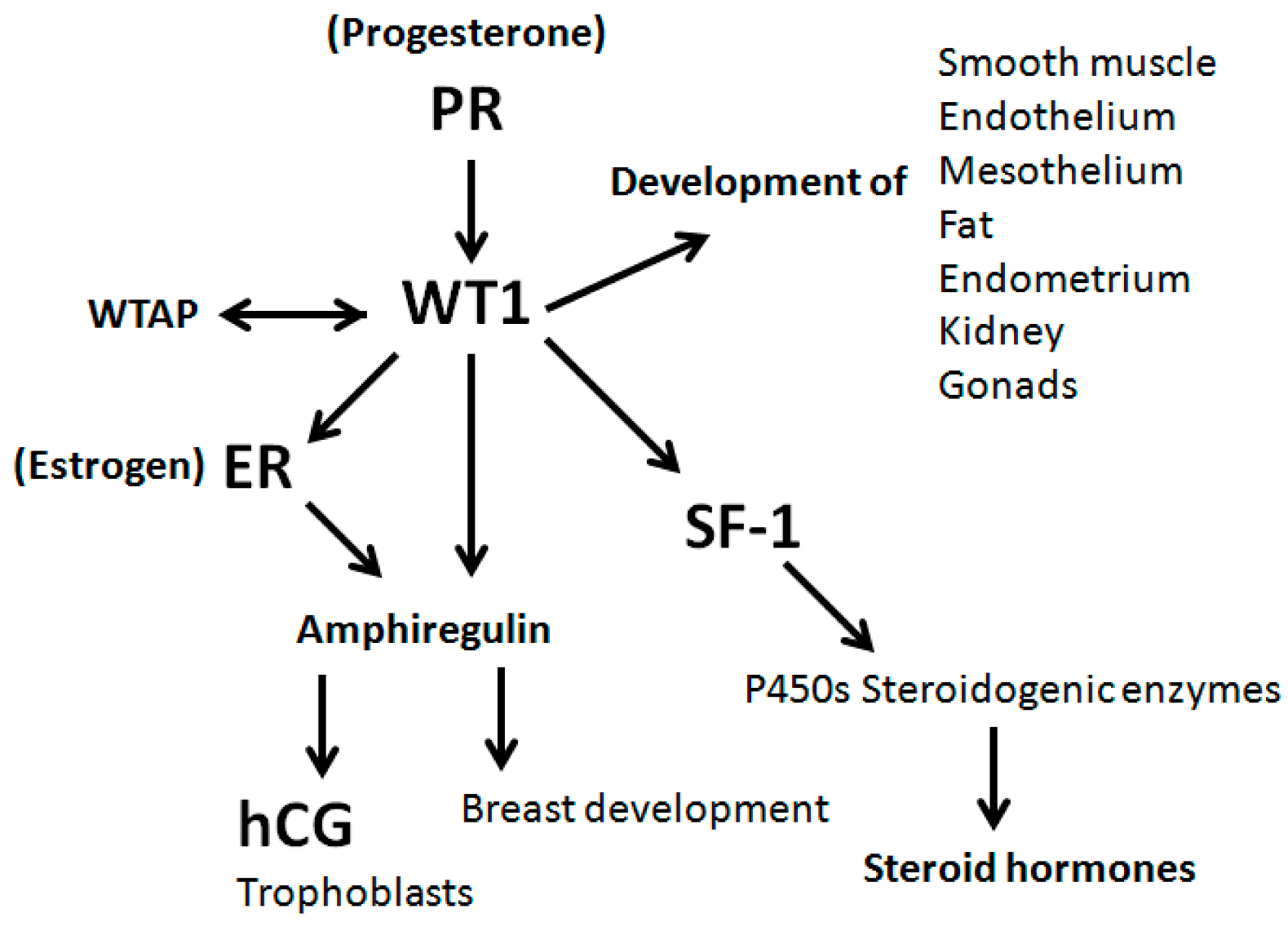
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 6th ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Cunningham, F.G. Williams Obstetrics, 25th ed.; McGraw-Hill: New York, NY, USA, 2018; Volume xvi, p. 1328. [Google Scholar]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.K.; Smith, S.D.; Keogh, R.J.; Jones, R.; Baker, P.; Knöfler, M.; Cartwright, J.E.; Whitley, G.S.; Aplin, J. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 2010, 177, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.K.; Benagiano, M.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019, 221, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Decidual vasculopathy and spiral artery remodeling revisited II: Relations to trophoblastic dependent and independent vascular transformation. J. Matern. Neonatal Med. 2020, 1–7. [Google Scholar] [CrossRef]
- Zhang, P. Phenotypic Switch of Endovascular Trophoblasts in Decidual Vasculopathy with Implication for Preeclampsia and Other Pregnancy Complications. Fetal Pediatr. Pathol. 2020, 1–20. [Google Scholar] [CrossRef]
- Zhang, P. Decidual Vasculopathy in Preeclampsia and Spiral Artery Remodeling Revisited: Shallow Invasion versus Failure of Involution. Am. J. Perinatol. Rep. 2018, 8, e241–e246. [Google Scholar] [CrossRef]
- Craven, C.M.; Morgan, T.; Ward, K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998, 19, 241–252. [Google Scholar] [CrossRef]
- Hastie, N.D. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef]
- Call, K.M.; Glaser, T.; Ito, C.Y.; Buckler, A.J.; Pelletier, J.; Haber, D.A.; Rose, E.A.; Kral, A.; Yeger, H.; Lewis, W.H.; et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990, 60, 509–520. [Google Scholar] [CrossRef]
- Haber, D.A.; Buckler, A.J.; Glaser, T.; Call, K.M.; Pelletier, J.; Sohn, R.L.; Douglass, E.C.; Housman, D.E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell 1990, 61, 1257–1269. [Google Scholar]
- Haber, D.A.; Housman, D.E. The genetics of Wilms’ tumor. Adv. Cancer Res. 1992, 59, 41–68. [Google Scholar]
- Haber, D.A.; Buckler, A.J. WT1: A novel tumor suppressor gene inactivated in Wilms’ tumor. New Biol. 1992, 4, 97–106. [Google Scholar]
- Van den Heuvel-Eibrink, M.M. Wilms Tumor. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK373360/?term=Wilms%20tumor (accessed on 30 April 2020).
- Buckler, A.J.; Pelletier, J.; Haber, D.A.; Glaser, T.; Housman, D.E. Isolation, characterization, and expression of the murine Wilms’ tumor gene (WT1) during kidney development. Mol. Cell. Boil. 1991, 11, 1707–1712. [Google Scholar] [CrossRef]
- Pelletier, J.; Schalling, M.; Buckler, A.J.; Rogers, A.; Haber, D.A.; Housman, D. Expression of the Wilms’ tumor gene WT1 in the murine urogenital system. Genes Dev. 1991, 5, 1345–1356. [Google Scholar] [CrossRef]
- Patek, C.E.; Little, M.H.; Fleming, S.; Miles, C.; Charlieu, J.-P.; Clarke, A.; Miyagawa, K.; Christie, S.; Doig, J.; Harrison, D.J.; et al. A zinc finger truncation of murine WT1 results in the characteristic urogenital abnormalities of Denys-Drash syndrome. Proc. Natl. Acad. Sci. USA 1999, 96, 2931–2936. [Google Scholar] [CrossRef]
- Francke, U.; Holmes, L.; Atkins, L.; Riccardi, V. Aniridia-Wilms’ tumor association: Evidence for specific deletion of 11p13. Cytogenet. Genome Res. 1979, 24, 185–192. [Google Scholar] [CrossRef]
- Chau, Y.-Y.; Brownstein, D.; Mjoseng, H.K.; Lee, W.-C.; Buza-Vidas, N.; Nerlov, C.; Jacobsen, S.E.W.; Perry, P.; Berry, R.; Thornburn, A.; et al. Acute multiple organ failure in adult mice deleted for the developmental regulator WT1. PLoS Genet. 2011, 7, e1002404. [Google Scholar] [CrossRef]
- Kaspar, H.G.; Crum, C.P. The utility of immunohistochemistry in the differential diagnosis of gynecologic disorders. Arch. Pathol. Lab. Med. 2015, 139, 39–54. [Google Scholar] [CrossRef]
- Husain, A.N.; Colby, T.V.; Ordóñez, N.G.; Allen, T.C.; Attanoos, R.L.; Beasley, M.B.; Butnor, K.J.; Chirieac, L.R.; Churg, A.M.; Dacic, S.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2018, 142, 89–108. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Gebrane-Younes, J.; Hoang, N.M.; Orcel, L. Ultrastructure of human umbilical vessels: A possible role in amniotic fluid formation? Placenta 1986, 7, 173–185. [Google Scholar] [CrossRef]
- Anthony, F.W.; Mukhtar, D.D.; Pickett, M.A.; Cameron, I.T. Progesterone up-regulates WT1 mRNA and protein, and alters the relative expression of WT1 transcripts in cultured endometrial stromal cells. J. Soc. Gynecol. Investig. 2003, 10, 509–516. [Google Scholar] [CrossRef]
- Werner, H.; Rauscher, F.J.; Sukhatme, V.P.; A Drummond, I.; Roberts, C.T.; Leroith, D. Transcriptional repression of the insulin-like growth factor I receptor (IGF-I-R) gene by the tumor suppressor WT1 involves binding to sequences both upstream and downstream of the IGF-I-R gene transcription start site. J. Boil. Chem. 1994, 269, 12577–12582. [Google Scholar]
- Roberts, C.T. Control of insulin-like growth factor (IGF) action by regulation of IGF-I receptor expression. Endocr. J. 1996, 43, S49–S55. [Google Scholar] [CrossRef]
- Moffett, P.; Bruening, W.; Nakagama, H.; Bardeesy, N.A.; Housman, D.; Housman, D.E.; Pelletier, J. Antagonism of WT1 activity by protein self-association. Proc. Natl. Acad. Sci. USA 1995, 92, 11105–11109. [Google Scholar] [CrossRef]
- Lee, S.; Huang, K.; Palmer, R.; Truong, V.B.; Herzlinger, D.; A Kolquist, K.; Wong, J.; Paulding, C.; Yoon, S.K.; Gerald, W.; et al. The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell 1999, 98, 663–673. [Google Scholar] [CrossRef]
- Ciarloni, L.; Mallepell, S.; Brisken, C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc. Natl. Acad. Sci. USA 2007, 104, 5455–5460. [Google Scholar] [CrossRef]
- Pronier, E.; Bowman, R.L.; Ahn, J.; Glass, J.; Kandoth, C.; Merlinsky, T.R.; Whitfield, J.T.; Durham, B.H.; Gruet, A.; Somasundara, A.V.H.; et al. Genetic and epigenetic evolution as a contributor to WT1-mutant leukemogenesis. Blood 2018, 132, 1265–1278. [Google Scholar] [CrossRef]
- Moser, G.; Windsperger, K.; Pollheimer, J.; Lopes, S.C.D.S.; Huppertz, B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Boil. 2018, 150, 361–370. [Google Scholar] [CrossRef]
- Scholz, H.; Kirschner, K.M. Oxygen-Dependent Gene Expression in Development and Cancer: Lessons Learned from the Wilms’ Tumor Gene, WT1. Front. Mol. Neurosci 2011, 4, 4. [Google Scholar] [CrossRef]
- Small, T.W.; Bolender, Z.; Bueno, C.; O’Neil, C.; Nong, Z.; Rushlow, W.; Rajakumar, N.; Kandel, C.; Strong, J.; Madrenas, J.; et al. Wilms’ tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells. Circ. Res. 2006, 99, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Small, T.W.; Penalva, L.O.; Pickering, J.G. Vascular biology and the sex of flies: Regulation of vascular smooth muscle cell proliferation by wilms’ tumor 1-associating protein. Trends Cardiovasc. Med. 2007, 17, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Estrada, O.M.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. WT1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Hastie, N.D. The role of WT1 in regulating mesenchyme in cancer, development, and tissue homeostasis. Trends Genet. 2012, 28, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.-Y.; Bandiera, R.; Serrels, A.; Estrada, O.M.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Hastie, N. WT1, the mesothelium and the origins and heterogeneity of visceral fat progenitors. Adipocyte 2015, 4, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Darcha, C.; Déchelotte, P.J.; Pouly, J.-L.; Mage, G. Expression of WT1 is down-regulated in eutopic endometrium obtained during the midsecretory phase from patients with endometriosis. Fertil. Steril. 2006, 86, 554–558. [Google Scholar] [CrossRef]
- Sbracia, M.; Scarpellini, F.; Zupi, E.; Manna, C.; Marconi, D.; Romanini, C.; Alo, P.; Tondo, U.; Grasso, J. Differential expression of IGF-I and IGF-II in eutopic and ectopic endometria of women with endometriosis and in women without endometriosis. Am. J. Reprod. Immunol. 1997, 37, 326–329. [Google Scholar] [CrossRef]
- Gurgan, T.; Bukulmez, O.; Yarali, H.; Tanir, M.; Akyildiz, S. Serum and peritoneal fluid levels of IGF I and II and insulinlike growth binding protein-3 in endometriosis. J. Reprod. Med. 1999, 44, 450–454. [Google Scholar]
- Reeve, A.E.; Eccles, M.R.; Wilkins, R.J.; Bell, G.I.; Millow, L.J. Expression of insulin-like growth factor-II transcripts in Wilms’ tumour. Nature 1985, 317, 258–260. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P. Expression of Wilm’s Tumor Gene (WT1) in Endometrium with Potential Link to Gestational Vascular Transformation. Reprod. Med. 2020, 1, 17-31. https://doi.org/10.3390/reprodmed1010003
Zhang P. Expression of Wilm’s Tumor Gene (WT1) in Endometrium with Potential Link to Gestational Vascular Transformation. Reproductive Medicine. 2020; 1(1):17-31. https://doi.org/10.3390/reprodmed1010003
Chicago/Turabian StyleZhang, Peilin. 2020. "Expression of Wilm’s Tumor Gene (WT1) in Endometrium with Potential Link to Gestational Vascular Transformation" Reproductive Medicine 1, no. 1: 17-31. https://doi.org/10.3390/reprodmed1010003
APA StyleZhang, P. (2020). Expression of Wilm’s Tumor Gene (WT1) in Endometrium with Potential Link to Gestational Vascular Transformation. Reproductive Medicine, 1(1), 17-31. https://doi.org/10.3390/reprodmed1010003




