1. Introduction
Infectious emboli originating in the endocardium and wreaking havoc in the vascular periphery have been noted as early as the 1850s [
1]. Later, in “Das Mycotisch Embolische Aneurysina” (1887), Eppinger documented an inflammatory arteritis interlinked with septic embolization [
2]. In his Gulstonian lectures in the same decade, Sir William Osler describes a 30-year-old patient admitted to the Montreal General Hospital who, on necropsy, was found with valvular vegetations [
3]. Referring to the aneurysms’ shape rather than the etiology, he coined this a “mycotic” process. Since Osler, the pathophysiology of the rare process known as a mycotic aneurysm (MA), sometimes called an infected aneurysm, has been studied in depth.
MAs can occur in a variety of vessels, including iliac, femoral, and cerebral arteries [
4]. Though the true pathogenesis is unknown, MAs likely develop from direct local invasion of the vessel wall in the setting of a known systemic infection, mostly bacterial [
5]. Today, we concentrate on the MA affecting the aorta, commonly known as a mycotic aortic aneurysm (MAA). Although predominantly affecting older adults, incredibly rare MAA cases have been described in children with aortic stenosis, even in the absence of underlying bacteremia [
4]. MAAs have also been described as iatrogenic complications of intravesical therapy for non-muscular invasive bladder cancer with Bacillus Calmette–Guerrin, the disease’s standard treatment [
6].
Predisposing factors to MAs include states of immune compromise, like diabetes, HIV, or high-dose glucocorticoid use, which lead to invasive infections [
7]. This rare disease, often presenting as a relentless fever, progresses very frequently to rupture without surgical intervention, with a high rate of morbidity and mortality [
7]. This aneurysm type is characterized by rapid growth, which likely underlies the high risk of rupture [
5]. In cases with extensive periaortic infection, poor outcomes and death are thought to be even greater [
7].
Although many aortopathies demand immediate surgical attention, MAAs are uniquely insidious and incredibly deadly—poor outcomes are reported from conservative management without surgery [
8,
9,
10]. MAAs’ radiographic appearance is diverse, with mycotic aneurysmal growths described in various locations, ranging from the ascending to the infrarenal aorta [
11].
The following case of a rapidly progressive mycotic aortic aneurysm exemplifies the rationale for heightened clinical suspicion in patients presenting atypically and close follow-up of such patients.
2. Case Presentation
The patient was a 72-year-old woman known for hypertension, dyslipidemia, diabetes mellitus type II, and hypothyroidism who presented with functional decline and vague back pain over three weeks. A broadly infectious picture led to an initial diagnosis of a urinary tract infection (UTI), especially given a urine culture positive for Group B Streptococcus (GBS). She had been transferred from a peripheral hospital for confusion. Labs showed leukocytosis (white blood cells or WBC 18.8 × 109/L), neutrophilia (absolute neutrophils 16.18 × 109/L), elevated C-reactive protein or CRP (64.7 mg/L), markedly elevated thyroid-stimulating hormone TSH (34.9 mIU/L), and mildly elevated high-sensitivity troponin (23.3 ng/L). She was initially managed with intravenous vancomycin 1 gram every 12 h and piperacillin–tazobactam 4.5 gram every 8 h, which she received empirically until eventual bacterial sensitivities were confirmed. Thoracic and abdominal computed tomography (CT) were nevertheless performed to eliminate other causes.
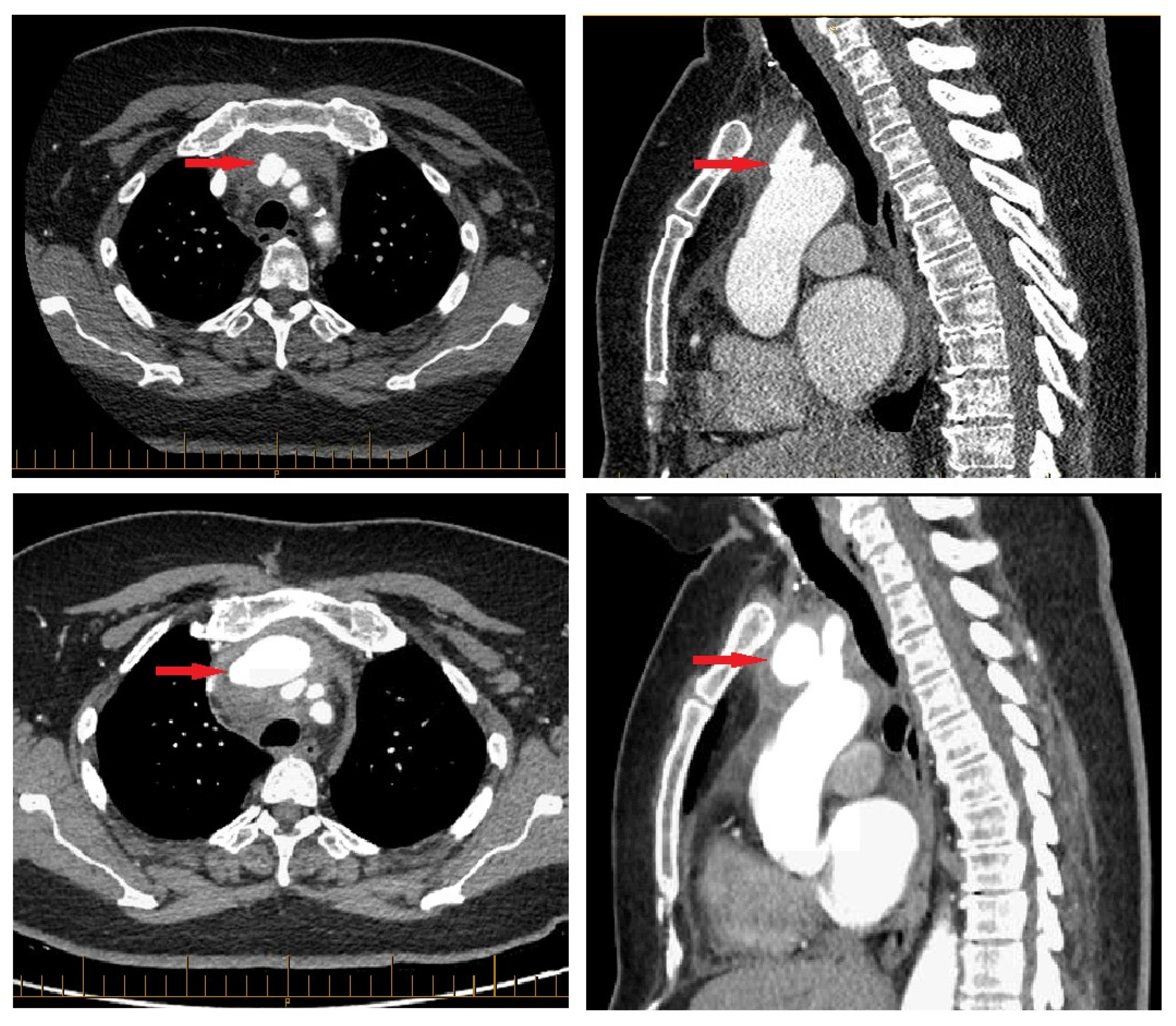
CT revealed a pseudoaneurysm (7 × 5.5 mm) at the common origin of the brachiocephalic trunk, and the left common carotid artery was incidentally found (
Figure 1). Surrounding periaortic fat stranding and the presence of axillary and retro-pectoral lymph nodes suggested an infectious process. A transthoracic echocardiogram demonstrated normal cardiac chamber dimensions, preserved systolic and diastolic function (left ventricular ejection fraction 60–65%), and mild degenerative valvular changes without intra-cardiac vegetations or significant valvulopathy.
Inflammatory markers remained elevated but gradually declined, as did WBCs. Troponins peaked mildly within 5 days of admission (34.0 ng/L) and eventually normalized, as did TSH by discharge. After a satisfactory decline in infectious and inflammatory biological parameters under intravenous piperacillin–tazobactam, the decision was made to pursue outpatient parenteral antibiotics. However, a CT angiogram performed before the patient’s discharge three weeks after admission revealed an interval increase in the mycotic aneurysm (41 × 26 mm) (
Figure 1). In light of this change, a staged surgical intervention was undertaken 2 weeks later.
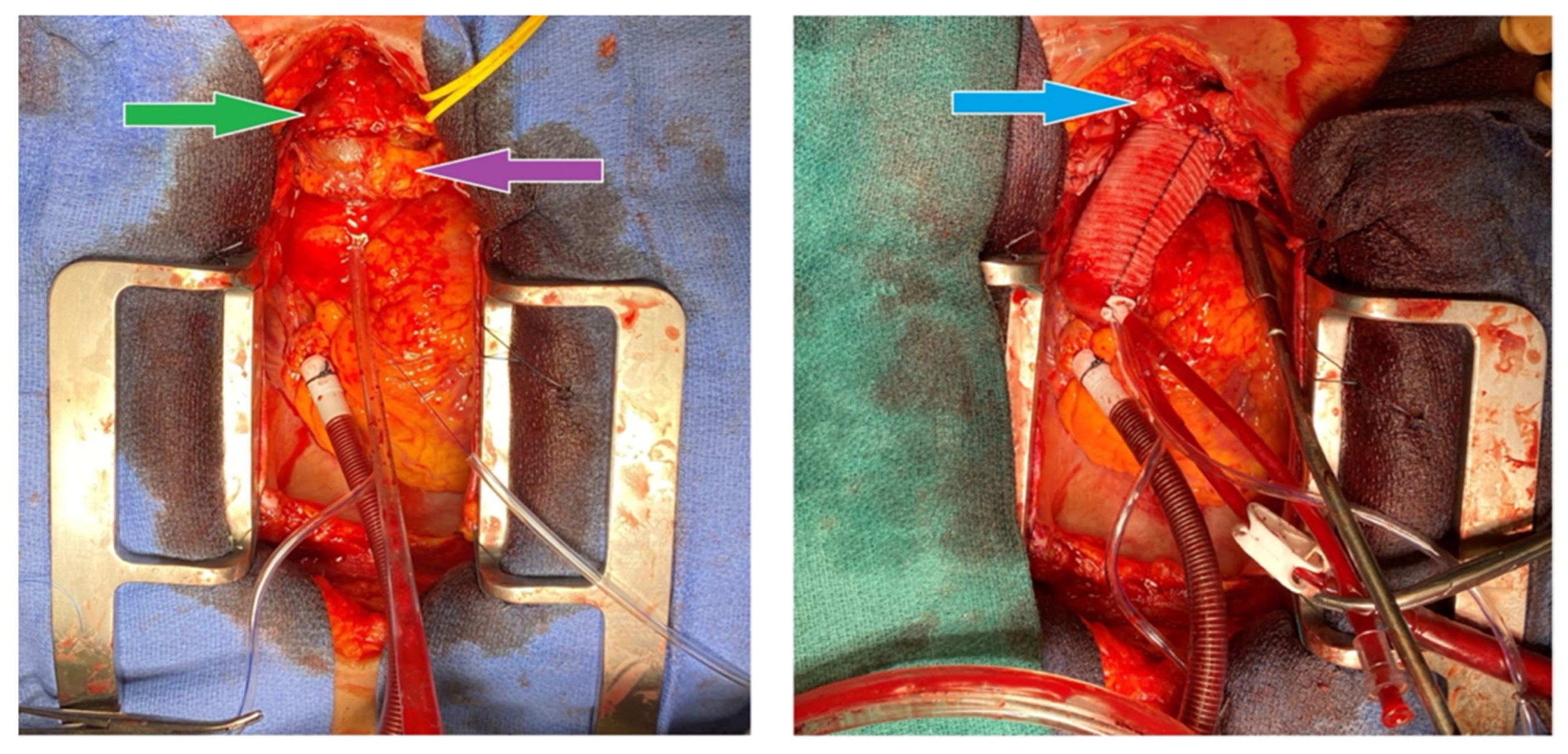
An index procedure debranching the left carotid artery was first performed by vascular surgery, via an end-to-side 8 mm Hemashield graft from the left subclavian to the left common carotid artery. Because catastrophic rupture at sternotomy or during arch clamping could abruptly interrupt antegrade flow to the left hemisphere, this prophylactic carotid–subclavian bypass was created before chest entry to guarantee cerebral perfusion. The extra-anatomic graft, in conjunction with right axillary arterial cannulation discussed in the following paragraph, provided bilateral brain protection while the infected proximal arch was excised and replaced in the second stage of the surgery (
Figure 2).
A second operation was performed under cardiopulmonary bypass using a double Y femoral configuration (arterial cannulation at the femoral and axillary sites; venous cannulation at the femoral and atrial sites). Right femoral and right axillary arterial inflows were established through 8 mm Hemashield grafts, while venous return was obtained from the right femoral vein (IVC position) and right atrium. Indeed, we prepared for a high risk of exsanguination intra-operatively. Femoral venous cannulation was instituted before sternotomy to permit the rapid initiation of cardiopulmonary bypass should the aneurysm rupture while opening the chest. After a successful sternotomy and no torrential bleeding, a right-atrial cannula was added to provide bicaval drainage, improve decompression, and maintain optimal perfusion during deep hypothermic circulatory arrest. Total cardiopulmonary bypass time was 200 min, aortic cross-clamp time was 46 min, deep-hypothermic circulatory arrest time was 37 min, selective cerebral perfusion time was 29 min, and the nadir temperature was 19.9 °C.
A pulsatile phlegmon encasing a thrombosed innominate vein was found originating from the base of the innominate artery. Critically, the totally clotted innominate vein was compressing—and thereby tamponading—the mycotic pseudoaneurysm, likely preventing catastrophic rupture before surgery; the vein was completely thrombosed, and when it was dissected, it resulted in an inconsequential rupture of the surrounding thrombus before its removal. No open venous structures were identified as the thrombosed vessel pieces were removed. All clots were removed to further expose the innominate structures, thus uncovering a perforation at the base of the mycotic aneurysm. The distal innominate artery was clamped to permit antegrade brain perfusion via the axillary graft while the patient was in circulatory arrest.
The proximal innominate artery was resected with ectatic tissue from the mid-ascending aorta to the proximal arch. When examined, the phlegmon was found to be perforated. A 30 mm Gelweave (Dacron) graft was sewn end to end to the distal ascending aorta using a running 3-0 Prolene suture; the posterior half of this distal anastomosis deliberately incorporated the rim of the innominate artery defect to create a single, hemostatic suture line (
Figure 3). Once the graft was de-aired, it was cross-clamped, systemic perfusion was re-established, and rewarming began. The proximal anastomosis between the trimmed native ascending aorta and the graft was then completed with continuous 4-0 Prolene following a single dose of antegrade cold-blood cardioplegia. Patch repairs were then performed on the right femoral and axillary arteriotomies; the right femoral venotomy was closed with a simple purse-string suture. Intra-operative and post-repair trans-esophageal echocardiography confirmed satisfactory ventricular function and no residual air. Aortic specimens were positive for
Streptococcus agalactiae (Group B Streptococcus) on ensuing analysis. Tissue from the distal ascending aorta and proximal arch was sent for histology and culture.
The patient was then transferred to the intensive care unit in stable condition, where she eventually progressed to the surgical ward and continued to progress favourably. Post-operative imaging (CT angiography) performed on day 12 post-operatively demonstrated a circumferential hematoma around the ascending aorta and hemiarch graft measuring 20 mm in thickness, with internal air bubbles attributed to surgical changes. Follow-up imaging confirmed the resolution. The patient completed a full course of intravenous antibiotics (ceftriaxone for 6 weeks post-operatively) with good recovery at three months post-operatively. Her total hospitalization at our center lasted 55 days, and she was discharged 18 days after surgery, returning to her hospital of origin to complete treatment.
3. Discussion
The described disease entity, the MAA, poses several clinical challenges. For one, the diagnosis is challenging. A wide variety of presentations have been described for arch aneurysms, ranging from seemingly benign dysphonia to life-threatening tamponade [
12,
13]. In the case of suspected infected aneurysms, even an infectious workup may prove unhelpful. As illustrated by our case, the patient remained afebrile and was not bacteremic throughout her admission; there was a quite benign presentation. In fact, her non-specific presentation was at first labeled uncomplicated UTI. The described modality of choice which has proved reliable for early diagnosis is computerized tomography (CT). The findings suggestive of an MA include the unusual location of the aneurysm, saccular morphology, fat stranding, and air in the aortic wall [
7]. Furthermore, the rapid evolution of the aneurysm on repeat CT heightens the clinical suspicion of infection [
14].
Secondly, the evolution of the pathology is highly unpredictable. Despite laboratory and clinical improvement in medical therapy, a repeat CT scan of the chest demonstrated a worsening of the disease process within the span of three weeks. Indeed, MAs are notorious for their rapid progression and increased tissue fragility compared to non-infected aneurysms, entailing an increased risk of rupture [
15]. As demonstrated by this case, growth is still possible despite clinical improvement. Critically, the rarity of the disease has precluded the development of high-quality evidence supporting diagnostic and management strategies [
16]. Therefore, serial imaging during admission if such rapidly growing pathology is suspected is needed, as well as intervals based on clinical response, such as on admission of a suspected patient, before discharge, and at 3 months, 6 months, and 1 year after discharge. This stringent follow-up is critical to monitor disease trajectory, and clinical or laboratory improvements alone cannot reliably exclude progression, especially if there is reason to believe the primary source was not well-controlled.
In fact, the timing of the intervention is not well explored in the literature, nor is it standardized. In this case, a radiographic sudden increase in the aneurysm’s size justified an early surgical approach, before the completion of antimicrobial therapy. Other patient factors, such as poor blood pressure control, may herald rupture and thus warrant expedited intervention [
17].
For the MA of the thoracic or abdominal aorta, a variety of surgical strategies are now described. Endovascular methods compete with open surgical synthetic graft implantation to replace the aneurysmal zone as the preferred treatment modality [
8]. Little is known with regard to the differences between the MA of the ascending aorta or aortic arch as compared to the more distal MAA. This is particularly of interest due to the disastrous consequences of disease of large, proximal branches of the aorta, such as the innominate.
A striking intra-operative finding in our patient was a completely thrombosed innominate vein densely adherent to—and actively compressing—the mycotic pseudoaneurysm. Unlike the more common scenario in which a contained rupture is sealed by a broad mantle of mediastinal or pleural tissues, here, a single, readily identifiable yet friable vascular structure served as the primary barrier to exsanguination. We believe this single structure’s “auto-tamponade” protected the pseudoaneurysm from catastrophic pre-operative rupture and effectively bought time for definitive repair. Because the vein lumen was totally occluded, it could be removed easily without reconstruction. Pre-operative CT can, in similar cases, show the intimate apposition of the vein to the aneurysmal sac, offering a valuable imaging clue that could alert surgeons to the risk of sudden hemorrhage when the vein is mobilized. We, therefore, recommend careful dissection and provisional vascular control before dividing such a vein to avoid precipitous blood loss. To our knowledge, such a fortuitous compressive mechanism has not been reported previously and underscores the heterogeneity of MAA presentations as well as the importance of meticulous operative exploration. This case poses the following questions: could the infection have originated in the innominate vein? Is venous contamination a likely source of infection?
4. Conclusions
MAAs are rare, dangerous, and diagnostically challenging. First, the diagnosis of a mycotic aortic aneurysm can easily be missed. Second, this pathology can evolve in unpredictable ways, and its worsening progression cannot be excluded on the basis of symptomatic improvement or serum markers alone. Third, serial imaging should play a critical role in monitoring disease trajectory and in determining both the need and timing of intervention. Finally, unexpected anatomic relationships—here, we suspect that a thrombosed innominate vein acted as a natural buttress—may transiently stabilize an otherwise lethal lesion and create a critical window for life-saving surgery. In such a serious condition, patient outcomes should not be left to chance. A high degree of suspicion and meticulous follow-up, including consideration for surgical intervention, are required, lest preventable mortality result.