1. Introduction
Pacemakers have evolved over the last 70 years [
1]. The programming in early single-lead pacemakers consisted of three parameters: amplitude, pulse width, and rate. Currently, pacemakers have multiple leads and over 233 programmable elements, as shown in
Table 1. Pacemakers utilize 170 elements of the seven main programmable features and select 3,875,040,000 programming options to optimize cardiac function at rest. Additional options are available to improve exercise capacity. Early device therapy was designed to treat the failure of electrical conduction. Current devices augment heart failure therapy by restoring the synchrony of atrial to ventricular (AV) conduction and ventricular septal depolarization. Early programming was designed to extend the life of the pacemaker, whereas current programming may extend the life of the patient at the expense of battery life. The combination of different pacing settings is an extremely large number; therefore, programming is often set to “out of the box” parameters. Many patients benefit from those parameters, but many others have continued symptoms. Optimization is for this group.
Based on the table, one selection from each row, with multiple choices per row the formula is
Implantation of the device is limited to the anatomical constraints of the patient, which can sometimes be improved by programming. The left ventricular lead evolved from unipolar to bipolar to four different longitudinal leads in part to diminish diaphragmatic stimulation. Multi-lead pacing, either His Bundle or Bundle pacing, adds additional permutations and complexity. Early randomized pacemaker trials limited the programming, resulting in a small defined group deemed improved. Many other individuals can benefit. The methodology in this paper is not designed around clinical trials. The optimization of echo parameters and physiology of cardiac disease are used to improve cardiac function by selecting pacemaker programming functions.
The spectrum of cardiac disease adds intricacy, requiring different optimization goals. Early pacemakers and more modern pacemakers have not accounted for two hearts, a right and a left heart. The failure of either heart will result in morbidity and mortality in the patient. Failure of the left heart eventually causes failure of the right heart. This means the pacing strategy may need to change as heart function evolves. The function of current devices is to improve right heart function by improving left heart function with a left ventricular lead. If this fails and right heart dysfunction is the dominant reason for symptoms, right ventricular pacing may be needed so the interventricular septum supports the right heart. The left-sided strategy leaves some patients with advanced disease and primary right heart failure with no options for improvement. Cascaded pacing, which is currently not available, could salvage some of these patients. Priming the left heart with right ventricular pacing for several beats and then switching to left ventricular pacing in an alternating sequence is one possible solution to combined left and right heart failure.
The purpose of this discussion is to provide an efficient rationale for optimizing pacemakers for the spectrum of cardiac disease. The goal is to recognize anatomical and functional abnormalities of the heart, correcting these malfunctions with electrical stimulation. The method uses echocardiography and Doppler principles to identify and modify abnormal cardiac structure and function.
2. Spectrum of Cardiac Disease
The spectrum of cardiac disease is presented in
Table 2.
Desired Outcomes of Pacing
Increase forward flow
Decrease backward flow (regurgitation)
Restore circumferential and longitudinal synchrony
Decrease the size of the heart
Support the failing ventricle with control of the interventricular septum
Narrow the QRS duration to increase the power of the heart
Increase the heart rate in individuals with fixed stroke volume
Reduce ventricular dyssynchrony from ectopic beats
Restore Regularity
Spectrum of Symptoms
Dyspnea, Shortness of breath—decrease filling pressures
Fatigability, Exercise Intolerance—increase cardiac output
Edema, Swelling, Fluid Retention—Improve right heart function
Arrhythmias—Improve function and decrease wall stress
Important Equations
Systemic Vascular Resistance
3. Echo Evaluation of Disease Processes
Left Ventricular Outflow Tract Velocity Time Integral (LVOT VTI)
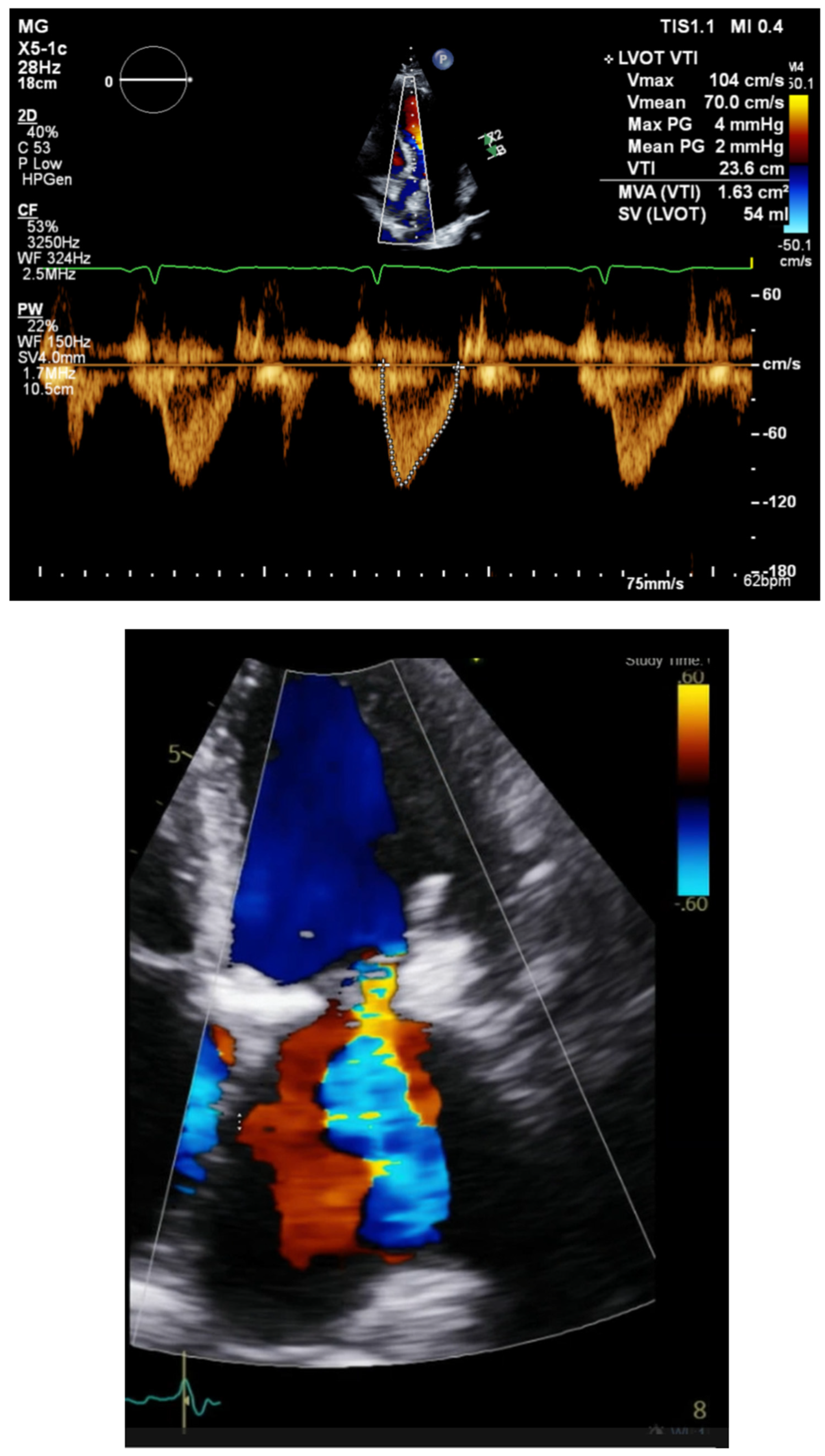
Pulse wave velocity measurement must be taken at the same location for each measurement. Error will be introduced if the measurement is taken too close to the aortic valve or too deep into the ventricle. If measurements are not consistent due to failure of consistent positioning of the sample volume (i.e., same location at each programming change), consider continuous wave Doppler as a more reliable measure. Ectopy and variable R-R intervals will also add to the measurement error. Choose measurement beats based on similar RR intervals. This parameter reflects stroke volume with the goal of increasing this value. Heart rate has an influence on this value. In normal hearts at rest, the cardiac output meets the demands of the body. Heart rate and stroke volume are inversely related. Raising the heart rate will reduce the stroke volume in hearts with normal filling. Stroke volume may not change in hearts with restrictive filling. Therefore, the first parameter in pacemaker optimization should be the selection of an appropriate heart rate dependent on the ventricular filling [
2].
Mitral Valve Inflow Velocity
E A Wave Separation
A restrictive inflow pattern occurs if the ventricle is already volume-loaded or the ventricle is stiff and fails to fill after the initial mitral valve opening. In either condition, stroke volume will not increase with greater diastolic filling times. The heart rate needs to increase to increase cardiac output. The selection of heart rate for restrictive disease must be an empirical estimate based on desired cardiac output. A tedious methodology includes titration of the heart rate until stroke volume begins to drop. This technique adds complexity, which is nearly impossible to perform, secondary to variations in respiration and sonographer attention span. For restrictive disease, a good place to start is 80 bpm. The heart rate is the most important parameter and has to be selected first. Unfortunately, little is known about the optimum heart rate for restrictive disease [
2].
For systolic dysfunction, a formula is available [
3].
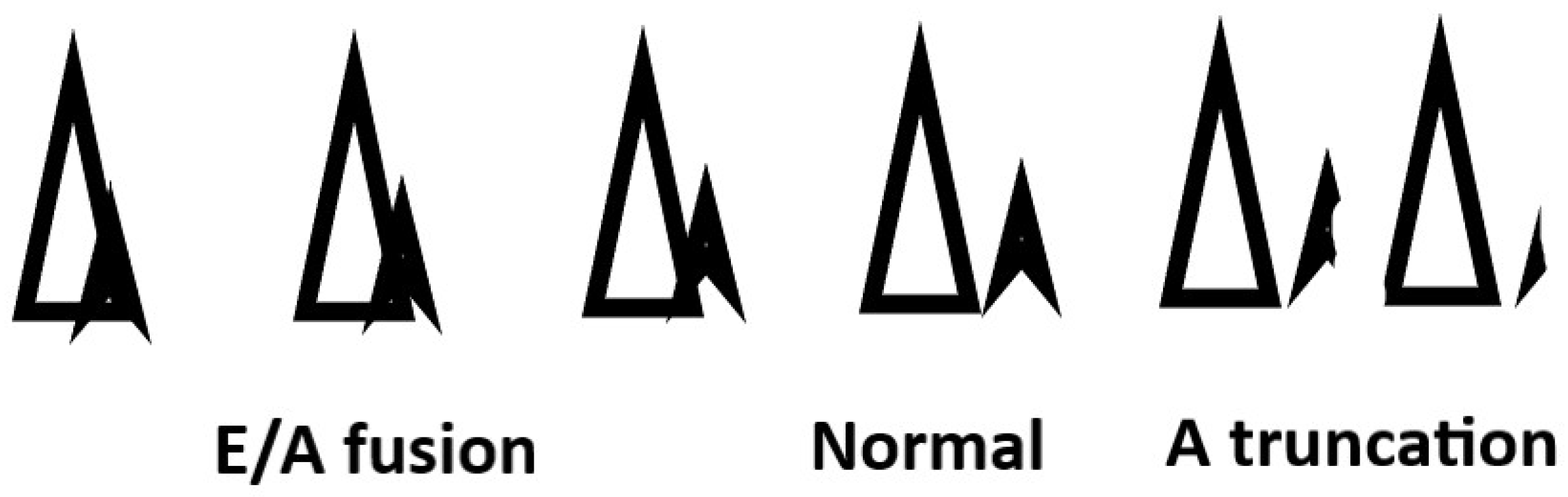
After the heart rate is selected, attention to atrial ventricular transport is performed by optimizing E and A wave separation. In systolic heart failure, guideline-directed medications with beta blockers usually make patients dependent on atrial pacing. When beta blockers are not used in restrictive disease or right ventricular failure, the sinus mechanism may be intact. In these cases, atrial pacing versus atrial sensing can make a big difference in AV transport. Thus, the AV delay will depend on atrial pacing or sensing. An intact AV node will allow intrinsic conduction to the ventricle. This is desired if there is no LBBB. These patients can maintain normal QRS configuration and avoid ventricular pacing dyssynchrony by programming a longer AV delay. The downside of this strategy is E and A wave fusion with greater filling pressure over a shorter time period. Long AV delays may harm some patients.
A short AV delay is desired if mandatory ventricular pacing is the goal. Ventricular pacing is desired if you have a bi-ventricular device and have pre-existing dyssynchrony from a left bundle branch block. Right ventricular pacing is desired in obstructive cardiomyopathy to induce paradoxical septal motion to relieve outflow obstruction. Right ventricular pacing is also desired in Cor Pulmonale. The short AV interval truncates the A wave with loss of atrial kick and reduced filling of the ventricle. The only way to manage individuals who are dependent on the atrial kick is to ablate the AV node to ensure ventricular pacing and optimize atrial ventricular transport.
Effective Mitral Valve Orifice—The iso-velocity curve orifice size is a visual assessment of the severity of mitral regurgitation. Optimization is achieved by reducing this area. An additional check on eccentric regurgitation or suspected occult regurgitation is to examine the height of the E wave of the mitral valve inflow. The reduction in the E wave is a signal of reduced mitral regurgitation
2-Dimensional Assessment of Dyssynchrony, Chamber Dimensions (Left Ventricular End Diastolic Volume), and Ejection Fraction—These standard echocardiographic measures should be performed initially and at the end of the optimizations to give further evidence of success.
Walk test—After the procedure, the patient should go for a walk to be sure the adjustments were beneficial clinically.
4. Pacing Goals for the Spectrum of Cardiac Disease
LBBB dyssynchrony right ventricular pacing dyssynchrony—Clinical trials support bi-ventricular pacing for these two disease processes. The abnormality is circumferential dyssynchrony, which causes a delay in septal activation, dyssynchrony, and widened QRS duration. The ejection fraction falls and mitral regurgitation increases. Late depolarization of the papillary muscle causes delayed repolarization with failure to relax the papillary muscle. The mitral valve remains slightly tethered open with the onset of systolic ejection. Early regurgitation begets more regurgitation. Restoring synchrony with left ventricular pacing requires the selection of the lead configuration and timing of the right and left ventricular leads.
Annular dilation and mitral regurgitation—A common final pathway in systolic dysfunction is chamber enlargement. As the annulus stretches from ventricular dilation or atrial dilation, the mitral valve can no longer co-apt. This results in mitral regurgitation and further atrial and ventricular dilation. Emptying the ventricle more frequently by increasing the heart rate can shrink the chamber dimensions. Programming the left lead with the earliest activation (LV before RV set to the largest interval) will ensure the mitral valve co-apt properly when systole begins. No clinical trial has addressed this solution.
Right heart failure in the setting of left heart failure or pulmonary hypertension—There are two hearts; the left and right heart. Between these two hearts is the interventricular septum, which can be programmed to help the right heart by right ventricular pacing. The phenotypic patient is an edematous patient who is lying flat with renal insufficiency, hypotensive, and with jugular venous distention and a positive Kussmaul’s sign.
Optimizing right heart function with LVAD—Left ventricular assist devices are dependent on the preload of the left ventricle. If the preload of the left ventricle is reduced due to the right heart’s inability to pump across the pulmonary bed; the LVAD will have suction events. The cause may be volume depletion or right heart failure. Pacing the right ventricle causes septal dyssynchrony, which favors the right ventricular function improving the filling of the left heart.
Annular dilation and tricuspid regurgitation—A further consequence of right heart failure is chamber enlargement with annular dilation. Tricuspid regurgitation secondary to pulmonary hypertension and annular dilation will cause more dilation and more regurgitation. Increasing the heart rate may shrink the volume of the right heart, providing better coaptation of the tricuspid valve.
Restrictive cardiac disease—Small hearts in amyloidosis or other infiltrative diseases have small stroke volumes, so these hearts are dependent on heart rate to increase cardiac output. Severe diastolic dysfunction also exists in large hearts with fixed stroke volumes. These large restrictive hearts require higher heart rates.
Valvular disease restricting stroke volume—Aortic stenosis restricts stroke volume alike peripheral vascular disease with fixed systemic vascular resistance. These conditions depend on heart rate to increase cardiac output.
Hypertrophic cardiomyopathy with obstruction—Paradoxical motion of the interventricular septum will reduce outflow tract obstruction. This can be accomplished with right ventricular pacing. These ventricles are also restrictive and dependent on atrial ventricular transport. Ideally, the best way to manage this condition is the ablation of the AV node so the optimum AV delay can be chosen. In these patients, atrial sensing versus atrial pacing must be assessed. The tradeoff is heart rate, AV delay, and atrial ventricular transport, which depend on atrial mechanics. Complete right ventricular pacing without fusion is mandatory to maintain paradoxical sepat motion.
Wide QRS duration dilated cardiomyopathy—Heart enlargement increases the distance the electrical signal has to travel. This is reflected in the electrocardiogram as a wide QRS duration. The QRS may or may not have bundle branch morphology. Multiple leads and the position of the leads can increase axial conduction velocity narrowing the QRS, potentially remodeling the heart into smaller chambers (see the Mathematical Model below). Longitudinal conduction velocity is the major determinant of dilation and sphericity of the heart. Wider QRS reduces the power of the heart.
Narrow QRS duration dilated cardiomyopathy—Trials investigating cardiac dyssynchrony found no benefit in biventricular pacing for the dilated ventricle with narrow complex < 130 ms QRS duration [
4,
5]. For this reason, the therapy is not offered. The reason for this failure has not been elucidated; however, programming was limited. The proposed pathophysiology of narrow complex dilated cardiomyopathy is presented in the following article [
6]. Houck PD, Jones B, Patel R, et al. Insight of the pathophysiology of narrow complex dilated cardiomyopathy derived from the velocity equation: velocity = distance/time BMJ Case Rep 2019. The article suggests that increasing the amplitude and pulse width can improve electromechanical coupling. Cardiac contractility modulation [
7,
8] is another proposed therapy.
Longitudinal versus circumferential dyssynchrony—With appropriately placed RV and LV leads, the optimum timing delay is simultaneous. Circumferential dyssynchrony may require a delay.
Ectopy-induced dyssynchrony—A high PVC burden with or without LBBB will cause dyssynchrony. In these cases, medications have to be added to reduce the burden of ectopy. Some of the PVCs may be suppressed by increasing the base rate, preventing premature beats from capturing the ventricle. Some pacing devices have the option to biventricular pace when an ectopic is sensed in an attempt to fuse ectopic beats with normal pacing.
Variable RR intervals—Resynchronization therapy depends on the patient being paced. Atrial fibrillation with rapid response must be controlled to ensure pacing therapy is delivered. Increasing the base rate, drugs to slow the rate of atrial fibrillation, or AV nodal ablation are methods to restore the irregularity of variable intervals.
Age—Pacemakers are a frequent occurrence in the aged cohort, providing improved quality of life [
9,
10]. The aged cohort has stiff blood vessels and, when combined with orthostatic hypotension, the most important parameter to adjust is a higher heart rate
5. Remodeling the Dilated Heart by Increasing Axial Propagation Velocity
Mathematical model
The following provides the mathematical relationships of lead placement and timing in relationship to QRS duration. Adding a third lead will increase circumferential propagation velocity by earlier initiation of impulses at the RV apex and lateral wall. Axial propagation velocity may be increased if the lead is placed between the base and apex. By placing multiple electrodes along the axis and timing them from base to apex, the axial velocity can be increased and should restore ventricular geometry. The optimal number of leads depends on the conduction velocity, the size of the heart (A defined as 1/2 the axial perimeter, circumferential length = C defined by 1/2 the circumferential perimeter), and the QRS duration. The new QRS duration can be calculated from the following formula for pacemaker leads placed equidistant from the apex and fired in sequential fashion from apex to base.
QRS = 0.196
N = Number of Leads—3
A = Axial Length (cm)—19
VA = Axial Conduction Velocity (cm/s) 196
QRSnew = 0.667 × 0.196 = 0.130 s
Effective Conduction Velocity = 146 cm/s
If the three leads were fired simultaneously, the following formula can be used:
For a 2-lead system firing simultaneously with one lead at the apex and a second lead located X cm from the apex along the axial perimeter with conduction velocity VA, the new QRS duration can be calculated from two formulas: one for X ≤ 2A/3 and one for X ≥ 2A/3.
There are two equations; since for any condition other than 2A/3, there will be an overlap of conduction either at the apex or base of the heart.
For X ≤ 2A/3—QRSnew = QRSold − X/(VA)
For X ≥ 2A/3—QRSnew = X/(2 × VA)
For X = 2A/3—There is no overlap—QRSnew = QRSold/3
For X = A—There is no overlap—QRSnew = QRSold/2
For X = 0—There is 100% overlap—QRSnew = QRSold
The QRS in the circumferential directions with one lead at the septal apex and one lead located Y cm away from the septum on the circumference; the conduction velocity VC is calculated from two formulas for Y ≥ C/2 and
For Y ≥ C/2—QRSnew = Y/(2 × VC)
For Y ≤ C/2—QRSnew = QRSold − Y/VC
For Y = C/2—There is no overlap—QRSnew = QRSold/2
For Y = 0 or Y = C—There is 100% overlap—QRSnew = QRSold
For a two-lead with a timing delay of T, the QRS duration in the axial direction is calculated from the following formula where T is the delay of the lateral lead, X is the apical displacement of the lateral lead from the apical lead, and A is 1/2 the axial perimeter.
Timing in the circumferential direction can be calculated from the following formula.
For the delay of the lateral lead of T, Y is the displacement of the lateral lead along the circumference and the Vc is the propagation velocity in the circumferential direction.
If the timing is reversed, the lateral lead fires first followed by the apical lead the results will be different.
For (A—VAT) ≤ X ≥ VAT—QRSnew = QRSold − X/(2 × VA) − T/2
For X ≤ VAT—QRSnew = QRSold − X/VA
For X ≥ (A − VAT)—QRSnew = X/(2 × VA) = T/2
For Y ≥ C/2—QRSnew = Y/(2 × VC) + T/2
For Y ≤ C/2—QRSnew = QRSold − Y/(2 × VC) + T/2
These equations demonstrate the following:
More leads can shorten the QRS than fewer leads;
Simultaneous stimulation of multiple leads will shorten the QRS more than sequential pacing;
For a bi-ventricular system, placing a lateral lead 2/3 of the distance from the apex and 180 degrees from the RV septal lead gives the shortest QRS duration for simultaneous pacing;
Lateral first versus apical first gives a wider QRS. A wider QRS duration is the cost of setting the left lead ahead of the RV lead. The lateral first setting favors a reduction in mitral regurgitation. A compromise must be made in these two parameters, which are at odds.
6. The Problem
Over three billion choices to improve 14 disease states with nine optimization goals (some of the optimization goals are diametrically opposed) to improve dyspnea, shortness of breath, fatigability, exercise intolerance, edema, swelling, fluid retention, and arrhythmia.
7. Method of Optimization of the Pacing Devices
7.1. Pre-Echo
Step 1 Evaluate symptoms
Symptoms—(fatigue, exertional intolerance) determine if the right heart or left heart is the culprit.
Symptoms—(shortness of breath) Evaluate for AV dyssynchrony, mitral regurgitation, and restriction of mitral inflow.
Step 2 Evaluate available data
Pick pacing lead from chest X-ray and test for capture or diaphragmatic stimulation.
ECG (rate, rhythm, PR interval, QRS duration, ectopy);
Echocardiogram (dyssynchrony, mitral valve inflow, mitral regurgitation, restrictive inflow, chamber dimensions, annular dilation, right heart function);
Chest X-ray Posterior, Anterior, and Lateral (determine ideal lead positions).
Examine lateral chest X-ray, determining which of the four lead positions are optimal (20 possible choices). To optimize longitudinal dyssynchrony, choose the best lead configuration—1/3 distance from the base or 2/3 distance from the apex
Figure 1.
Step 3 Echo Examination
Step 3a Pulse wave mitral valve inflow to choose heart rate. This is not a trivial task, as illustrated by the editorial Heart Rate [
2].
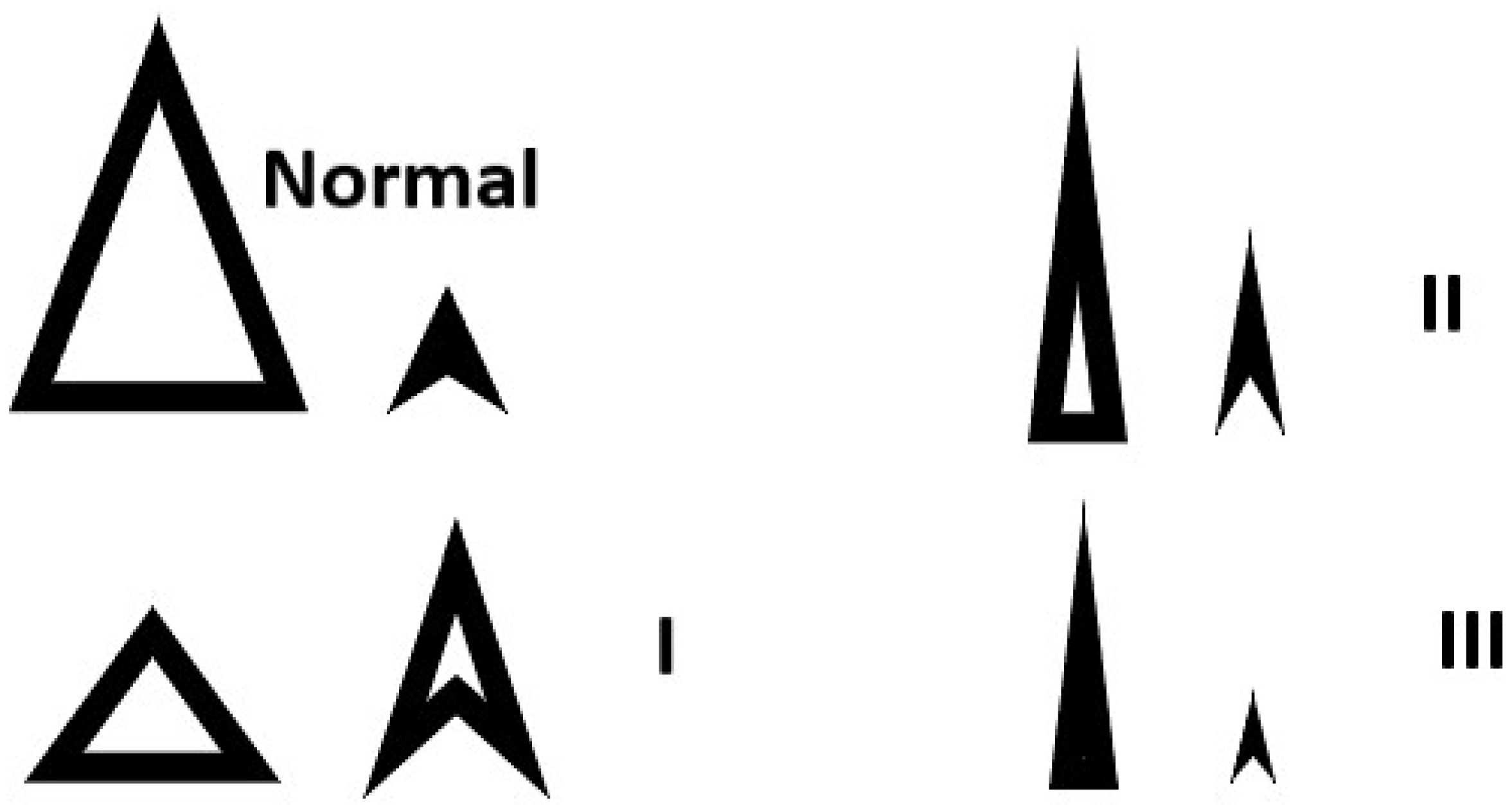
If the inflow is restrictive, grade 2 or 3 (
Figure 2), the ventricle is already full and needs to be emptied more frequently. Increase heart rate to 80 [
2].
For systolic dysfunction, one may choose a base heart rate with the following formula [
3]:
All others have an intact sinus node atrial sense.
Step 3b Select the AV interval from the mitral valve inflow at the new heart rate.
Set pacing and sensed interval to allow separation of the E and A waves and do not truncate the A wave.
Figure 3.
Step 3c Examine mitral regurgitation. If moderate or severe, choose the left lead first with the longest lead time. The earliest depolarization allows for earlier relaxation and better coaptation of leaflets before systole begins.
Step 3d Timing of the LV and RV goal is to decrease mitral regurgitation and increase the LVOT integral. Evaluate the LVOT integral and adjust LV/RV timing to maximize the LVOT integral and reduce mitral ERO. The RV LV timing delay is chosen by iteration of an interval to optimize LVOT VTI
Figure 4.
Step 4 Walk the patient and assess for symptom improvement. As the heart remodels to the new settings, a repeat optimization may be required.
7.2. Specific Cardiac Pathology
Annular dilation and mitral regurgitation If step 3c above does not reduce the ERO due to the primary dilated annulus, the pacing heart rate should be increased to make the ventricle smaller. Return to step 3b and re-optimize the AV interval for the new higher heart rate.
Left versus right heart failure and Optimizing right heart function in the setting of an LVAD If the right is failing, turn off the left lead and ensure right ventricular pacing to reassess for mitral regurgitation and the LVOT integral.
Annular dilation and tricuspid regurgitation Similar to mitral annular dilation, increasing the heart rate to shrink the heart with a return to step 3b AV optimization will optimize for a higher heart rate.
Restrictive cardiac disease, Valvular disease restricting stroke volume, Peripheral vascular disease with fixed systemic vascular resistance restricting stroke volume Increased heart rate compensates for reduced stroke volume. Returning to step 3b AV optimization is critical since these patients depend on atrioventricular transport.
Hypertrophic cardiomyopathy with obstruction Ensure that right ventricular pacing ablates the AV node in order to optimize AV transport.
Wide QRS duration dilated cardiomyopathy Pacemaker activation of multiple sites can increase the axial and circumferential conduction velocities, reducing the size of the heart. Greater lead number and simultaneous pacing can accomplish this feat. Depending on the lead position, circumferential dyssynchrony can be induced.
Optimize exercise parameters
Evaluate the Ramp (sensitivity of piezoelectric crystal) increase if there is orthostatic or exertional intolerance.
Why has this protocol not been studied or recommended?
Designing a study to test three billion choices to improve 14 disease states with nine optimization goals (some of the optimization goals are diametrically opposed) is impossible. Studies designed to prove the clinical benefit of bi-ventricular pacing allowed only minimal programming options, as noted in the following
Table 3. The presented method requires knowledge of physiology and hemodynamics. Pacemaker companies have used algorithms to minimize programming; however, one size does not fit all. An easy and common device algorithm is for the device to automatically minimize the QRS duration. If mitral regurgitation is present, symptoms may worsen. If symptoms persist, individualization is required and those algorithms should be turned off.
8. Conclusions
The problem can be stated as over three billion choices to improve 14 disease states with nine optimization goals (some of the optimization goals are diametrically opposed) to improve dyspnea, shortness of breath, fatigability, exercise intolerance, edema, swelling, fluid retention, and arrhythmias. The goal is to increase the Left Ventricular Outflow Integral, reduce mitral regurgitation, increase longitudinal conduction velocities, and restore synchrony of the septum to the ventricle that needs it the most. Pacemakers are sophisticated devices that should be optimized to meet the goals of individual patients. Pacing trials provided the basic justification for an additional pacing lead but fell short in optimizing individual patients. The physician needs to recognize the spectrum of disease and use the protocol to improve the quality of life of the individual patient. Age and pacemakers are highly correlated. The aged cohort has increased heart rate requirements to prevent falls from orthostatic hypotension and fatigue due to reduced cardiac output. The protocol will help select the most optimum pacing parameters out of the 3,875,040,000 choices. Caveat—As the heart remodels with the new pacing parameters, repeat optimization may be required. Do not trust device manufacturer’s algorithms in symptomatic patients.