Implementation of Minimally Invasive Mitral Valve Surgery in a Novice Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Implementation
2.2. Procedure
2.2.1. Anesthesia
2.2.2. Surgery
2.2.3. Post-Surgical-/ICU Care
2.2.4. CS Patients
2.3. Statistics
2.4. Data Acquisition
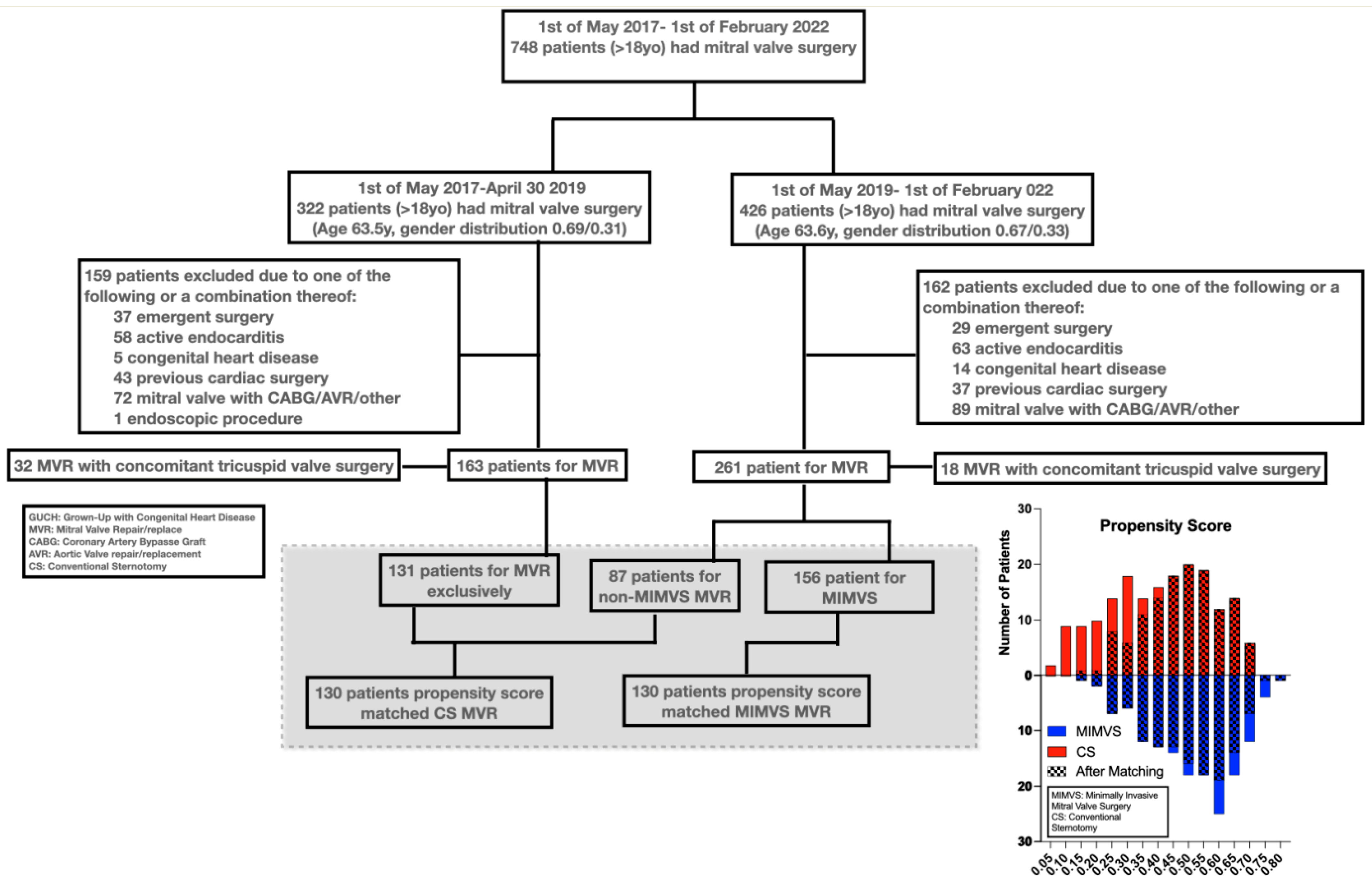
3. Results
3.1. Matching
- Mean and median propensity score margins were both 0.006 (range −0.015–0.05, interquartile range −0.002–0.014).
- Matching mitigated pre-existing significant differences (Table 1) in gender (34% vs. 32%, p = 0.7), size (BSA of 1.93 m2 vs. 1.94 m2, p = 0.7), age (64 vs. 64 years, p = 0.6), rate of atrial fibrillation (30% vs. 34%, p = 0.6) and Euroscore 2 (1.38% vs. 1.43%, p = 0.7) in patients receiving CS compared to MIMVS. Significant differences persisted in CAD frequency after matching (19% vs. 36%, p = 0.003).
3.2. Surgical Technique
- More chord placement (57% vs. 83%, p < 0.001) and smaller ring sizes (34 mm vs. 36 mm, p < 0.001)
- Less left atrial appendage closure (28% vs. 43%, p = 0.009)
3.3. Patient Outcome
- Operating, CPB (180 vs. 102 min, p < 0.001) and aortic cross-clamp times (98 vs. 81 min, p < 0.001) (5.5 vs. 4.3 h, p < 0.001) increased.
- Longer procedure time appeared to affect extubation in MIMVS slightly (10 vs. 9 h, p = 0.009).
- However, MIMVS in-hospital time decrased significantly.
- ICU re-admissions occurred less (0 vs. 3.1%, p = 0.045) and hospital discharge shortened (p < 0.001, median 5 vs. 7) after MIMVS.
- Patient-centered outcomes such as neurologic-, effusion- and reintervention endpoints showed non-significant small differences.
- Postoperative atrial fibrillation and endocarditis/mediastinitis occurred significantly less in MIMVS (42% vs. 70%, p < 0.001/0 vs. 3.1%, p = 0.044)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Activated clotting time |
| BSA | Body surface area |
| CAD | Coronary artery disease |
| CPB | Cardiopulmonary bypass |
| CS | Conventional sternotomy |
| CTCA | Computer tomography coronary angiogram |
| CVP | Central venous pressure |
| EMR | Electronic medical record |
| ICU | Intensive care unit |
| LVEF | Left ventricular ejection fraction |
| NIRS | Near infra-read spectrometry |
| MAP | Mean arterial pressure |
| MIMVS | Minimally invasive mitral valve surgery |
| MR | Mitral regurgitation |
| MV | Mitral valve |
| MVR | Mitral valve repair/replacement |
| SAM | Systolic anterior motion |
References
- Modi, P.; Hassan, A.; Chitwood, W.R. Minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur. J. Cardio-Thoracic Surg. 2008, 34, 943–952. [Google Scholar] [CrossRef]
- Goldstone, A.B.; Atluri, P.; Szeto, W.Y.; Trubelja, A.; Howard, J.L.; MacArthur, J.W.; Newcomb, C.; Donnelly, J.P.; Kobrin, D.M.; Sheridan, M.A.; et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: A propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 2013, 145, 748–756. [Google Scholar] [CrossRef]
- Iribarne, A.; Easterwood, R.; Russo, M.J.; Wang, Y.C.; Yang, J.; Hong, K.N.; Smith, C.R.; Argenziano, M. A minimally invasive approach is more cost-effective than a traditional sternotomy approach for mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2011, 142, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Shaw, M.; Toolan, C.; Palmer, K.; Al-Rawi, O.; Modi, P. Cost Analysis of Minimally Invasive Mitral Valve Surgery in the UK National Health Service. Ann. Thorac. Surg. 2021, 112, 124–131. [Google Scholar] [CrossRef]
- Svensson, L.G.; Atik, F.A.; Cosgrove, D.M.; Blackstone, E.H.; Rajeswaran, J.; Krishnaswamy, G.; Jin, U.; Gillinov, A.M.; Griffin, B.; Navia, J.L.; et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J. Thorac. Cardiovasc. Surg. 2010, 139, 926–932.e2. [Google Scholar] [CrossRef] [PubMed]
- Moscarelli, M.; Fattouch, K.; Casula, R.; Speziale, G.; Lancellotti, P.; Athanasiou, T. What Is the Role of Minimally Invasive Mitral Valve Surgery in High-Risk Patients? A Meta-Analysis of Observational Studies. Ann. Thorac. Surg. 2016, 101, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Martin, J.; Lal, A.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J.; et al. Minimally Invasive Versus Conventional Open Mitral Valve Surgery. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2011, 6, 84–103. [Google Scholar] [CrossRef]
- Hussain, S.; Swystun, A.G.; Caputo, M.; Angelini, G.D.; Vohra, H.A. A review and meta-analysis of conventional sternotomy versus minimally invasive mitral valve surgery for degenerative mitral valve disease focused on the last decade of evidence. Perfusion 2023, 39, 988–997. [Google Scholar] [CrossRef]
- Gammie, J.S.; Bartlett, S.T.; Griffith, B.P. Small-Incision Mitral Valve Repair: Safe, Durable, and Approaching Perfection. Ann. Surg. 2009, 250, 409–415. [Google Scholar] [CrossRef]
- Han, J.J.; Smood, B.; Atluri, P. Commentary: Transitioning to Minimally Invasive Mitral Valve Repair—Navigating the Gauntlet. Semin. Thorac. Cardiovasc. Surg. 2021, 32, 838–839. [Google Scholar] [CrossRef]
- Abu-Omar, Y.; Fazmin, I.T.; Ali, J.M.; Pelletier, M.P. Minimally invasive mitral valve surgery. J. Thorac. Dis. 2020, 13, 1960–1970. [Google Scholar] [CrossRef]
- Holzhey, D.M.; Seeburger, J.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Learning minimally invasive mitral valve surgery: A cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013, 128, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.C. Is minimally invasive mitral valve surgery equal to or worse than a full sternotomy: A perspective from the Netherlands National Heart Registration. Eur. J. cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 61, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Barbero, C.; Marchetto, G.; Ricci, D.; Rinaldi, M. Temporary Neurological Dysfunction After Minimal Invasive Mitral Valve Surgery: Influence of Type of Perfusion and Aortic Clamping Technique. Ann. Thorac. Surg. 2017, 103, 691–692. [Google Scholar] [CrossRef]
- Olsthoorn, J.R.; Heuts, S.; Houterman, S.; Maessen, J.G.; Sardari Nia, P. Effect of minimally invasive mitral valve surgery compared to sternotomy on short- and long-term outcomes: A retrospective multicentre interventional cohort study based on Netherlands Heart Registration. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 61, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.; Lorenzen, U.; Borzikowsky, C.; Schoeneich, F.; Cremer, J.; Haneya, A.; Hensler, J.; Panholzer, B.; Huenges, K.; Broch, O. Unilateral pulmonary oedema after minimally invasive mitral valve surgery: A single-centre experience. Eur. J. Cardio-Thoracic Surg. 2017, 53, 764–770. [Google Scholar] [CrossRef]
- Hunter, S. How to start a minimal access mitral valve program. Ann. Cardiothorac. Surg. 2013, 2, 774–778. [Google Scholar] [CrossRef]
- Gammie, J.S.; Chikwe, J.; Badhwar, V.; Thibault, D.P.; Vemulapalli, S.; Thourani, V.H.; Gillinov, M.; Adams, D.H.; Rankin, J.S.; Ghoreishi, M.; et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann. Thorac. Surg. 2018, 106, 716–727. [Google Scholar] [CrossRef]
- Dreyfus, G.D.; Dulguerov, F.; Marcacci, C.; Haley, S.R.; Gkouma, A.; Dommerc, C.; Albert, A. “Respect when you can, resect when you should”: A realistic approach to posterior leaflet mitral valve repair. J. Thorac. Cardiovasc. Surg. 2018, 156, 1856–1866.e3. [Google Scholar] [CrossRef]
- Sá, M.P.; Cavalcanti, L.R.P.; Eynde, J.V.D.; Amabile, A.; Neto, A.C.E.; Perazzo, A.M.; Weymann, A.; Ruhparwar, A.; Sicouri, S.; Bisleri, G.; et al. Respect versus resect approaches for mitral valve repair: A study-level meta-analysis. Trends Cardiovasc. Med. 2023, 33, 225–239. [Google Scholar] [CrossRef]
- Maier, R.H.; Kasim, A.S.; Zacharias, J.; Vale, L.; Graham, R.; Walker, A.; Laskawski, G.; Deshpande, R.; Goodwin, A.; Kendall, S.; et al. Minimally invasive versus conventional sternotomy for Mitral valve repair: Protocol for a multicentre randomised controlled trial (UK Mini Mitral). BMJ Open 2021, 11, e047676. [Google Scholar] [CrossRef] [PubMed]
- Chikwe, J.; Toyoda, N.; Anyanwu, A.C.; Itagaki, S.; Egorova, N.N.; Boateng, P.; El-Eshmawi, A.; Adams, D.H. Relation of Mitral Valve Surgery Volume to Repair Rate, Durability, and Survival. J. Am. Coll. Cardiol. 2017, 69, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
| MIMVS (130) | CS (130) | p | |
|---|---|---|---|
| Age | 64.0 y (130) | 64.0 y (130) | 0.556 |
| Female | 32.3% (130) | 34.6% (130) | 0.695 |
| Body surface area | 1.94 m2 (130) | 1.93 m2 (130) | 0.720 |
| Diabetes | 4.6% (130) | 3.8% (130) | 0.760 |
| Hypertension | 42.3% (130) | 40.1% (130) | 0.802 |
| Cerebrovascular disease | 6.9% (130) | 5.4% (130) | 0.607 |
| Mild CAD on angiogram/CTCA | 36.2% (130) | 19.4% (129) | 0.003 * |
| History of smoking | 39.2% (130) | 42.3% (130) | 0.615 |
| Hyperlipidemia | 31.5% (130) | 31.5% (130) | 0.999 |
| Atrial fibrillation | 30.1% (130) | 33.9% (130) | 0.600 |
| EuroScore 2 | 1.38% (130) | 1.43% (130) | 0.676 |
| MIMVS (130) | CS (130) | p | |
|---|---|---|---|
| Repair | 84.6% (110 ptt) | 78.5% (102 ptt) | 0.202 |
| Tissue valve | 10.8% (14 ptt) | 16.9% (22 ptt) | 0.152 |
| Mechanical valve | 4.6% (6 ptt) | 3.1% (4 ptt) | 0.521 |
| Chords placed | 83.1% (108 ptt) | 56.7% (74 ptt) | <0.001 * |
| Mitral ring size (M, range) | 34 (28–40) mm | 36 (28–40) mm | <0.001 * |
| Left atrial closure | 27.7% (36 ptt) | 43.1% (56 ptt) | 0.009 * |
| Cryomaze | 21.5% (28 ptt) | 17.7% (23 ptt) | 0.437 |
| MIMVS (130) | CS (130) | p | |
|---|---|---|---|
| Cardiopulmonary bypass time (m) | 180 (96–391) min (130) | 102 (43–214) min (129) | <0.001 * |
| Aortic cross-clamp time (m) | 98 (58–204) min (125) | 81 (28–160) min (129) | <0.001 * |
| Time in operating room (anesthesia time) | 5.5 (3.75–9.0) h (130) | 4.3 (2.8–7.0) h (129) | <0.001 * |
| Time on ventilator | 10 (7–120) h (130) | 9 (4–46) h (130) | 0.009 * |
| ICU discharge (m/range) | 1 d (1–16 d) (130) | 1 d (1–6 d) (130) | 0.974 |
| ICU re-admission | 0% (130) | 3.1% (4 ptt) (130) | 0.045 * |
| Hospital discharge postoperatively (m/range) | 5 (3–29) d (130) | 7 (4–72) d (130) | <0.001 * |
| Bleeding total (m/range) | 450 (105–4675) mL (130) | 465 (40–5357) mL (130) | 0.5136 |
| Urine output 24 h (m/range) | 3398 (1665–7610) mL (130) | 2893 (270–5700) mL (130) | <0.001 * |
| Red blood cell transfusion per- or postoperatively | 24.6 (32 ptt) (130) | 23.8 (31 ptt) (130) | 0.886 |
| Neuro-scan postoperatively | 12.3% (16 ptt) (130) | 7.7% (10 ptt) (130) | 0.2163 |
| Neuro-scan abnormal | 4.6% (6 ptt) (130) | 4.6% (6 ptt) (130) | 0.998 |
| Neuro sequela | 3.9% (5 ptt) (130) | 3.9% (5 ptt) (130) | 0.998 |
| Major neurologic injury | 0 (130) | 1.5% (2 ptt) (130) | 0.158 |
| Permanent cardiac implanted electronic device (CIED) | 4.6% (6 ptt) (130) | 5.4% (7 ptt) (130) | 0.778 |
| Pericardial effusion requiring intervention | 8.5% (11 ptt) (130) | 13.4% (18 ptt) (130) | 0.169 |
| Pleural effusion requiring intervention | 6.9% (9 ptt) (130) | 10.8% (14 ptt) (130) | 0.277 |
| Postoperative atrial fibrillation | 42.3% (55 ptt) (130) | 66.9% (87 ptt) (130) | <0.001 * |
| Re-exploration for bleeding | 2.3% (3 ptt) (130) | 4.6% (6 ptt) (130) | 0.331 |
| Re-op medias-/endocarditis | 0 (130) | 3.1 (4 ptt) (130) | 0.044 * |
| One year mortality | 1.5% (2 ptt) (130) | 1.5% (2 ptt) (130) | 0.999 |
| One year mortality cardiac | 0.8 (1 ptt) (130) | 1.5% (2 ptt) (130) | 0.974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korshin, A.; Møller-Sørensen, P.H.; Møller, J.E.; Carranza, C.L. Implementation of Minimally Invasive Mitral Valve Surgery in a Novice Center. Hearts 2025, 6, 11. https://doi.org/10.3390/hearts6020011
Korshin A, Møller-Sørensen PH, Møller JE, Carranza CL. Implementation of Minimally Invasive Mitral Valve Surgery in a Novice Center. Hearts. 2025; 6(2):11. https://doi.org/10.3390/hearts6020011
Chicago/Turabian StyleKorshin, Andre, Peter Hasse Møller-Sørensen, Jacob Eifer Møller, and Christian Lildal Carranza. 2025. "Implementation of Minimally Invasive Mitral Valve Surgery in a Novice Center" Hearts 6, no. 2: 11. https://doi.org/10.3390/hearts6020011
APA StyleKorshin, A., Møller-Sørensen, P. H., Møller, J. E., & Carranza, C. L. (2025). Implementation of Minimally Invasive Mitral Valve Surgery in a Novice Center. Hearts, 6(2), 11. https://doi.org/10.3390/hearts6020011






