Abstract
Aim and Background: To determine whether occupational physical activity (OPA) and physical fitness (Fitscore) predict cardiovascular disease (CVD) mortality and its components. Methods: Among middle-aged men (N = 5482) of seven cohorts of the Seven Countries Study (SCS), several baseline risk factors were measured, and there was a follow-up for 60 years until virtual extinction. OPA was estimated from the type of work while Fitscore was derived from linear combinations of levels of arm circumference, heart rate and vital capacity computed as a factor score by principal component analysis. The predictive adjusted power of these characteristics was obtained by Cox models for coronary heart disease (CHD), heart diseases of uncertain etiology (HDUE), stroke and CVD outcomes. Results: Single levels of the three indicators of fitness were highly related to the three levels of OPA and Fitscore. High levels of both OPA and Fitscore forced into the same models were associated with lower CVD, CHD, HDUE and stroke mortality. When assessed concomitantly in the same models, hazard ratios (high versus low) for 60-year CVD mortality were 0.88 (OPA: 95% CI: 0.78–0.99) and 0.68 (Fitscore 95% CI: 0.61–0.75), and the predictive power of Fitscore outperformed that of OPA for CHD, HDUE and stroke outcomes. Similar results were obtained in individual outcome models in the presence of risk factors. Segregating the first 30 from the second 30 years of follow-up indicated that people dying earlier had lower arm circumference and vital capacity, whereas heart rate was higher for CVD and most of its major components (all p < 0.0001). Conclusions: OPA was well related to the indicators of fitness involving muscular mass, cardio-circulatory and respiratory functions, thus adding predictive power for CVD events. The Fitscore derived from the above indicators represents another powerful long-term predictor of CHD, HDUE and stroke mortality.
1. Introduction
A substantial body of scientific evidence supports the beneficial impact of physical activity on health and the prevention of premature mortality [1,2,3,4,5]. As a result, promoting physical activity has become a common strategy in both community and clinical settings [1,2]. However, confusion can arise when no distinction is made between various forms of physical activity [1]. Apart from the energy expended through bodily movement, attention can be directed toward physical fitness, which is the (potentially inherited) ability of the body to perform activities efficiently and effectively [6,7,8,9,10,11,12,13,14,15,16]. Only a limited number of studies have thoroughly examined the distinct roles of muscular, cardio-circulatory, and respiratory functions as indicators of physical activity in relation to cardiovascular disease and life expectancy [13,14,15,16]. Objectively measured physical fitness derived from linearly combined arm circumference, heart rate and vital capacity (by Fitscore) may represent an improvement over classes of physical activity estimated from the type of work performed. It was comparatively assessed among 5482 middle-aged men examined with the measurement of several risk factors in a previous analysis whereby these measured parameters of functions had a significant predictive role on all-cause mortality and age at death in European population cohorts of middle-aged men followed up until extinction [17]. Physical fitness is defined as “the ability to carry out daily tasks with vigor and alertness, without undue fatigue and with ample energy to enjoy leisure-time pursuits and to meet unforeseen emergencies” and might thus help to better assess the long-term risk of all-cause mortality and higher age at death when Fitscore based on arm circumference, heart rate and vital capacity is in the upper tertile. This is an improvement compared to physical activity classified by occupation (OPA) as “any bodily movement produced by skeletal muscles that results in energy expenditure” and should be applied in day-to-day clinical/preventive cardiology practice.
The purpose of the present analysis is therefore to explore the possible relationship between OPA and physical fitness for their joint and/or independent roles in the prediction of major cardiovascular disease mortality subtypes and overall, in the same European cohorts for 60 years [17], thus to practical extinction.
2. Material and Methods
2.1. Population and Measurements
A total of 5482 middle-aged men (40 to 59 years) were enrolled in 7 European cohorts of the Seven Countries Study, identified as East Finland, West Finland, Zutphen in the Netherlands, Crevalcore and Montegiorgio in Italy and Crete and Corfu Islands in Greece. All cohorts were of rural nature with men largely engaged in occupations of heavy physical activity, except for Zutphen, a small trade town in the Netherlands. Participation rate at baseline examination was, on average, 95% of all men aged 40–59 in the defined regions. Details of these cohorts can be found elsewhere [18,19].
The main variables were as follows: (A) Occupational (at work) physical activity (OPA) classified as low, intermediate or high and derived from the type of occupation and a few extra non-standardized questions; leisure physical activity was not considered since it was extremely rare in those communities in the early 1960s. (B) Indicators of physical fitness were as follows: (B1) arm circumference (in mm) following the technique reported in the WHO Survey Methods Manual [20] (WHO Manual) with the crude measurement adjusted for the contribution of subcutaneous tissue [21], to represent muscle mass; (B2) heart rate (in beats/min) derived from a standard resting ECG, to potentially represent cardio-circulatory fitness; and (B3) vital capacity (in L/m2) based on the technique reported in the WHO Manual [20] using the best value of 2 attempts, to represent fitness derived from the respiratory function. Vital capacity in Zutphen, the Netherlands, was measured a few years after the entry examination. Therefore, computations were performed by regressing the measurements on age to reach the levels of the entry date. The combination of arm circumference, heart rate and vital capacity was used to obtain a fitness score (Fitscore) by running principal component analyses and expressing it individually by the consequent factor score (arbitrary units). Factor score coefficients were 0.6433 for arm circumference, −0.1404 for heart rate and 0.6812 for vital capacity. In analysis, Fitscore was divided into tertile classes (low, intermediate and high). The term “fitness score” is not fully appropriate since the variables were derived from functions likely related to fitness score but not measuring its properties. However, it was previously adopted to identify these characteristics and used in analyses [17].
Other baseline variables were used as possible confounders in the multivariate predictive analyses, as follows: (a) age in years: the nearest birthday was used to approximate this; (b) average number of cigarettes smoked per day (n/day), after having shown that ex-smokers could be classified as non-smokers [3,4,5]; (c) body mass index (kg/m2) using the measurement technique reported in the WHO Manual [20]; (d) systolic blood pressure (mmHg) measured at the end of a physical examination, in supine position, using a mercury sphygmomanometer, following the technique reported in the WHO Manual [20]; the average of two measurements taken one minute apart was adopted; e) serum cholesterol (mg/dL) using the technique described by Anderson and Keys [22] on measurements of casual blood samples. About 4 per 1000 of the above measurements were missing, and thus, multivariate normal procedures were adopted for imputations using a program of the NCSS 12 computer statistical package.
End-points for testing the predictive power of OPA and Fitscore were 3 cardiovascular mortality end-points classified following the WHO International Classification of Diseases, 8th Revision [23] (ICD-8): (1) Coronary heart disease (CHD) included only cases of explicit coronary syndromes such as myocardial infarction, acute ischemic attack and sudden coronary death, after reasonable exclusion of other possible causes. (2) Stroke included any type of cerebrovascular disease. (3) Heart diseases of uncertain etiology (HDUE) included a pool of symptomatic heart diseases manifesting as heart failure, arrhythmia and blocks in the absence of a clear etiology as well as cases including chronic coronary heart disease and hypertensive heart disease, in the absence of typical coronary syndromes. (4) Cardiovascular diseases (CVD) correspoding to the sum of CHD plus HDUE and stroke.
The reasons for keeping CHD mortality well separated from that due to HDUE is bound to extensive documentation we have provided about their differences, at least for risk factors (serum cholesterol definitely higher for CHD), and age at death (definitely higher for HDUE) [24,25]. During the 60 years of follow-up, out of 5482 men examined at baseline, there were 5471 deaths (99.8%), while 3 men were still alive and 8 were lost to follow-up and censored at defined times.
2.2. Statistical Analysis
OPA was used as defined and classified into 3 classes (low, intermediate and high), as Fitscore was divided into low, intermediate and high tertiles. Baseline levels of OPA and Fitscore were computed for each cohort together with those of arm circumference, heart rate, vital capacity and risk factors used as confounding variables in the multivariate models. They were then all compared in the 3 classes of OPA and in tertile classes of Fitscore. Death rates in 60 years for the 3 groups of CVD and their pool were also computed and tabulated. More details might be found in a previous paper [17].
Tests of predictive power were performed as follows: (1) Kaplan–Meier survival curves for mortality from CHD, HDUE, stroke and CVD separately versus the 3 original OPA classes and the 3 tertiles of Fitscore distribution; (2) Cox proportional hazard models separately for 3 major groups of cardiovascular mortality and their pool including the following predictors in different models: OPA plus Fitscore alone. Another 4 Cox models were solved, for each CVD end-point, including OPA, Fitscore, and a series of possible confounding variables such as age, cigarette smoking, body mass index, systolic blood pressure and serum cholesterol along with dummy variables identifying countries (Finland as reference); in all cases, OPA and Fitscore were divided into 3 classes: low level (used as reference), intermediate and high levels for OPA, whereas Fitscore was divided into 3 tertile classes (low level used a reference). Data from cohorts belonging to the same country were combined in all previous analyses since their characteristics were similar [17]. Using the Schoenfeld residuals versus time, we tested the proportional hazard (PH) assumptions. We also tested log(time) and covariate interaction for statistical significance.
Finally, the mean levels of 3 indexes of fitness were compared among those who died during the first 30 years of follow-up versus those who died in the second 30 years.
3. Results
3.1. Baseline Variables and Death Rates
Death rates from CVD are shown in Table 1: CHD were more common in the Northern European countries while HDUE and stroke were more common in the Southern European countries. The proportion of all deaths (not reported in detail) was similar among the various countries since the cohorts reached practical extinction as documented elsewhere [4,5]. Table 1 does not include the proportions of OPA distributed according to low, intermediate and high activities, nor does it include individual fitness indicators (arm circumference, heart rate and vital capacity) with confounding factors since these results were previously published in each of the four European countries of the SCS and in the overall population [17]. The class of high OPA was particularly large due to the rural occupations in six out of the seven cohorts [17], whereas we previously reported large differences across countries for serum cholesterol [19,20].

Table 1.
Death rates per 1000 (standard errors) of CHD, HDUE, stroke and CVD in the European cohorts of the SCS (*).
3.2. OPA and Fitscore versus Indicators of Fitness
Increasing levels of arm circumference and vital capacity across the three classes of OPA were seen in Table 2, and the reverse was true for heart rate. Mean levels of Fitscore were significantly different in the three classes of OPA. The same picture was seen in the relationship of the three fitness indexes with tertile classes of Fitscore. The arm circumference gradient across OPA classes was not very large nor monotonic. The associations of the Fitscore components with the overall score are useful for understanding. However, they are larger because they are components of the score. Heart rate was more closely related to OPA than to Fitscore and its components. In all cases, ANOVA was highly significant for heterogeneity.

Table 2.
Mean values of indicators of fitness in 3 classes of OPA and Fitscore (standard deviations) (*).
3.3. Prediction of 60-Year Mortality from CHD, HDUE, Stroke and CVD by OPA and Fitscore
In Figure 1, Kaplan–Meier survival curves for CHD mortality groups in the three classes of OPA did not show clear separations of curves, confirmed by a non-significant p of the log-rank test, while the curves of the three classes of Fitscore were somewhat diverging and associated with a significant log-rank test. The situation for HDUE and stroke (Figure 2 and Figure 3) was similar since the curves from Phayc were not significantly separated while this was true for Fitscore. The curves for CVD, instead, were better separated (mainly for Fitscore), and both OPA and Fitscore had significant log-rank tests (Figure 4).

Figure 1.
Kaplan–Meier survival curves for CHD mortality as a function of 3 classes of OPA (p of log rank = 0.2252) and 3 tertiles of Fitscore (p = 0.0006).
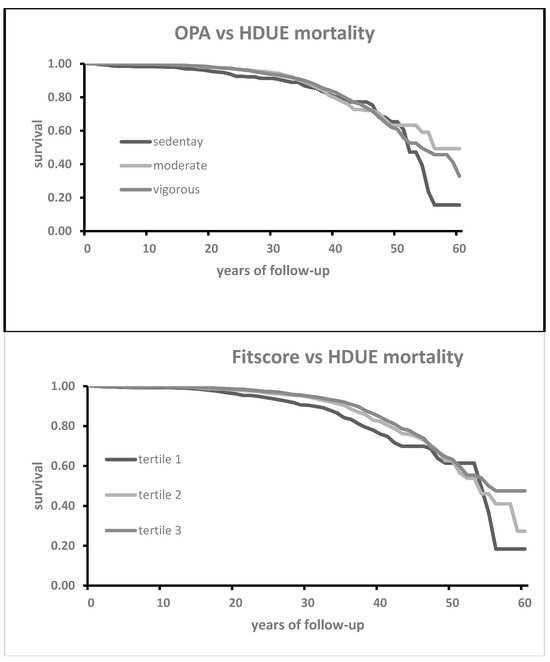
Figure 2.
Kaplan–Meier survival curves for HDUE mortality as a function of 3 classes of OPA (p of log rank = 0.0907) and 3 tertiles of Fitscore (p < 0.0001).

Figure 3.
Kaplan–Meier survival curves for stroke mortality as a function of 3 classes of OPA (p of log rank = 0.0765) and 3 tertiles of Fitscore (p < 0.0001).
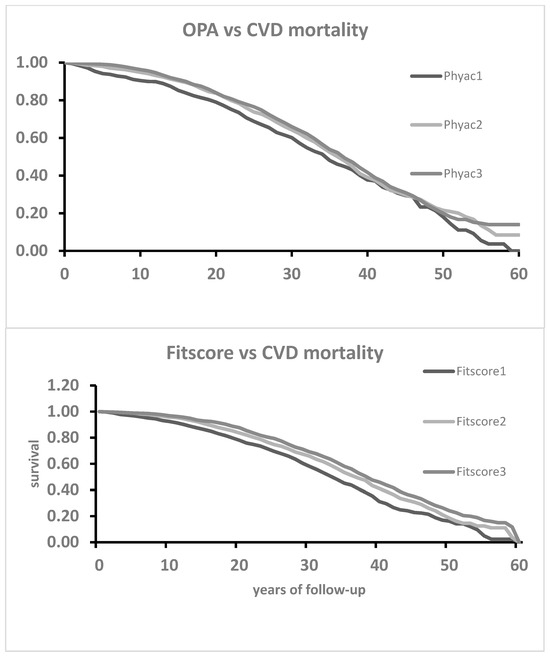
Figure 4.
Kaplan–Meier survival curves for CVD mortality as a function of 3 classes of OPA (p of log rank = 0.0032) and 3 tertiles of Fitscore (p < 0.0001).
In Table 3, OPA and Fitscore used in combination in the same model had, for each end-point, four options to be significantly associated with mortality of the respective CVD groups. In the case of CHD, OPA was always not significant while Fitscore was inversely related to mortality when comparing class 3 with class 1. For HDUE and stroke, again, OPA was not significant while Fitscore was significant on all four occasions. Only in CVD, both OPA and Fitscore were always inversely related to mortality. This presentation mimics, multivariately, those given by the Kaplan–Meier survival curves of Figure 1, Figure 2, Figure 3 and Figure 4. Overall, in all four tests comparing high with low levels, Fitscore produced negative and significant coefficients corresponding to hazard ratios ranging from 0.58 to 0.79 while, for the same comparisons, OPA did so only two times with hazard ratios of 0.88 (only in CVD significantly so).

Table 3.
Multivariate Cox models predicting CHD, HDUE, stroke or CVD 60-year mortality as a function of OPA (3 classes) and Fitscore (3 classes), both combined in the same model.
Cox models reported in Table 4, Table 5, Table 6 and Table 7 summarize the situation for 60-year mortality from CHD, HDUE, stroke and CVD, respectively, feeding as covariate the levels of OPA, Fitscore plus a few classic cardiovascular risk factors and dummy variables for the identification of four countries (with Finland as references). For CHD, OPA was not significant, while Fitscore was significant comparing high levels with low levels (hazard ratio = 0.81). For HDUE, OPA was significant in comparing high with low levels (hazard ratio = 0.74), while Fitscore did so (hazard ratio = 0.72) also comparing intermediate with low levels (hazard ratio = 0.79). The same outcome occurred in the model having stroke as the end-point. In the model of CVD, all coefficients were negative, and the related hazard ratios did not include 1, except the comparison of intermediate OPA versus low OPA. Overall, the hazard ratios of the significant coefficients were somewhat smaller than in the previous analysis of Table 3, due to the coexistence of many other covariates, most of which presented, expectedly, significant hazard ratios (not commented on in detail). PH assumptions were not violated notwithstanding some crossing among several Kaplan-Meier (univariate) survival curves which was however less evident visually for Fitscore and CVD as outcome also comparing to OPA (Figure 4).

Table 4.
Multivariate Cox model predicting CHD 60-year mortality as a function of 3 classes of OPA and Fitscore adjusted for 5 confounding variables and country (Finland is the reference).

Table 5.
Multivariate Cox model predicting HDUE 60-year mortality as a function of 3 classes of OPA and Fitscore adjusted for 5 confounding variables and country (Finland is the reference).

Table 6.
Multivariate Cox model predicting stroke 60-year mortality as a function of 3 classes of OPA and Fitscore adjusted for 5 confounding variables and country (Finland is the reference).

Table 7.
Multivariate Cox model predicting CVD mortality as a function of 3 classes of OPA and Fitscore adjusted for 5 confounding variables and country (Finland is the reference).
The mean levels of the three indexes of fitness for those who died from the three CVD groups (separately and pooled) during the first 30 years of follow-up versus those who died during the second 30 years are shown in Table 8. Lower levels were seen for arm circumference and vital capacity when men died early while the opposite was true for heart rate. The findings were similar in the various outcome groups, and t tests between the two time periods were highly significant in 11 out of 12 comparisons.

Table 8.
Mean levels (standard deviations) of 3 indexes of fitness in men who died during the first 30 years versus those who died in the second 30 years of follow-up.
4. Discussion
In this investigation, we tried to disentangle the role of OPA from that of physical fitness in predicting CVD and its subtypes to go deeper than what we showed in relation to all-cause deaths and age at death [17]. OPA was subdivided into three levels, whereas Fitscore—expressed as arbitrary units of a factor score derived from a principal component analysis and also subdivided into three levels—was equally or even better predictive than OPA when fed in the same models. This was so when these two parameters were challenged alone yet together (Table 3 and Figure 1, Figure 2, Figure 3 and Figure 4) or when they were considered concomitantly and by adding classic risk factors for each respective subtype (Table 4, Table 5 and Table 6) or as CVD (Table 7), and there were also covariates defining countries. This suggests that OPA and Fitscore are relatively independent from each other and that Fitscore [probably due to being the result of actual measurements of muscular (arm circumference) [21,26], cardio-circulatory (heart rate) [11,27,28,29] and respiratory (vital capacity) [11,20,30,31,32] capacities] seems more intimately related to physical fitness [7,13,14,15,16]. Although some connections between OPA and Fitscore may exist, there were no mathematical connections between them, and they performed in different ways when used to predict events. On one hand, Fitscore was created in an entirely different and independent way from OPA, simply derived from the type of occupation at work and a few extra non-standardized questions. On the other hand, Fitscore outperformed OPA in all comparisons (Table 3, Table 4, Table 5, Table 6 and Table 7). Finally, multicollinearity across the covariates may be reasonably excluded since the tolerance was always very high.
The results confirm, however, that working physical activity classes, although obtained by a relatively rough procedure, have a good predictive power for fatal CVD events [3,4,5] while the Fitscore adds something extra by being more strongly predictive and to a greater extent than OPA. Therefore, it is logical that despite some inter-relations, OPA and Fitscore have a differential impact in predicting CVD outcomes, also shown in other studies [8,12,14,15,16]. At year zero, at baseline, OPA measurements were taken along those of indexes used to compute Fitscore. Although there was an expectation that they could change along the long-term follow-up of the SCS [18,19], later systematic measurements were not obtained after entry for all variables and countries. It is still possible that characteristics measured at year 0 are predictive of events spread along an unusually long follow-up of 60 years, since by segregating the first 30 from the second 30 years of follow-up, the existence was clearly shown of long-term associations with fatal events. This was so not only for overall CVD mortality but also for its major components of CHD, HDUE and stroke mortalities. Although people dying earlier had lower arm circumference and vital capacity, their heart rate was higher (Table 8). This points to the importance of considering functional parameters measured at baseline by Fitscore that might indeed represent a pathophysiologically based predictive index of CVD outcomes [26,27,28,29,30,31,32]. In regards to physical activity, it is important to underline that people with sedentary habits [10] were not classified correctly using a single question when true time spent in physical activity seemed a more proper way [9]. In the majority of reported cohort studies, however, physical activity was simply classified as self-reported [16] or derived from activity pattern questionnaires [12] or from caloric expenditure [8] or, in a recent large review, from these and other ways like complex questionnaires or estimates of metabolic equivalents [15].
All original investigations and their meta-analyses adopted exercise testing as an indicator of physical fitness when physical activity with physical fitness was compared [12,14,15,16] or when only physical fitness was taken into account [6,7,13]. We were unable to trace any study that used arm circumference, heart rate and vital capacity as baseline variables to define fitness levels, and accordingly, comparisons with results from other investigations are impossible. There was only one report whereby the association of physical activity was shown on the levels of forced expiratory volume [11]. On the other hand, studies that have directly tackled the problem have systematically shown that physical fitness classification is a better predictor than physical activity classification for cardiovascular and all-cause mortality [12,14,15,16] which is in line with the findings from our analysis.
There are also negative results in relation to OPA and its capacity to predict CVD and all-cause mortalities as reported in a systematic review and meta-analysis covering 23 studies and 655,892 individuals, although leisure physical activity appeared more powerfully predictive [33]. The findings of our study seem thus an exception or may simply belong to the minority group of those reviewed [33]. However, in studies with three activity categories (mildly, moderately, and highly active) and multivariate-adjusted models, men with high activities had 22% less risk for all-cause (including CVD) mortality (RR = 0.78; 95% CI: 0.72 to 0.84) compared to men who were mildly active [34]. For women, the relative risk was 0.69 (95% CI: 0.53 to 0.90), and the association with all-cause mortality was similar and statistically significant only in older subjects [34]. There was therefore a dose–response curve especially from sedentary subjects to those with mild and moderate exercise with only a minor additional reduction with further increase in activity level.
Our study was undertaken in a time period when leisure physical activity practically did not exist among rural men occupied in high physical activity. The hazard ratios of a few risk factors were calculated in the respective CVD outcomes, providing expected results, (Table 4, Table 5, Table 6 and Table 7). On the other hand, in more recent years, when leisure physical activity is widespread, it seems that comparatively work-related physical activity does not prevent CVD, but only leisure time activity does [35]. This was observed among 7058 outpatients with CVD (age 61 ± 10 years, 75% male) from the prospective Utrecht Cardiovascular Cohort-Second Manifestations of ARTerial disease cohort, where self-reported leisure physical activity and OPA were investigated in relation to all-cause mortality and incidences of CVD and type 2 diabetes, a completely different situation compared to the one explored in our study where younger apparently healthy people were followed up for mortality predictions over 50 years. In a much shorter period of 8.6 years (IQR: 4.6–12.5) of follow-up, 1088 vascular events (15.4%), 1254 deaths (17.8%) and 447 incident type 2 diabetes cases occurred [35]. Again, objective measurements were not performed, primary versus secondary preventive approaches may not be comparable, and very long versus quite short follow-up durations may not compensate for the comparability of results. Finally, the four levels that assessed the physical activity intensity during participants’ last active employment were [35] predominantly sedentary work, standing work, manual work and heavy manual work, and the OPA was probably not so all-consuming as in our study.
Longitudinal associations of OPA and left ventricular structure and function were examined among 1462 participants {50.0% female, 56.4% white, aged 30.4 ± 3.4 years at baseline [Year 5 exam (1990–91)]} from the Coronary Artery Risk Development in Young Adults study, to test the explanatory hypothesis that unfavorable cardiac remodeling may result from chronic OPA-induced cardiovascular strain [36]. An OPA exposure of 25 years was not significantly associated with left ventricular functionality parameters especially those referred to left ventricular contractility among those in the high- versus no-OPA trajectories [36] which points to the need to obtain objective parameters in the future to assess comparatively how leisure physical activity versus OPA affect survival, either all-cause or CVD related.
5. Conclusions
From our residential cohort study of middle-aged European men enrolled in the middle of the last century, the majority in rural areas of hard work and followed for a very long term, the three classes of OPA of the original Seven Countries Study classification were strongly associated with the indicators of fitness involving muscular mass (arm circumference), circulatory (heart rate), and respiratory (vital capacity) functions. Fitscore derived from the latter indicators of fitness has valuable predictive power for mortality occurrence in the CVD area subdivided into its major components. A major limitation was, however, that we had no women involved at the time this study was started when financial constraints negated the possibility of enrolling individuals with a low probability of CVD also long-term. The Fitscore represents, nevertheless, at least among men, another powerful predictor of three major classes of long-term CVD mortality. It should be appropriate to use Fitscore for the very long-term prediction of CVD and all-cause [17] mortality. Fitscore thus represents a new arrow to be applied in primary prevention and should be used largely, since it is based on easy-to-obtain and relatively low-cost measurements. It is wise to repeat this investigation in other investigations enrolling both genders.
Author Contributions
Conceptualization, P.E.P. and A.M.; methodology and analysis, A.M. and P.E.P.; writing—original draft, P.E.P. and A.M.; review, J.M.G., A.K. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
For the initiation of the Seven Countries Study of Cardiovascular Diseases, Prof. Ancel Keys, University of Minnesota, USA, obtained research grants from the National Heart Institute (later NHLBI) and the American Heart Association, and funds were distributed to all the national research groups of the study. Moreover, the national research groups responsible for the present paper obtained other local funds as follows: (1) Finland: Finnish Heart Association; Finnish State Science Board; Sigrid Juselius Fund; Yrjö Jahnsson Foundation. (2) The Netherlands: Netherlands Prevention Foundation (Preventiefonds); National Institute of Public Health and the Environment (RIVM); Royal Netherlands Academy of Arts and Sciences (KNAW); Ministry of Public Health; Nutrition Council; Organisation for Food and Nutrition Research (TNO); Netherlands Heart Foundation; Netherlands Cancer Foundation. (3) Italy: Association for Cardiac Research, Rome; Centre of Cardiovascular Disease, S. Camillo Hospital, Rome; City of Naples; National Institute of Public Health (ISS); National Research Council (CNR); European Union; Centre for the fight against infarction, Rome. (4) Greece: Royal Institute for Research; Elais Oil Company. The analysis and writing of this paper were not covered by the above funds.
Institutional Review Board Statement
The board of directors of the various institutions involved in data collection were de facto playing the role of ethical committee approving the execution of the study on the basis of the local existing legislation by the date this investigation started.
Informed Consent Statement
Baseline measurements were taken before the era of the Helsinki Declaration, and approval was implied in participation, while verbal or written consent was obtained for the collection of follow-up data.
Data Availability Statement
The original data are not publicly available. However, research projects are evaluated centrally by an ad hoc committee.
Acknowledgments
The authors acknowledge the following collaborators for their professional and enthusiastic action in collecting the most recent follow-up data: Cleo Dontas in Corfu; Manolis Linardakis in Crete; and Giovina Catasta in the Italian Areas. Moreover, appreciation is expressed to Daan Kromhout, for his long-term responsibility for the Zutphen data, the Dutch cohort of the Seven Countries Study and his general leadership in the SCS. Henry Blackburn, a long-term co-investigator of the SCS, is also acknowledged for encouragement and help with this MS along with David R. Jacobs Jr. who provided useful suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Powell, K.E.; Paffenbarger, R.S., Jr. Workshop on Epidemiologic and Public Health Aspects of Physical Activity and Exercise: A summary. Public. Health Rep. 1985, 100, 118–126. [Google Scholar]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Menotti, A.; Keys, A.; Kromhout, D.; Nissinen, A.; Blackburn, H.; Fidanza, F.; Giampaoli, S.; Karvonen, M.; Pekkanen, J.; Punsar, S. All-cause mortality and its determinants in middle-aged men in Finland, the Netherlands and Italy in a 25-year follow-up. J. Epidemiol. Commun. Health 1991, 45, 125–130. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E.; Tolonen, H.; Kafatos, A. Age at death in cohorts of middle-aged men followed-up until nearly extinction: The European Areas of the Seven Countries Study. Ann. Med. 2018, 50, 620–633. [Google Scholar] [CrossRef]
- Puddu, P.E.; Menotti, A.; Jacobs, D.R., Jr.; Adachi, H.; Kafatos, A.; Tolonen, H. Cardiovascular risk factors predict age at death in 60-year follow-up of the Seven Countries Study. Aging Clin. Exp. Res. 2023, 35, 193–202. [Google Scholar] [CrossRef]
- Sandvik, L.; Erikssen, J.; Thaulow, E.; Erikssen, G.; Mundal, R.; Rodahl, K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N. Engl. J. Med. 1993, 328, 533–537. [Google Scholar] [CrossRef]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W., 3rd; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.S.; Gibbons, L.W. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Morrison, H.I.; Craig, C.L.; Schaubel, D.E. Physical activity, physical fitness, and risk of dying. Epidemiology 1998, 9, 626–631. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Bassett, D.R., Jr.; Strath, S.J.; Swartz, A.M.; O’Brien, W.L.; Thompson, R.W.; Jones, D.A.; Macera, C.A.; Kimsey, C.D. Comparison of three methods for measuring the time spent in physical activity. Med. Sci. Sports Exerc. 2000, 32 (Suppl. S9), S457–S464. [Google Scholar] [CrossRef]
- Macera, C.A.; Ham, S.A.; Jones, D.A.; Kimsey, C.D.; Ainsworth, B.E.; Neff, L.J. Limitations on the use of a single screening question to measure sedentary behavior. Am. J. Public Health 2001, 91, 2010–2012. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Macera, C.A.; Addy, C.L.; Sy, F.S.; Wieland, D.; Blair, S.N. Effects of physical activity on exercise tests and respiratory function. Br. J. Sports Med. 2003, 37, 521–528. [Google Scholar] [CrossRef]
- Myers, J.; Kaykha, A.; George, S.; Abella, J.; Zaheer, N.; Lear, S.; Yamazaki, T.; Froelicher, V. Fitness versus physical activity patterns in predicting mortality in men. Am. J. Med. 2004, 117, 912–918. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- De Fina, L.F.; Haskell, W.L.; Willis, B.L.; Barlow, C.E.; Finley, C.E.; Levine, B.D.; Cooper, K.H. Physical activity versus cardiorespiratory fitness: Two (partly) distinct components of cardiovascular health? Prog. Cardiovasc. Dis. 2015, 57, 324–329. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Davidson, T.; Vainshelboim, B.; Kokkinos, P.; Myers, J.; Ross, R. Cardiorespiratory fitness versus physical activity as predictors of all-cause mortality in men. Am. Heart J. 2018, 196, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Geleijnse, J.M.; Kafatos, A.; Tolonen, H. Physical activity and physical fitness in prediction of all-cuse mortality and age at death in European extinct cohorts of middle-aged men followef for 60 years. Eur. J. Prev. Cardiol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Keys, A.; Aravanis, C.; Blackburn, H.W.; Van Buchem, F.S.; Buzina, R.; Djordjević, B.D.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40-59 in seven countries. Acta Med. Scand. 1967, 460, 1–392. [Google Scholar] [CrossRef]
- Kromhout, D.; Menotti, A.; Blackburn, H. Prevention of coronary heart disease. In Diet, Lifestyle and Risk Factors in the Seven Countries Study; Kluwer Public: Norwell, MA, USA; Dordrecht, NL, USA, 2022; pp. 1–267. [Google Scholar]
- Rose, G.; Blackburn, H. Cardiovascular Survey Methods; World Health Organization: Geneva, Switzerland, 1968; pp. 1–188. [Google Scholar]
- Hemsfield, S.B.; MacManus, C.; Smith, J.; Stevens, V.; Nixon, D.W. Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am. J. Clin. Nutr. 1982, 36, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.T.; Keys, A. Cholesterol in serum and lipoprotein fractions: Its measurement and stability. Clin. Chem. 1956, 2, 145–159. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases and Causes of Death, 8th Revision; World Health Organization: Geneva, Switzerland, 1965; ICD-8. [Google Scholar]
- Menotti, A.; Puddu, P.E. Heart Diseases of uncertain etiology: Epidemiological observations compared with clinical experience. Med. Res. Arch. 2022, 10, 9. [Google Scholar]
- Puddu, P.E.; Menotti, A. Heart disease of uncertain etiology: A new definition of heart failure for epidemiological studies. J. Cardiovasc. Dev. Dis. 2023, 10, 132. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Lopez-Lopez, J.P.; Tole, M.C.; Cohen, D.D. Increasing muscular strength to improve cardiometabolic risk factors. Clin. Investig. Arterioscler. 2023, 35, 144–154. [Google Scholar]
- Puddu, P.E.; Menotti, A.; Tolonen, H.; Nedeljkovic, S.; Kafatos, A. Determinants of 40-year all-cause mortality in the European cohorts of the Seven Countries Study. Eur. J. Epidemiol. 2011, 26, 595–608. [Google Scholar] [CrossRef]
- Palatini, P.; Parati, G.; Virdis, A.; Reboldi, G.; Masi, S.; Mengozzi, A.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; et al. High heart rate amplifies the risk of cardiovascular mortality associated with elevated uric acid. Eur. J. Prev. Cardiol. 2023, 30, 2047–2048. [Google Scholar] [CrossRef]
- Delgado-Betancourt, V.; Chinda, K.; Mesirca, P.; Barrère, C.; Covinhes, A.; Gallot, L.; Vincent, A.; Bidaud, I.; Kumphune, S.; Nargeot, J.; et al. Heart rate reduction after genetic ablation of L-type Cav1.3 channels induces cardioprotection against ischemia-reperfusion injury. Front. Cardiovasc. Med. 2023, 10, 1134503. [Google Scholar] [CrossRef]
- Weinmayr, G.; Schulz, H.; Klenk, J.; Denkinger, M.; Duran-Tauleria, E.; Koenig, W.; Dallmeier, D.; Rothenbacher, D.; Böhm, B.; Geiger, H.; et al. Association of lung function with overall mortality is independent of inflammatory, cardiac, and functional biomarkers in older adults: The ActiFE-study. Sci. Rep. 2020, 10, 11862. [Google Scholar] [CrossRef]
- Costanzo, S.; Magnacca, S.; Bonaccio, M.; Di Castelnuovo, A.; Piraino, A.; Cerletti, C.; de Gaetano, G.; Donati, M.B.; Iacoviello, L. Reduced pulmonary function, low-grade inflammation and increased risk of total and cardiovascular mortality in a general adult population: Prospective results from the Moli-sani study. Respir. Med. 2021, 184, 106441. [Google Scholar] [CrossRef]
- Puddu, P.E.; Menotti, A.; Kromhout, D.; Kafatos, A.; Tolonen, H. Chronic bronchitis in the 50-year follow-up of the European cohorts of the Seven Countries Study: Prevalence, mortality and association with cardiovascular diseases. Respir. Med. 2021, 181, 106385. [Google Scholar] [CrossRef]
- Cillekens, B.; Huysmans, M.A.; Holtermann, A.; van Mechelen, W.; Straker, L.; Krause, N.; van der Beek, A.J.; Coenen, P. Physical activity at work may not be health enhancing. A systematic review with meta-analysis on the association between occupational physical activity and cardiovascular disease mortality covering 23 studies with 655892 participants. Scand. J. Work. Environ. Health 2022, 48, 86–98. [Google Scholar] [CrossRef]
- Löllgen, H.; Böckenhoff, A.; Knapp, G. Physical activity and all-cause mortality: An updated meta-analysis with different intensity categories. Int. J. Sports Med. 2009, 30, 313–324. [Google Scholar] [CrossRef]
- Bonekamp, N.E.; Visseren, F.L.J.; Ruigrok, Y.; Cramer, M.J.M.; de Borst, G.J.; May, A.M.; Koopal, C. Leisure-time and occupational physical activity and health outcomes in cardiovascular disease. Heart 2023, 109, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.D.; Lane, A.; Gabrie, K.P.; Sternfeld, B.; Jacobs, D.R.; Smith, P.; Gibbs, B.B. Associations between occupational physical activity and left ventricular structure and function over 25 years in CARDIA. Eur. J. Prev. Cardiol. 2024, 31, 425–433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).