Technical Quality of Contemporary Endoscopic Sinus Surgery: An Assessment by Study of Anatomical Features Needing Attention at Revision Surgery
Abstract
1. Introduction
2. Materials and Methods
- Residual uncinate process (Figure 1);
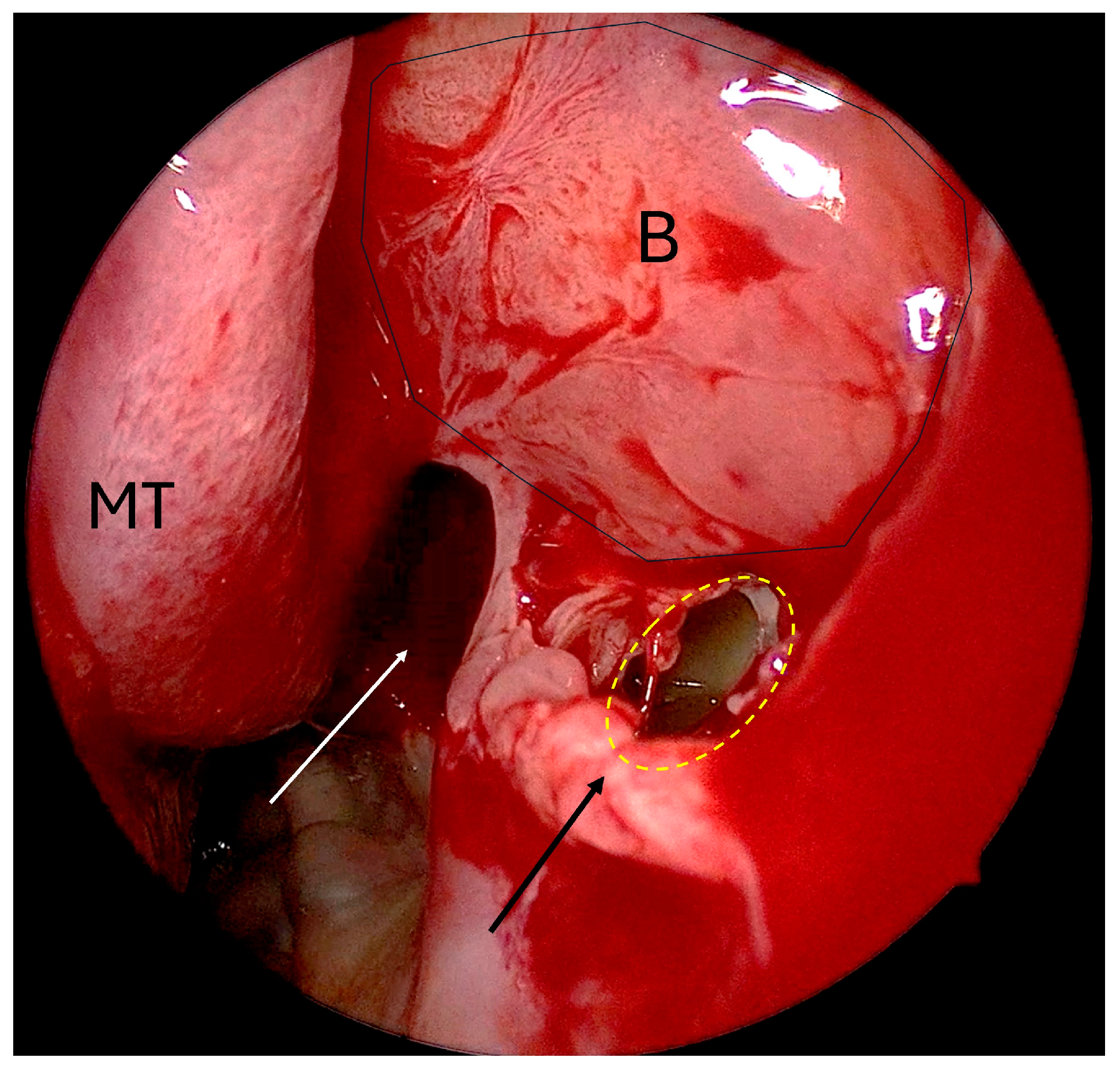
- Non-incorporated natural ostium/posteriorly placed maxillary antrostomy (Figure 2);
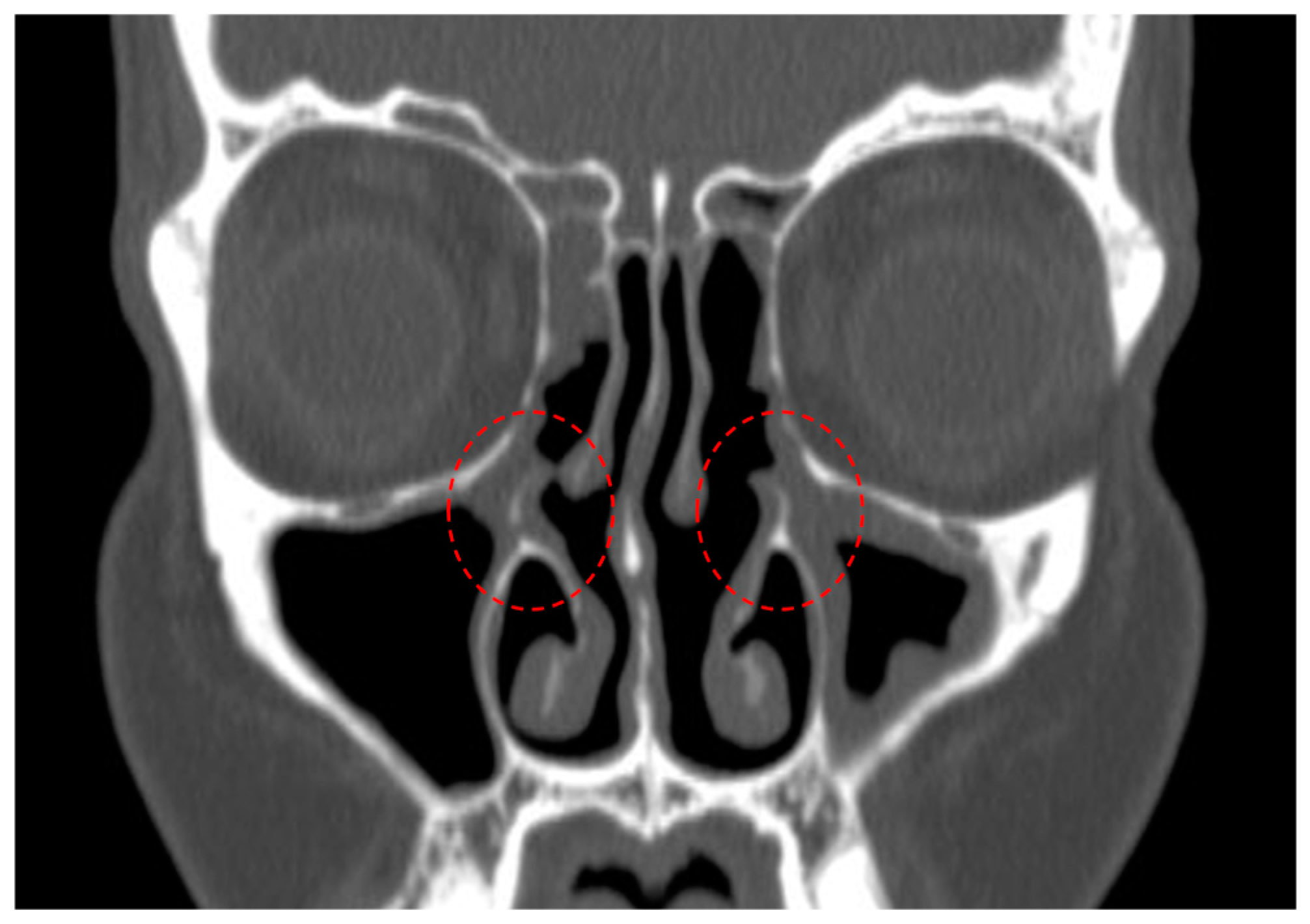
- Residual bony septae in anterior ethmoids, which were defined as >3 bony septae measuring 3 mm along lamina papyracea/skull base/middle turbinate on preoperative sinus CT (Figure 3) [residual agger nasi cells were excluded from this category and considered as residual frontoethmoidal cells];
- Residual bony septae in posterior ethmoids, which were defined as >3 bony septae measuring 3 mm along lamina papyracea/skull base/middle turbinate on preoperative sinus CT (Figure 3);
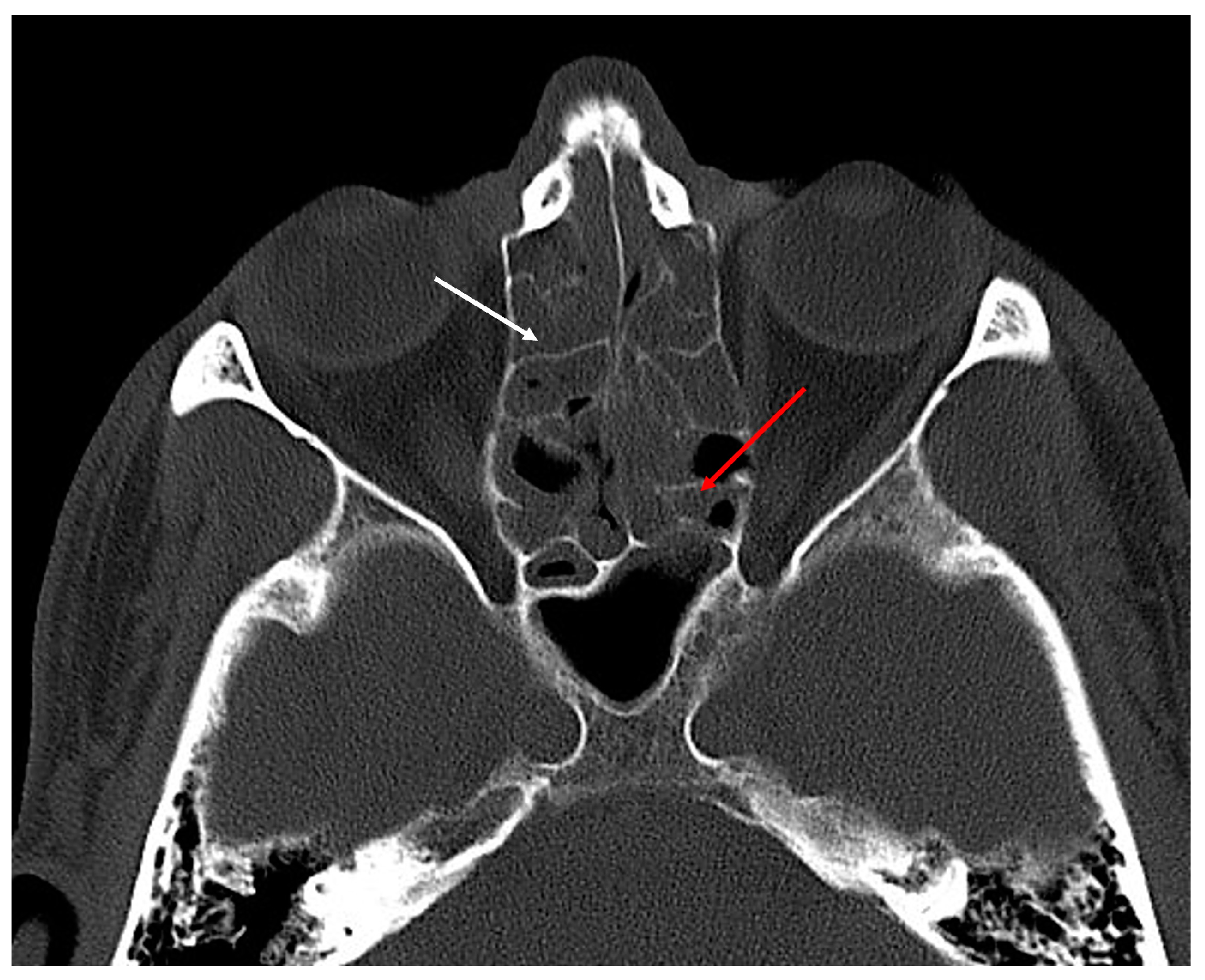
- Presence of residual frontoethmoidal cells (anterior or posterior group) in the frontal sinus outflow tract (Figure 4).
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESS | Endoscopic sinus surgery |
| CRS | Chronic rhinosinusitis |
| CRSwNP | Chronic rhinosinusitis with nasal polyposis |
| CRSsNP | Chronic rhinosinusitis without nasal polyposis |
| IRB | Institutional review board |
| CT | Computed tomography |
| SNOT-22 | 22-item sinonasal outcome test |
| HPE | Histopathology examination |
References
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis 2021; International Forum of Allergy and Rhinology; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021; Volume 11, pp. 213–739. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef] [PubMed]
- Snidvongs, K.; Chin, D.; Sacks, R.; Earls, P.; Harvey, R.J. Eosinophilic rhinosinusitis is not a disease of ostiomeatal occlusion. Laryngoscope 2013, 123, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Slack, R.; Bates, G. Functional Endoscopic Sinus Surgery. Am. Fam. Physician 1998, 58, 707–718. [Google Scholar] [PubMed]
- Snidvongs, K.; Pratt, E.; Chin, D.; Sacks, R.; Earls, P.; Harvey, R.J. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2012, 2, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Snidvongs, K.; Kalish, L.; Sacks, R.; Sivasubramaniam, R.; Cope, D.; Harvey, R.J. Sinus surgery and delivery method influence the effectiveness of topical corticosteroids for chronic rhinosinusitis: Systematic review and meta-analysis. Am. J. Rhinol. Allergy 2013, 27, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.A.; Donzelli, J.J.; Chow, J.M. Failures of functional endoscopic sinus surgery and their surgical correction. Oper. Tech. Otolaryngol.—Head Neck Surg. 1996, 7, 297–304. [Google Scholar] [CrossRef]
- DeConde, A.S.; Mace, J.C.; Levy, J.M.; Rudmik, L.; Alt, J.A.; Smith, T.L. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 2017, 127, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.S.; Stivers, F.E.; Talbot, A.R. The missed ostium sequence and the surgical approach to revision functional endoscopic sinus surgery. Otolaryngol. Clin. N. Am. 1996, 29, 169–183. [Google Scholar] [CrossRef]
- Gore, M.R.; Ebert, C.S.; Zanation, A.M.; Senior, B.A. Beyond the “central sinus”: Radiographic findings in patients undergoing revision functional endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2013, 3, 139–146. [Google Scholar] [CrossRef]
- Mechor, B.; Javer, A.R. Revision endoscopic sinus surgery: The St. paul’s sinus centre experience. J. Otolaryngol.—Head Neck Surg. 2008, 37, 676–680. [Google Scholar] [CrossRef]
- Musy, P.Y.; Kountakis, S.E. Anatomic findings in patients undergoing revision endoscopic sinus surgery. Am. J. Otolaryngol.—Head Neck Med. Surg. 2004, 25, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Bewick, J.; Egro, F.M.; Masterson, L.; Javer, A.R.; Philpott, C.M. Anatomic Findings in Revision Endoscopic Sinus Surgery: Case Series and Review of Contributory Factors. Allergy Rhinol. 2016, 7, ar-2016. [Google Scholar] [CrossRef] [PubMed]
- Cekic, E. Host and Surgical Factors Affecting the Frequency and Duration of Revision Endoscopic Sinus Surgery. Cureus 2022, 15, e29209. [Google Scholar] [CrossRef] [PubMed]
- Baban, M.I.A.; Mirza, B.; Castelnuovo, P. Radiological and endoscopic findings in patients undergoing revision endoscopic sinus surgery. Surg. Radiol. Anat. 2020, 42, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Englhard, A.S.; Ledderose, G.J. Anatomical findings in patients with chronic rhinosinusitis without nasal polyps requiring revision surgery. Braz. J. Otorhinolaryngol. 2023, 89, 101287. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.A.; Soler, Z.M.; Koochakzadeh, S.; Desiato, V.M.; Yoo, F.; Nguyen, S.A.; Schlosser, R.J. Revision surgery rates in chronic rhinosinusitis with nasal polyps: Meta-analysis of risk factors. Int. Forum Allergy Rhinol. 2020, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Weitzel, E.K.; Lin, E.; Feldt, B.; Kriete, B.; McMains, K.C.; Thwin, M.; Wormald, P.J. Effect of head position and surgical dissection on sinus irrigant penetration in cadavers. Laryngoscope 2010, 120, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Kane, K.J. The early history and development of functional endoscopic sinus surgery. J. Laryngol. Otol. 2020, 134, 8–13. [Google Scholar] [CrossRef]
- Tajudeen, B.A.; Kennedy, D.W. Thirty years of endoscopic sinus surgery: What have we learned? World J. Otorhinolaryngol.—Head Neck Surg. 2017, 3, 115–121. [Google Scholar] [CrossRef]
- Kennedy, D.W. Technical innovations and the evolution of endoscopic sinus surgery. Ann. Otol. Rhinol. Laryngol. 2006, 196, 3–12. [Google Scholar] [CrossRef]
- Govindaraj, S.; Adappa, N.D.; Kennedy, D.W. Endoscopic sinus surgery: Evolution and technical innovations. J. Laryngol. Otol. 2010, 124, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cain, R.B.; Lal, D. Update on the management of chronic rhinosinusitis. Infect. Drug Resist. 2013, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.R.; Jafari, A.; DeConde, A.S. Revision rates and time to revision following endoscopic sinus surgery: A large database analysis. Laryngoscope 2018, 128, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Miglani, A.; Divekar, R.D.; Azar, A.; Rank, M.A.; Lal, D. Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int. Forum Allergy Rhinol. 2018, 8, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Slack, R.; Lund, V.; Brown, P.; Copley, L.; Browne, J. Long-term outcomes from the english national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope 2009, 119, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Lee, D.; Yip, J.; Jamal, A.; Lee, J.M. Impact of Septal Deviation on Recurrent Chronic Rhinosinusitis after Primary Surgery: A Matched Case-Control Study. Otolaryngol.—Head Neck Surg. 2019, 160, 922–927. [Google Scholar] [CrossRef]
- Denison, M.E.; Awad, K.; Gillen, J.R.; Nussbaum, M.S.; Collier, B.R. Issues and Strategies in Training Left-Handed Surgeons. Am. Surg. 2023, 89, 5107–5111. [Google Scholar] [CrossRef]

| Total patients | 69 |
| CRSwNP/CRSsNP | 40/29 |
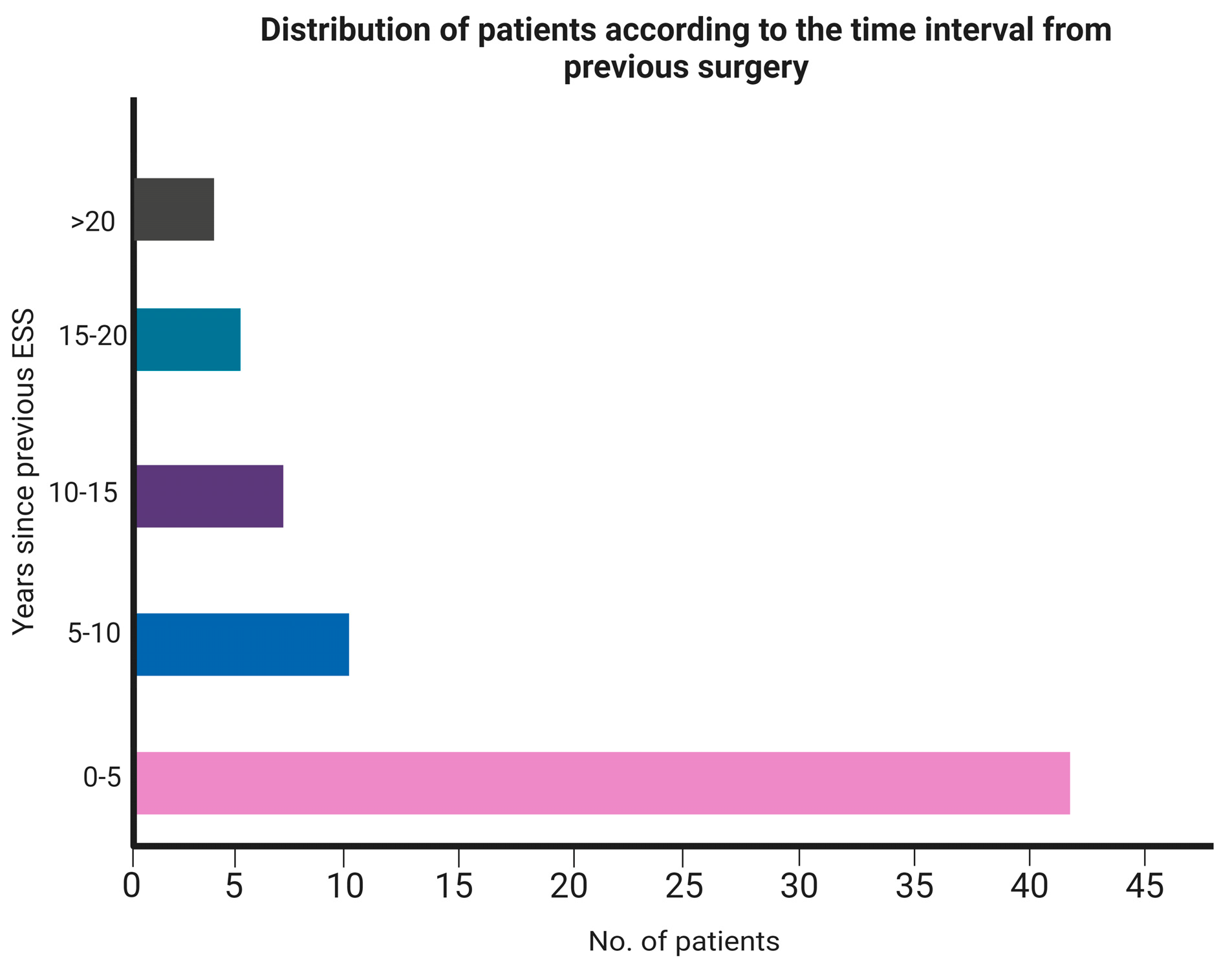
| Median time from last procedure (IQR) | 3.5 yrs. (8.5) |
| Range | 6 months-26 yrs. |
| • <5 years | 42 |
| • 5–10 years | 10 |
| • 10–15 years | 8 |
| • 15–20 years | 5 |
| • >20 years | 4 |
| Median pre-operative SNOT-22 (IQR): available for 62 patients | 43 (30) |
| Histopathology findings: Eosinophil count/hpf (available for 66 patients) | |
| • <10 | 38 (57.57%) |
| • 10–100 | 9 (13.63%) |
| • >100 | 19 (28.78%) |
| Anatomical Features | No. of Patients in Which Identified = n (Out of 69) | No. of Patients with Bilateral Presence of the Same Feature (Out of n) |
|---|---|---|
| Deviated nasal septum | 37 (53.6%) | - |
| Residual uncinate tissue | 35 (50.7%) | 15 (42.8%) |
| Missed natural maxillary ostium in maxillary antrostomy | 27 (39.13%) | 23 (85.18%) |
| Residual bony septae in anterior ethmoids | 43 (62.3%) | 40 (93.02%) |
| Residual bony septae in posterior ethmoids | 46 (66.67%) | 43 (93.47%) |
| Inadequate sphenoid dissection | 44 (63.76%) | 19 (43.18%) |
| Residual frontoethmoidal cells | 50 (72.4%) | 47 (94%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Lanca Gomes, P.; Marino, M.J.; Miglani, A.; Lal, D. Technical Quality of Contemporary Endoscopic Sinus Surgery: An Assessment by Study of Anatomical Features Needing Attention at Revision Surgery. Sinusitis 2024, 8, 28-36. https://doi.org/10.3390/sinusitis8020005
Kumar N, Lanca Gomes P, Marino MJ, Miglani A, Lal D. Technical Quality of Contemporary Endoscopic Sinus Surgery: An Assessment by Study of Anatomical Features Needing Attention at Revision Surgery. Sinusitis. 2024; 8(2):28-36. https://doi.org/10.3390/sinusitis8020005
Chicago/Turabian StyleKumar, Nitish, Pedro Lanca Gomes, Michael J. Marino, Amar Miglani, and Devyani Lal. 2024. "Technical Quality of Contemporary Endoscopic Sinus Surgery: An Assessment by Study of Anatomical Features Needing Attention at Revision Surgery" Sinusitis 8, no. 2: 28-36. https://doi.org/10.3390/sinusitis8020005
APA StyleKumar, N., Lanca Gomes, P., Marino, M. J., Miglani, A., & Lal, D. (2024). Technical Quality of Contemporary Endoscopic Sinus Surgery: An Assessment by Study of Anatomical Features Needing Attention at Revision Surgery. Sinusitis, 8(2), 28-36. https://doi.org/10.3390/sinusitis8020005







