A Review on Atrial Fibrillation (Computer Simulation and Clinical Perspectives)
Abstract
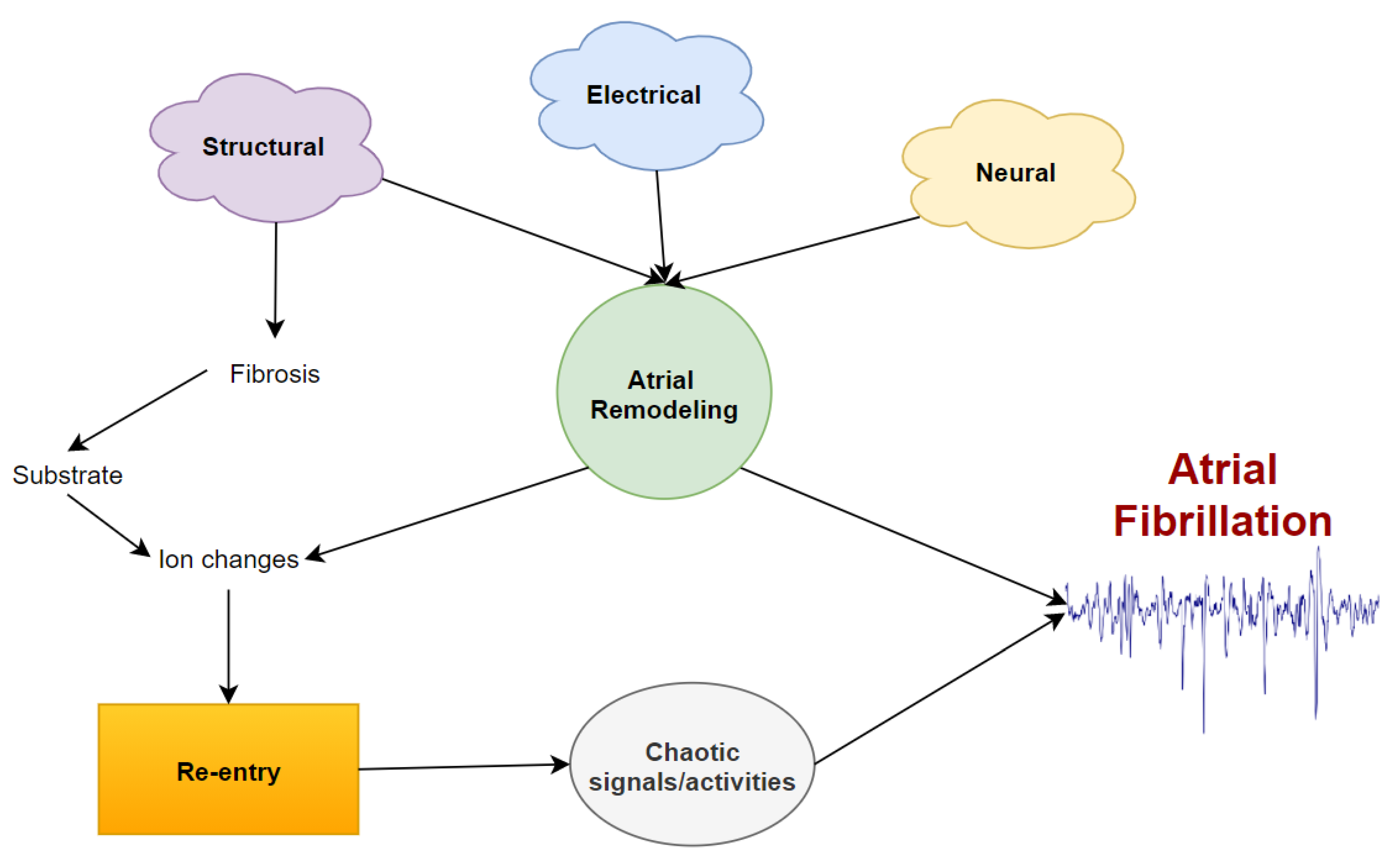
1. Introduction and Background
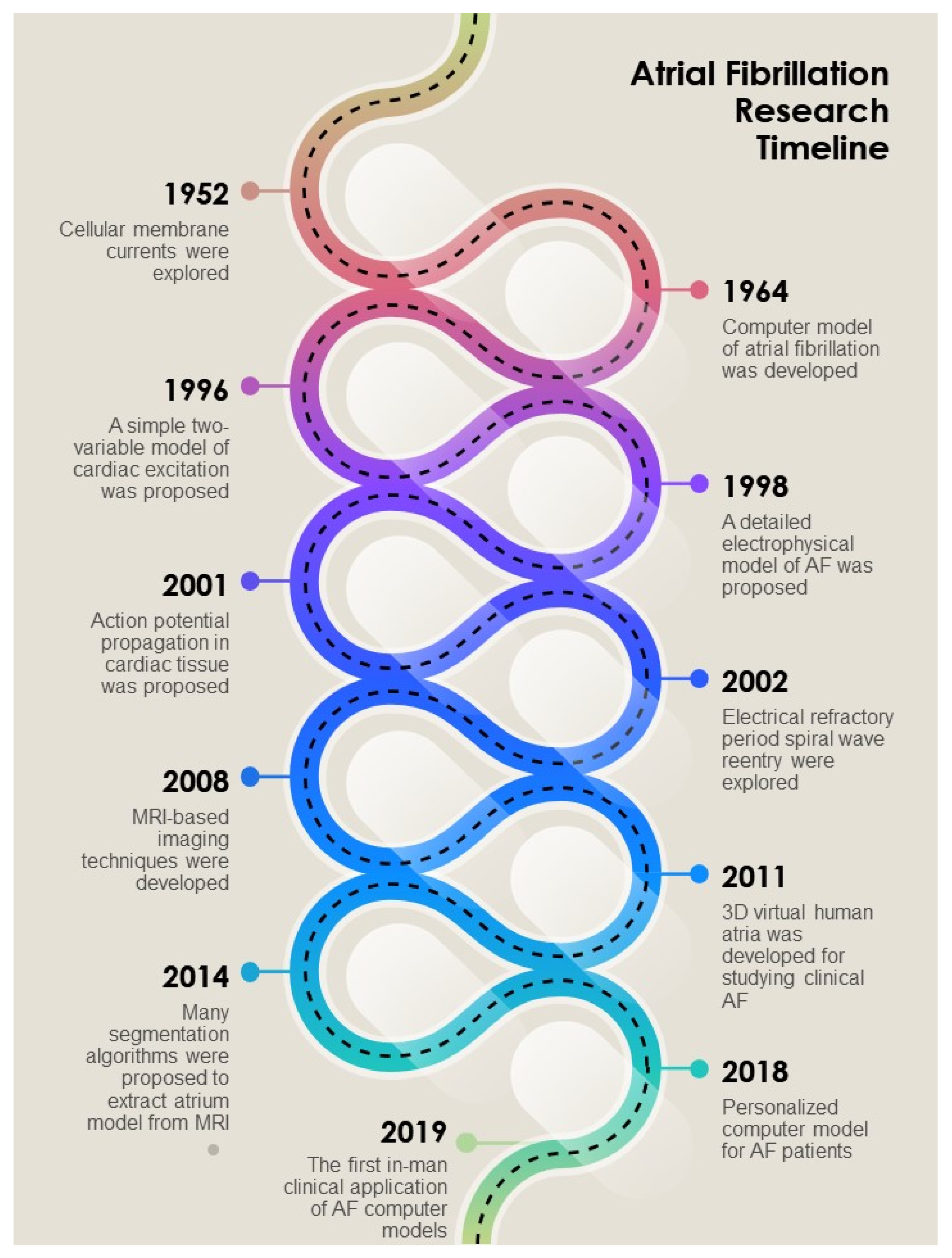
2. Simulation and Modeling
2.1. Cellular Modeling
2.2. Simulation in Atrium
2.3. Simulation of Ablation and Clinical Observation
3. Rotor/Trigger Identification
3.1. Dominant Frequency (DF) and Signal Processing (SP)
3.2. Wave Propagation (WP)
4. Biledical Image Processing
Image Segmentation
5. Post-Treatment Actions
5.1. Statistical Analysis
5.2. Cardiac Rhythm Control and Maintenance
6. Limitations
7. Conclusions and Future Scopes
Author Contributions
Funding
Conflicts of Interest
References
- Trayanova, N.A. Mathematical approaches to understanding and imaging atrial fibrillation: Significance for mechanisms and management. Circ. Res. 2014, 114, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.K.; Nishida, K.; Kato, T.; Nattel, S. Atrial fibrillation pathophysiology: Implications for management. Circulation 2011, 124, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Zaman, J.A.; Sauer, W.H.; Alhusseini, M.I.; Baykaner, T.; Borne, R.T.; Kowalewski, C.A.; Wang, P.J. Identification and characterization of sites where persistent atrial fibrillation is terminated by localized ablation. Circ. Arrhythmia Electrophysiol. 2018, 11, e005258. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef]
- Nygren, A.; Leon, L.J.; Giles, W.R. Simulations of the human atrial action potential. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2001, 359, 1111–1125. [Google Scholar] [CrossRef]
- Moe, G.K.; Rheinboldt, W.C.; Abildskov, J.A. A computer model of atrial fibrillation. Am. Heart J. 1964, 67, 200–220. [Google Scholar] [CrossRef]
- Du, D.; Du, Y. Detection of the propagating direction of electrical wavefront in atrial fibrillation. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2749–2752. [Google Scholar]
- Simitev, R.D.; Biktashev, V.N. Conditions for propagation and block of excitation in an asymptotic model of atrial tissue. Biophys. J. 2006, 90, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, T.; Abbott, G.W.; Christini, D.J. Effects of electrical and structural remodeling on atrial fibrillation maintenance: A simulation study. PLoS Comput. Biol. 2012, 8, e1002390. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, V.; Virag, N.; Kappenberger, L. Wavelength and vulnerability to atrial fibrillation: Insights from a computer model of human atria. EP Eur. 2005, 7, S83–S92. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chang, E.T.; Eatock, J.; Galla, T.; Clayton, R.H. Mechanisms of stochastic onset and termination of atrial fibrillation studied with a cellular automaton model. J. R. Soc. Interface 2017, 14, 20160968. [Google Scholar] [CrossRef]
- McDowell, K.S.; Vadakkumpadan, F.; Blake, R.; Blauer, J.; Plank, G.; MacLeod, R.S.; Trayanova, N.A. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys. J. 2013, 104, 2764–2773. [Google Scholar] [CrossRef]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Berenfeld, O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009, 6, 33–40. [Google Scholar] [CrossRef]
- Lazar, S.; Dixit, S.; Callans, D.J.; Lin, D.; Marchlinski, F.E.; Gerstenfeld, E.P. Effect of pulmonary vein isolation on the left-to-right atrial dominant frequency gradient in human atrial fibrillation. Heart Rhythm. 2006, 3, 889–895. [Google Scholar] [CrossRef]
- Fitzgerald, T.N.; Brooks, D.H.; Triedman, J.K. Identification of cardiac rhythm features by mathematical analysis of vector fields. IEEE Trans. Biomed. Eng. 2005, 52, 19–29. [Google Scholar] [CrossRef]
- Deedwania, P.C.; Lardizabal, J.A. Atrial fibrillation in heart failure: A comprehensive review. Am. J. Med. 2010, 123, 198–204. [Google Scholar] [CrossRef]
- Rodrigo, M.; Climent, A.M.; Liberos, A.; Fernandez-Aviles, F.; Berenfeld, O.; Atienza, F.; Guillem, M.S. Atrial sources identification by causality analysis during atrial fibrillation. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3783–3786. [Google Scholar]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Breithardt, G. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sanders, P. Long-term outcomes of catheter ablation of atrial fibrillation: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004549. [Google Scholar] [CrossRef] [PubMed]
- Baykaner, T.; Rogers, A.J.; Meckler, G.L.; Zaman, J.; Navara, R.; Rodrigo, M.; Clopton, P. Clinical implications of ablation of drivers for atrial fibrillation: A systematic review and meta-analysis. Circ. Arrhythmia Electrophysiol. 2018, 11, e006119. [Google Scholar] [CrossRef] [PubMed]
- Verma, A. The techniques for catheter ablation of paroxysmal and persistent atrial fibrillation: A systematic review. Curr. Opin. Cardiol. 2011, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Khairy, P.; Guerra, P.G.; Deyell, M.W.; Rivard, L.; Macle, L.; Dubuc, M. Efficacy and safety of cryoballoon ablation for atrial fibrillation: A systematic review of published studies. Heart Rhythm. 2011, 8, 1444–1451. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zghaib, T.; Zahid, S.; Ali, R.L.; Deng, D.; Franceschi, W.H.; Trayanova, N.A. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat. Biomed. Eng. 2019, 3, 870–879. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Bifulco, S.F.; Akoum, N.; Boyle, P.M. Translational applications of computational modelling for patients with cardiac arrhythmias. Heart 2021, 107, 456–461. [Google Scholar] [CrossRef]
- Corrado, C.; Avezzù, A.; Lee, A.W.; Mendoca Costa, C.; Roney, C.H.; Strocchi, M.; Niederer, S.A. Using cardiac ionic cell models to interpret clinical data. WIREs Mech. Dis. 2021, 13, e1508. [Google Scholar] [CrossRef]
- Heijman, J.; Sutanto, H.; Crijns, H.J.; Nattel, S.; Trayanova, N.A. Computational models of atrial fibrillation: Achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Harrild, D.M.; Henriquez, C.S. A computer model of normal conduction in the human atria. Circ. Res. 2000, 87, 25–36. [Google Scholar]
- Grandi, E.; Pandit, S.V.; Voigt, N.; Workman, A.J.; Dobrev, D.; Jalife, J.; Bers, D.M. Human atrial action potential and Ca2+ model: Sinus rhythm and chronic atrial fibrillation. Circ. Res. 2011, 109, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Krummen, D.E.; Bayer, J.D.; Ho, J.; Ho, G.; Smetak, M.R.; Clopton, P.; Narayan, S.M. Mechanisms of human atrial fibrillation initiation: Clinical and computational studies of repolarization restitution and activation latency. Circ. Arrhythmia Electrophysiol. 2012, 5, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Berenfeld, O.; Jalife, J. Mechanisms of atrial fibrillation: Rotors, ionic determinants, and excitation frequency. Heart Fail. Clin. 2016, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kalifa, J.; Tanaka, K.; Zaitsev, A.V.; Warren, M.; Vaidyanathan, R.; Auerbach, D.; Atienza, F. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation 2006, 113, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.P.; Kaur, K.; Hwang, E.; Ramirez, R.J.; Willis, B.C.; Filgueiras-Rama, D.; O’connell, R.P. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014, 129, 1472. [Google Scholar] [CrossRef]
- Addis, A.; Vanosi, G.; Manasse, E.; Mainetti, M.; Monaco, A.; Addis, F. An experimental sheep model used to develop an ablation procedure for chronic atrial fibrillation. Surg. Endosc. 2007, 21, 1626–1630. [Google Scholar] [CrossRef]
- Butters, T.D.; Aslanidi, O.V.; Zhao, J.; Smaill, B.; Zhang, H. A novel computational sheep atria model for the study of atrial fibrillation. Interface Focus 2013, 3, 20120067. [Google Scholar] [CrossRef]
- Doll, N.; Kornherr, P.; Aupperle, H.; Fabricius, A.M.; Kiaii, B.; Ullmann, C.; Mohr, F.W. Epicardial treatment of atrial fibrillation using cryoablation in an acute off-pump sheep model. Thorac. Cardiovasc. Surg. 2003, 51, 267–273. [Google Scholar] [CrossRef]
- Doll, N.; Suwalski, P.; Aupperle, H.; Walther, T.; Borger, M.A.; Schoon, H.A.; Mohr, F.W. Endocardial laser ablation for the treatment of atrial fibrillation in an acute sheep model. J. Card. Surg. 2008, 23, 198–203. [Google Scholar] [CrossRef]
- Everett, T.H.T.; Li, H.; Mangrum, J.M.; McRury, I.D.; Mitchell, M.A.; Redick, J.A.; Haines, D.E. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 2000, 102, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Sharifov, O.F.; Beloshapko, G.G.; Yushmanova, A.V.; Rosenshtraukh, L.V. Effects of a new class III antiarrhythmic drug nibentan in a canine model of vagally mediated atrial fibrillation. J. Cardiovasc. Pharmacol. 2000, 36, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Kusunose, K.; Qamruddin, S.; Rodriguez, L.L.; Mazgalev, T.N.; Griffin, B.P.; Van Wagoner, D.R.; Zhang, Y.; Popovic, Z.B. Left Atrial Size and Function in a Canine Model of Chronic Atrial Fibrillation and Heart Failure. PLoS ONE 2016, 11, e0147015. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, S.; Sun, Q.; Huang, C. Alterations in electrophysiology and tissue structure of the left atrial posterior wall in a canine model of atrial fibrillation caused by chronic atrial dilatation. Circ. J. 2007, 71, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Feng, J.; Gaspo, R.; Li, G.R.; Wang, Z.; Nattel, S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997, 81, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, R.L.; Clark, J.W.; Giles, W.R.; Robinson, K.; Clark, R.B.; Shibata, E.F.; Campbell, D.L. A mathematical model of electrophysiological activity in a bullfrog atrial cell. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H370–H389. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Noble, D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: Reconstruction of basic cellular mechanisms. Proc. R. Soc. Lond. B 1987, 230, 163–205. [Google Scholar]
- Bukowska, A.; Felgendreher, M.; Scholz, B.; Wolke, C.; Schulte, J.S.; Fehrmann, E.; Wardelmann, E.; Seidl, M.D.; Lendeckel, U.; Himmler, K.; et al. CREM-transgene mice: An animal model of atrial fibrillation and thrombogenesis. Thromb. Res. 2018, 163, 172–179. [Google Scholar] [CrossRef]
- Kneller, J.; Zou, R.; Vigmond, E.J.; Wang, Z.; Leon, L.J.; Nattel, S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ. Res. 2002, 90, e73–e87. [Google Scholar] [CrossRef]
- Correa de Sa, D.D.; Thompson, N.; Stinnett-Donnelly, J.; Znojkiewicz, P.; Habel, N.; Müller, J.G.; Spector, P.S. Electrogram fractionation: The relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ. Arrhythmia Electrophysiol. 2011, 4, 909–916. [Google Scholar] [CrossRef]
- Kuo, S.R.; Trayanova, N.A. Action potential morphology heterogeneity in the atrium and its effect on atrial reentry: A two-dimensional and quasi-three-dimensional study. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2006, 364, 1349–1366. [Google Scholar] [CrossRef] [PubMed]
- Maleckar, M.M.; Greenstein, J.L.; Giles, W.R.; Trayanova, N.A. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1398–H1410. [Google Scholar] [CrossRef] [PubMed]
- Aslanidi, O.V.; Colman, M.A.; Stott, J.; Dobrzynski, H.; Boyett, M.R.; Holden, A.V.; Zhang, H. 3D virtual human atria: A computational platform for studying clinical atrial fibrillation. Prog. Biophys. Mol. Biol. 2011, 107, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Colman, M.A.; Aslanidi, O.V.; Kharche, S.; Boyett, M.R.; Garratt, C.; Hancox, J.C.; Zhang, H. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodeling: Insights from the three dimensional virtual human atria. J. Physiol. 2013, 591, 4249–4272. [Google Scholar] [CrossRef] [PubMed]
- Seemann, G.; Bustamante, P.C.; Ponto, S.; Wilhelms, M.; Scholz, E.P.; Dössel, O. Atrial fibrillation-based electrical remodeling in a computer model of the human atrium. Comput. Cardiol. 2010, 37, 417–420. [Google Scholar]
- Adeniran, I.; MacIver, D.H.; Garratt, C.J.; Ye, J.; Hancox, J.C.; Zhang, H. Effects of Persistent Atrial Fibrillation-Induced Electrical Remodeling on Atrial Electro-Mechanics—Insights from a 3D Model of the Human Atria. PLoS ONE 2015, 10, e0142397. [Google Scholar] [CrossRef]
- Gong, Y.; Gao, Y.; Lu, Z.; Zheng, D.; Deng, D.; Xia, L. Preliminary simulation study of atrial fibrillation treatment procedure based on a detailed human atrial model. J. Clin. Trial. Cardiol. 2015, 2, 1–9. [Google Scholar]
- Santangeli, P.; Zado, E.S.; Hutchinson, M.D.; Riley, M.P.; Lin, D.; Frankel, D.S.; Marchlinski, F.E. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016, 13, 374–382. [Google Scholar] [CrossRef]
- Rappel, W.J.; Zaman, J.A.; Narayan, S.M. Mechanisms for the termination of atrial fibrillation by localized ablation: Computational and clinical studies. Circ. Arrhythmia Electrophysiol. 2015, 8, 1325–1333. [Google Scholar] [CrossRef]
- Hansen, B.J.; Zhao, J.; Csepe, T.A.; Jayne, L.A.; Li, N.; Moore, B.; Weiss, R. Human atrial fibrillation terminated by targeted ablation of localized reentrant drivers guided by dual-sided simultaneous epicardial and endocardial optical mapping. Heart Rhythm. 2014, 11, 2129–2130. [Google Scholar] [CrossRef]
- Dang, L.; Virag, N.; Ihara, Z.; Jacquemet, V.; Vesin, J.M.; Schlaepfer, J.; Kappenberger, L. Evaluation of ablation patterns using a biophysical model of atrial fibrillation. Ann. Biomed. Eng. 2005, 33, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kharche, S.R.; Hansen, B.J.; Csepe, T.A.; Wang, Y.; Stiles, M.K.; Fedorov, V.V. Optimization of catheter ablation of atrial fibrillation: Insights gained from clinically-derived computer models. Int. J. Mol. Sci. 2015, 16, 10834–10854. [Google Scholar] [CrossRef] [PubMed]
- Reumann, M.; Bohnert, J.; Seemann, G.; Osswald, B.; Dossel, O. Preventive ablation strategies in a biophysical model of atrial fibrillation based on realistic anatomical data. IEEE Trans. Biomed. Eng. 2008, 55, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, V. Lessons from computer simulations of ablation of atrial fibrillation. J. Physiol. 2016, 594, 2417–2430. [Google Scholar] [CrossRef]
- Zahid, S.; Whyte, K.N.; Schwarz, E.L.; Blake III, R.C.; Boyle, P.M.; Chrispin, J.; Calkins, H. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm. 2016, 13, 1687–1698. [Google Scholar] [CrossRef]
- Boyle, P.M.; Zahid, S.; Trayanova, N.A. Using personalized computer models to custom-tailor ablation procedures for atrial fibrillation patients: Are we there yet? Expert Rev. Cardiovasc. Ther. 2017, 15, 339–341. [Google Scholar] [CrossRef]
- Bayer, J.D.; Roney, C.H.; Pashaei, A.; Jaïs, P.; Vigmond, E.J. Novel radiofrequency ablation strategies for terminating atrial fibrillation in the left atrium: A simulation study. Front. Physiol. 2016, 7, 108. [Google Scholar] [CrossRef]
- Bajorek, B.V.; Krass, I.; Ogle, S.J.; Duguid, M.J.; Shenfield, G.M. Optimizing the Use of Antithrombotic Therapy for Atrial Fibrillation in Older People: A Pharmacist-Led Multidisciplinary Intervention. J. Am. Geriatr. Soc. 2005, 53, 1912–1920. [Google Scholar] [CrossRef]
- Kautzner, J.; Neuzil, P.; Lambert, H.; Peichl, P.; Petru, J.; Cihak, R.; Kuck, K.H. EFFICAS II: Optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. EP Eur. 2015, 17, 1229–1235. [Google Scholar] [CrossRef]
- Quintanilla, J.G.; Perez-Villacastin, J.; Perez-Castellano, N.; Pandit, S.V.; Berenfeld, O.; Jalife, J.; Filgueiras-Rama, D. Mechanistic approaches to detect, target, and ablate the drivers of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e002481. [Google Scholar] [CrossRef]
- Jadidi, A.S.; Lehrmann, H.; Keyl, C.; Sorrel, J.; Markstein, V.; Minners, J.; Potocnik, C. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ. Arrhythmia Electrophysiol. 2016, 9, e002962. [Google Scholar] [CrossRef] [PubMed]
- Bonso, A.; Fantinel, M.; Scalchi, G.; Ferrara, S.; Indiani, S.; Calore, F.; Licciardello, C. Left atrial model reconstruction in atrial fibrillation ablation: Reliability of new mapping and complex impedance systems. EP Eur. 2016, 19, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Manani, K.A.; Peters, N.S. Simple model for identifying critical regions in atrial fibrillation. Phys. Rev. Lett. 2015, 114, 028104. [Google Scholar] [CrossRef]
- Ciaccio, E.J.; Biviano, A.B.; Wan, E.Y.; Peters, N.S.; Garan, H. Development of an automaton model of rotational activity driving atrial fibrillation. Comput. Biol. Med. 2017, 83, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Chugh, A.; Good, E.; Crawford, T.; Myles, J.; Veerareddy, S.; Jongnarangsin, K. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010, 7, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Javad, H.; Saeed, G.; Redfearn, D.P. Regional Dominant Frequency: A New Tool for Wave Break Identification During Atrial Fibrillation. Front. Cardiovasc. Med. 2018, 5, 79. [Google Scholar]
- Lin, Y.J.; Tsao, H.M.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chang, C.J.; Wu, T.J. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: Insights from high-density frequency and fractionation mapping. Heart Rhythm. 2010, 7, 1255–1262. [Google Scholar] [CrossRef]
- Slocum, J.; Sahakian, A.; Swiryn, S. Diagnosis of atrial fibrillation from surface electrocardiograms based on computer-detected atrial activity. J. Electrocardiol. 1992, 25, 1–8. [Google Scholar] [CrossRef]
- Jalife, J. Rotors and spiral waves in atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003, 14, 776–780. [Google Scholar] [CrossRef]
- Zhao, J.; Trew, M.L.; Legrice, I.J.; Smaill, B.H.; Pullan, A.J. A Tissue-Specific Model of Reentry in the Right Atrial Appendage. J. Cardiovasc. Electrophysiol. 2009, 20, 675–684. [Google Scholar] [CrossRef]
- Guillem, M.S.; Climent, A.M.; Rodrigo, M.; Hernandez-Romero, I.; Liberos, A.; Fernandez-Aviles, F.; Atienza, F. Noninvasive identification of atrial fibrillation drivers: Simulation and patient data evaluation. In Proceedings of the Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 1–14 September 2016; pp. 121–124. [Google Scholar]
- Tobon, C.; Rodriguez, J.F.; Ferrero, J.M., Jr.; Hornero, F.; Saiz, J. Dominant frequency and organization index maps in a realistic three-dimensional computational model of atrial fibrillation. Europace 2012, 14, v25–v32. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.M.; Schilling, C.; Seemann, G.; Luik, A.; Schmitt, C.; Lorenz, C.; Dossel, O. Wave-direction and conduction-velocity analysis from intracardiac electrograms: A single-shot technique. IEEE Trans. Biomed. Eng. 2010, 57, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Shariat, M.H.; Gazor, S.; Redfearn, D. Cardiac conduction velocity estimation from sequential mapping assuming known Gaussian distribution for activation time estimation error. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 505–508. [Google Scholar]
- Cantwell, C.D.; Roney, C.H.; Ng, F.S.; Siggers, J.H.; Sherwin, S.J.; Peters, N.S. Techniques for automated local activation time annotation and conduction velocity estimation in cardiac mapping. Comput. Biol. Med. 2015, 65, 229–242. [Google Scholar] [CrossRef]
- Markl, M.; Fluckiger, J.; Lee, D.C.; Ng, J.; Goldberger, J.J. Velocity quantification by electrocardiography-gated phase contrast magnetic resonance imaging in patients with cardiac arrhythmia: A simulation study based on real time transesophageal echocardiography data in atrial fibrillation. J. Comput. Assist. Tomogr. 2015, 39, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.M.; Hakim, J.B.; Zahid, S.; Franceschi, W.H.; Murphy, M.J.; Vigmond, E.J.; Trayanova, N.A. Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation. Front. Physiol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Boyle, P.M.; Hakim, J.B.; Zahid, S.; Franceschi, W.H.; Murphy, M.J.; Prakosa, A.; Chrispin, J. The Fibrotic Substrate in Persistent Atrial Fibrillation Patients: Comparison Between Predictions from Computational Modeling and Measurements from Focal Impulse and Rotor Mapping. Front. Physiol. 2018, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.R.; Jarrett, T.R.; Burgon, N.S.; Kholmovski, E.G.; Akoum, N.W.; Hu, N.; Ranjan, R. Comparison of left atrial area marked ablated in electroanatomical maps with scar in MRI. J. Cardiovasc. Electrophysiol. 2014, 25, 457–463. [Google Scholar] [CrossRef]
- Crozier, A.; Augustin, C.M.; Neic, A.; Prassl, A.J.; Holler, M.; Fastl, T.E.; Niederer, S.A. Image-based personalization of cardiac anatomy for coupled electromechanical modeling. Ann. Biomed. Eng. 2016, 44, 58–70. [Google Scholar] [CrossRef]
- Larburu, N.; Lopetegi, T.; Romero, I. Comparative study of algorithms for atrial fibrillation detection. In Proceedings of the Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 265–268. [Google Scholar]
- McGillivray, M.F.; Cheng, W.; Peters, N.S.; Christensen, K. Machine learning methods for locating re-entrant drivers from electrograms in a model of atrial fibrillation. R. Soc. Open Sci. 2018, 5, 172434. [Google Scholar] [CrossRef]
- Chorro, F.J.; Mainar, L.; Sanchis, J.; Canoves, J.; Porres, J.C.; Guerrero, J.; Millet, J.; Llavador, E.; Such, L.M.; Egea, S.; et al. The activation patterns during atrial fibrillation in an experimental model. Rev. Esp. Cardiol. 1999, 52, 327–338. [Google Scholar] [CrossRef]
- Ugarte, J.P.; Orozco-Duque, A.; Tobon, C.; Kremen, V.; Novak, D.; Saiz, J.; Oesterlein, T.; Schmitt, C.; Luik, A.; Bustamante, J. Dynamic approximate entropy electroanatomic maps detect rotors in a simulated atrial fibrillation model. PLoS ONE 2014, 9, e114577. [Google Scholar] [CrossRef] [PubMed]
- Lahdenoja, O.; Hurnanen, T.; Iftikhar, Z.; Nieminen, S.; Knuutila, T.; Saraste, A.; Koivisto, T. Atrial fibrillation detection via accelerometer and gyroscope of a smartphone. IEEE J. Biomed. Health Inform. 2018, 22, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Brasier, N.; Raichle, C.J.; Dörr, M.; Becke, A.; Nohturfft, V.; Weber, S.; Eckstein, J. Detection of atrial fibrillation with a smartphone camera: First prospective, international, two-centre, clinical validation study (DETECT AF PRO). Ep Eur. 2019, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Lin, Y.T.; Galla, T.; Clayton, R.H.; Eatock, J. A Stochastic Individual-Based Model of the Progression of Atrial Fibrillation in Individuals and Populations. PLoS ONE 2016, 11, e0152349. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.M.; Yu, W.C.; Cheng, H.C.; Wu, M.H.; Tai, C.T.; Lin, W.S.; Chen, S.A. Pulmonary vein dilation in patients with atrial fibrillation: Detection by magnetic resonance imaging. J. Cardiovasc. Electrophysiol. 2001, 12, 809–813. [Google Scholar] [CrossRef]
- McGann, C.J.; Kholmovski, E.G.; Oakes, R.S.; Blauer, J.J.; Daccarett, M.; Segerson, N.; DiBella, E.V. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2008, 52, 1263–1271. [Google Scholar] [CrossRef]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; MacLeod, R.S. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef]
- Sohns, C.; Lemes, C.; Metzner, A.; Fink, T.; Chmelevsky, M.; Maurer, T.; Mathew, S. First-in-man analysis of the relationship between electrical rotors from noninvasive panoramic mapping and atrial fibrosis from magnetic resonance imaging in patients with persistent atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2017, 10, e004419. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Deneke, T. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef]
- Cochet, H.; Dubois, R.; Yamashita, S.; Al Jefairi, N.; Berte, B.; Sellal, J.M.; Denis, A. Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial fibrillation. JACC Clin. Electrophysiol. 2018, 4, 17–29. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Alves, P.; Inacio, N.; Marto, J.P.; Viana-Baptista, M.; Pinho-e-Melo, T.; Almeida, A.G. Patients With Undetermined Stroke Have Increased Atrial Fibrosis: A Cardiac Magnetic Resonance Imaging Study. Stroke 2018, 49, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Gal, P.; Marrouche, N.F. Magnetic resonance imaging of atrial fibrosis: Redefining atrial fibrillation to a syndrome. Eur. Heart J. 2015, 38, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cochet, H.; Mouries, A.; Nivet, H.; Sacher, F.; Derval, N.; Denis, A.; Laurent, F. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol. 2015, 26, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Geremia, E.; Clatz, O.; Menze, B.H.; Konukoglu, E.; Criminisi, A.; Ayache, N. Spatial decision forests for MS lesion segmentation in multi-channel magnetic resonance images. NeuroImage 2011, 57, 378–390. [Google Scholar] [CrossRef]
- Kandwal, R.; Kumar, A.; Bhargava, S. Existing Image Segmentation Techniques. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2014, 4, 166–170. [Google Scholar]
- Zaitoun, N.M.; Aqel, M.J. Survey on image segmentation techniques. Procedia Comput. Sci. 2015, 65, 797–806. [Google Scholar] [CrossRef]
- Sharma, P.; Suji, J. A review on image segmentation with its clustering techniques. Int. J. Signal Process. Image Process. Pattern Recognit. 2016, 9, 209–218. [Google Scholar] [CrossRef]
- Erdt, M.; Steger, S.; Sakas, G. Regmentation: A new view of image segmentation and registration. J. Radiat. Oncol. Inform. 2017, 4, 1–23. [Google Scholar]
- Camara, O.; Mansi, T.; Pop, M.; Rhode, K.; Sermesant, M.; Young, A. Statistical Atlases and Computational Models of the Heart-Imaging and Modeling Challenges. Stat. Atlases Comput. Model. Heart 2014, 8896, 296. [Google Scholar]
- Fastl, T.E.; Tobon-Gomez, C.; Crozier, A.; Whitaker, J.; Rajani, R.; McCarthy, K.P.; Bishop, M.J. Personalized computational modeling of left atrial geometry and transmural myofiber architecture. Med. Image Anal. 2018, 47, 180–190. [Google Scholar] [CrossRef]
- Fastl, T.E.; Tobon-Gomez, C.; Crozier, W.A.; Whitaker, J.; Rajani, R.; McCarthy, K.P.; Bishop, M.J. Personalized modeling pipeline for left atrial electromechanics. In Proceedings of the Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 225–228. [Google Scholar]
- Taneja, A.; Ranjan, P.; Ujjlayan, A. A performance study of image segmentation techniques. In Proceedings of the 2015 4th International Conference on Reliability, Infocom Technologies and Optimization (ICRITO) (Trends and Future Directions), Noida, India, 2–4 September 2015; pp. 1–6. [Google Scholar]
- Sohns, C.; Metzner, A.; Chmelevsky, M.; Kuck, K.H. A new algorithm to visualize the individual relationship between electrical rotors from non-invasive panoramic mapping and atrial fibrosis to guide ablation of persistent atrial fibrillation. Clin. Res. Cardiol. 2018, 107, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Maryam, H.; Mustapha, A.; Younes, J. A multilevel thresholding method for image segmentation based on multiobjective particle swarm optimization. In Proceedings of the 2017 International Conference on Wireless Technologies, Embedded and Intelligent Systems (WITS), Fez, Morocco, 19–20 April 2017; pp. 1–6. [Google Scholar]
- Duraisamy, S.P.; Kayalvizhi, R. A new multilevel thresholding method using swarm intelligence algorithm for image segmentation. J. Intell. Learn. Syst. Appl. 2010, 2, 126. [Google Scholar] [CrossRef]
- Datta, N.S.; Dutta, H.S.; Majumder, K.; Chatterjee, S.; Wasim, N.A. A Survey on the Application of Multi-Objective Optimization Methods in Image Segmentation. In Multi-Objective Optimization; Springer: Singapore, 2018; pp. 269–278. [Google Scholar]
- Chen, L.C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected crfs. IEEE Trans. Pattern Anal. Mach. Intell. 2018, 40, 834–848. [Google Scholar] [CrossRef]
- Rahkar Farshi, T.; Demirci, R.; Feizi-Derakhshi, M.R. Image Clustering with Optimization Algorithms and Color Space. Entropy 2018, 20, 296. [Google Scholar] [CrossRef] [PubMed]
- Nakib, A.; Oulhadj, H.; Siarry, P. Non-supervised image segmentation based on multiobjective optimization. Pattern Recognit. Lett. 2008, 29, 161–172. [Google Scholar] [CrossRef]
- Piccini, J.P.; Fauchier, L. Rhythm control in atrial fibrillation. Lancet 2016, 388, 829–840. [Google Scholar] [CrossRef]
- Friberg, J.; Buch, P.; Scharling, H.; Gadsbøll, N.; Jensen, G.B. Rising rates of hospital admissions for atrial fibrillation. Epidemiology 2003, 14, 666–672. [Google Scholar] [CrossRef]
- Deshmukh, A.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-Hospital Complications Associated With Catheter Ablation of Atrial Fibrillation in the United States Between 2000 and 2010. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef]
- Tayal, A.H.; Tian, M.; Kelly, K.M.; Jones, S.C.; Wright, D.G.; Singh, D.; Gupta, R. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008, 71, 1696–1701. [Google Scholar] [CrossRef]
- Ng, J.; Gordon, D.; Passman, R.S.; Knight, B.P.; Arora, R.; Goldberger, J.J. Electrogram morphology recurrence patterns during atrial fibrillation. Heart Rhythm. 2014, 11, 2027–2034. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Albenque, J.P. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, F.; Zhang, Z.; Qin, Z.; Dudley, S.C.; Wang, Y.; Dabhadkar, K.; Heist, E. Impact of treatment crossovers on clinical outcomes in the rate and rhythm control strategies for atrial fibrillation: Insights from the affirm (atrial fibrillation follow-up investigation of rhythm management) trial. J. Am. Coll. Cardiol. 2017, 69, 476. [Google Scholar] [CrossRef]
- Rajeswaran, J.; Blackstone, E.H.; Ehrlinger, J.; Li, L.; Ishwaran, H.; Parides, M.K. Probability of atrial fibrillation after ablation: Using a parametric nonlinear temporal decomposition mixed effects model. Stat. Methods Med. Res. 2018, 27, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Ittu, R.; Abrahamowicz, M.; Jackevicius, C.A.; Essebag, V.; Eisenberg, M.J.; Wynant, W.; Pilote, L. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch. Intern. Med. 2012, 172, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Filgueiras-Rama, D.; Jalife, J. Mechanisms and Drug Development in Atrial Fibrillation. Pharmacol. Rev. 2018, 70, 505–525. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Smith, R.L. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef]
- Lafuente-Lafuente, C.; Valembois, L.; Bergmann, J.F.; Belmin, J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Abraham, J.M.; Saliba, W.I.; Vekstein, C.; Lawrence, D.; Bhargava, M.; Bassiouny, M.; Poe, S. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ. Arrhythmia Electrophysiol. 2015, 8, 772–776. [Google Scholar] [CrossRef]
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Trivedi, C.; Lakkireddy, D.; Casella, M. Ablation vs. amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef]
- Frommeyer, G.; Schmidt, M.; Clauss, C.; Kaese, S.; Stypmann, J.; Pott, C.; Milberg, P. Further insights into the underlying electrophysiological mechanisms for reduction of atrial fibrillation by ranolazine in an experimental model of chronic heart failure. Eur. J. Heart Fail. 2012, 14, 1322–1331. [Google Scholar] [CrossRef]
- Milberg, P.; Frommeyer, G.; Ghezelbash, S.; Rajamani, S.; Osada, N.; Razvan, R.; Belardinelli, L.; Breithardt, G.; Eckardt, L. Sodium channel block by ranolazine in an experimental model of stretch-related atrial fibrillation: Prolongation of interatrial conduction time and increase in post-repolarization refractoriness. Europace 2013, 15, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Lugenbiel, P.; Wenz, F.; Syren, P.; Geschwill, P.; Govorov, K.; Seyler, C.; Bruehl, C. TREK-1 (K2p2.1) K+ channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res. Cardiol. 2017, 112, 8. [Google Scholar] [CrossRef] [PubMed]
- Inada, S.; Shibata, M.D.; Ashihara, M.D.; Ikeda, M.D.; Mark, R. Simulation of ventricular rate control during atrial fibrillation using ionic channel blockers. J. Arrhythmia 2017, 33, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.W.; Rhode, K.S.; O’Neill, M.D.; Rinaldi, C.A.; Gill, J.; Razavi, R.; Doessel, O. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. J. Electrocardiol. 2014, 47, 324–328. [Google Scholar] [CrossRef]
- Shah, R.; Al-Darzi, W.; Greenbaum, A.; Eng, M.; Wang, D.D.; Greenberg, J.; Singh, G. Safety of rhythm control and cardioversion for patients with recurrent symptomatic atrial fibrillation in the absence of anticoagulation following percutaneous left atrial appendage occlusion. J. Am. Coll. Cardiol. 2018, 71, A293. [Google Scholar] [CrossRef]
- Ouyang, Z.L.; Sun, L.P.; Chi, H.; Xia, L.; Gong, Y.L.; Fan, Y.B. Potential Maintenance Mechanism in Atrial Fibrillation Patients Subject to Different Treatment Procedures: A Preliminary Study Based on a Human Atrial Model. Curr. Med. Sci. 2018, 38, 422–426. [Google Scholar] [CrossRef]
- Hamada, R.; Muto, S. Simple risk model and score for predicting of incident atrial fibrillation in Japanese. J. Cardiol. 2019, 73, 65–72. [Google Scholar] [CrossRef]
- Sears, S.F.; Anthony, S.; Harrell, R.; Tripp, C.; Bowman, J.; Khan, S.; Naniwadekar, A. Managing atrial fibrillation: The intersection of cardiology, health psychology, and the patient experience. Health Psychol. 2021. [Google Scholar] [CrossRef]


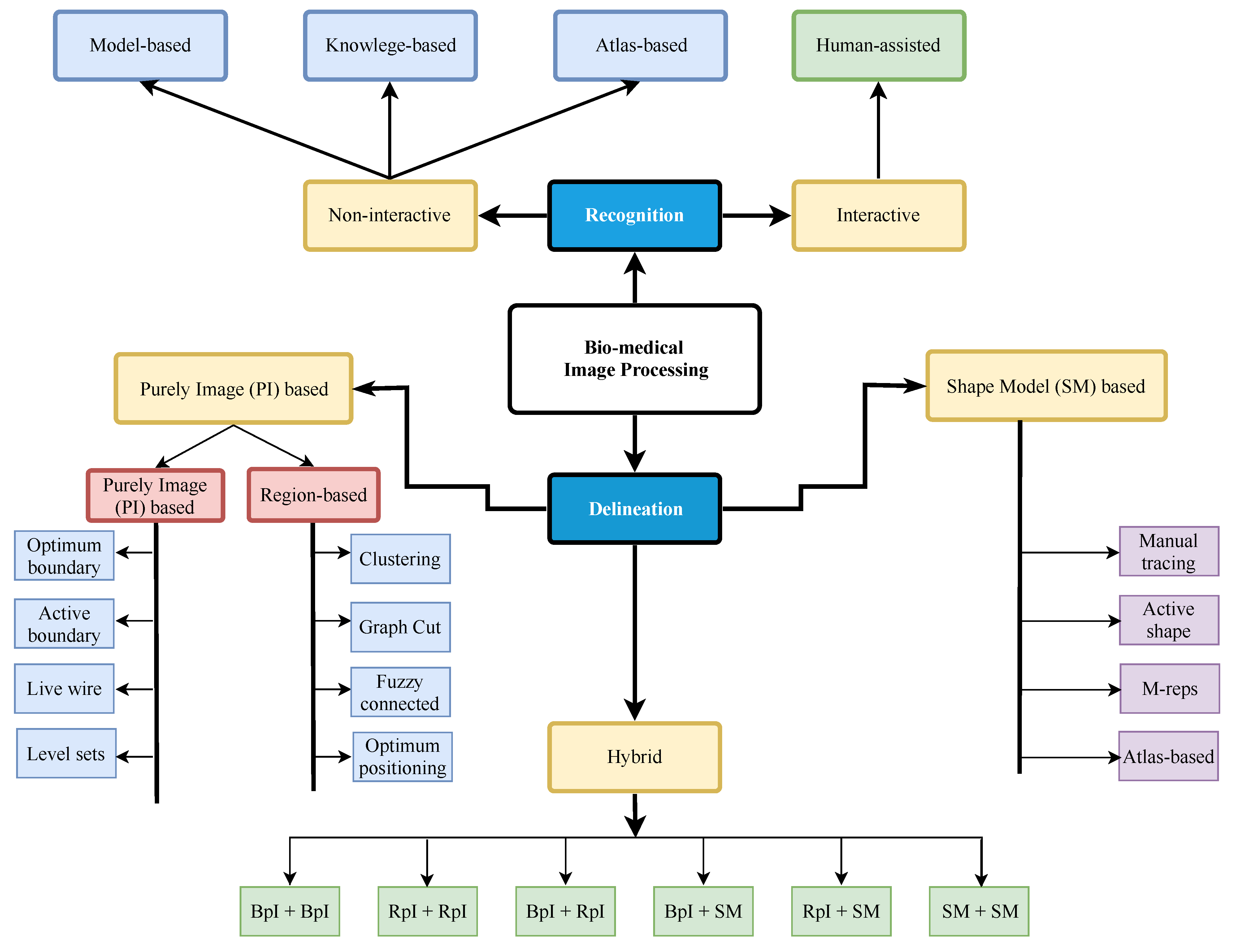
| Purely Image (PI) Based Image Segmentation | |
|---|---|
| Merits | Demerits |
| It works well when image information is good. | It does not do well if image information is bad/absent. |
| It can determine degree of match of model to image. | It cannot perform recognition well. |
| It does not require best match information | It generally lacks object shape and information. |
| Shape Model (SM)-Based Image Segmentation | |
| Merits | Demerits |
| Model might work well even if the image info is bad. | Accuracy might suffer even with good image info. |
| It helps with image recognition | It generally requires best match info. |
| Good model has object shape and geographic info | It might not be suitable for object delineation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, M.A.U.; Du, D. A Review on Atrial Fibrillation (Computer Simulation and Clinical Perspectives). Hearts 2022, 3, 20-37. https://doi.org/10.3390/hearts3010005
Zaman MAU, Du D. A Review on Atrial Fibrillation (Computer Simulation and Clinical Perspectives). Hearts. 2022; 3(1):20-37. https://doi.org/10.3390/hearts3010005
Chicago/Turabian StyleZaman, Muhammad Adib Uz, and Dongping Du. 2022. "A Review on Atrial Fibrillation (Computer Simulation and Clinical Perspectives)" Hearts 3, no. 1: 20-37. https://doi.org/10.3390/hearts3010005
APA StyleZaman, M. A. U., & Du, D. (2022). A Review on Atrial Fibrillation (Computer Simulation and Clinical Perspectives). Hearts, 3(1), 20-37. https://doi.org/10.3390/hearts3010005






