Fabrication of High-Aspect-Ratio Cylindrical Micro-Structures Based on Electroactive Ionogel/Gold Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
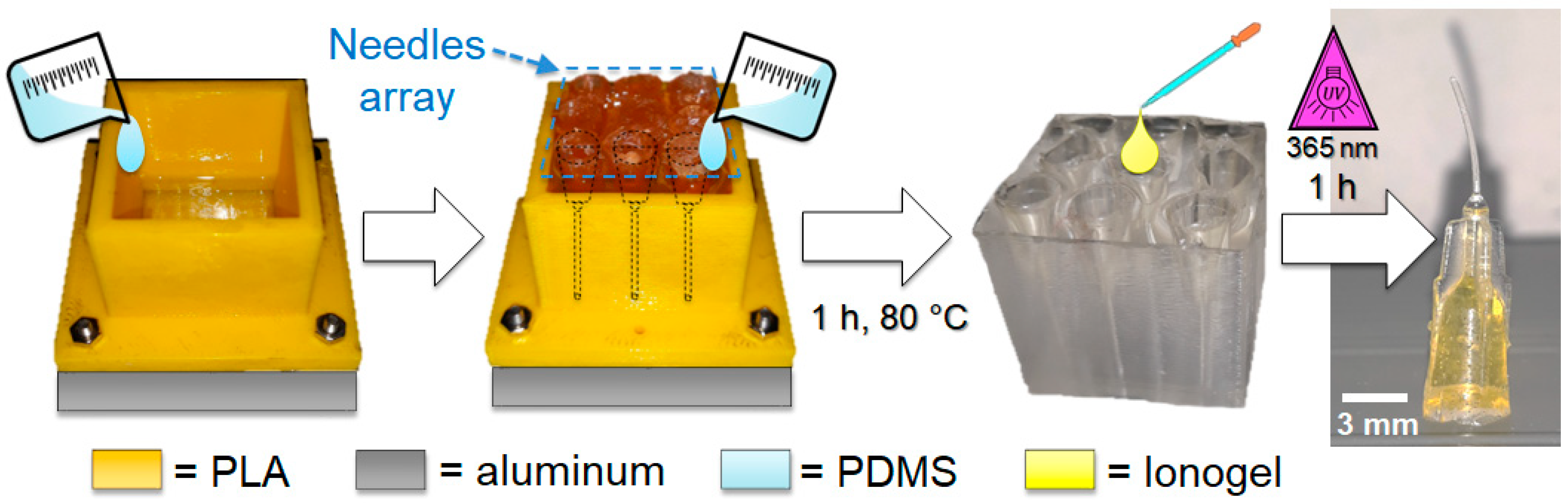
2.1. Ionogel Synthesis and Thin Films Fabrication
2.2. Replica Molding
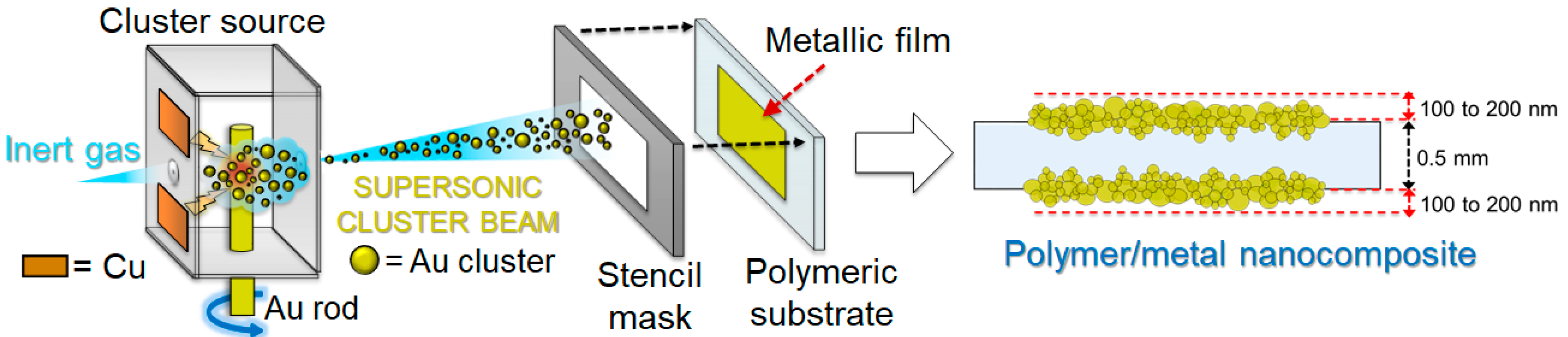
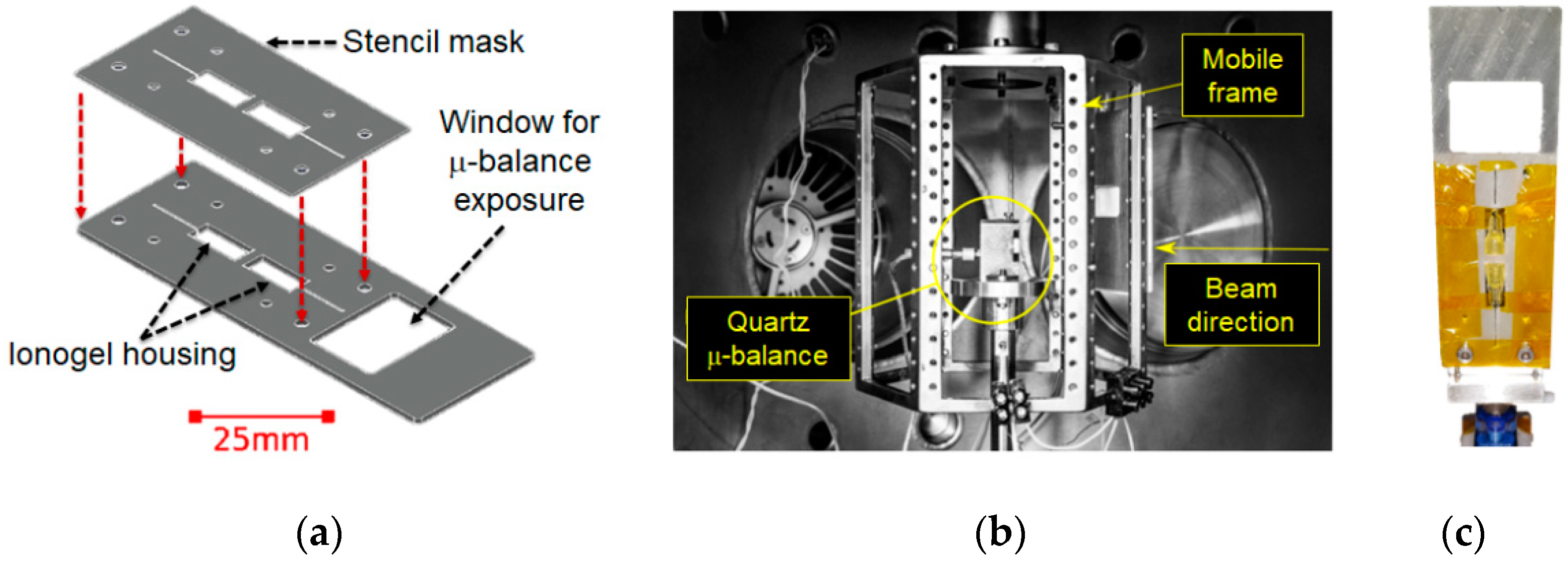
2.3. Supersonic Cluster Beam Deposition for Ionogel/Gold Nanocomposites Fabrication
2.4. Electrochemical and Electromechanical Characterization
3. Results and Discussions
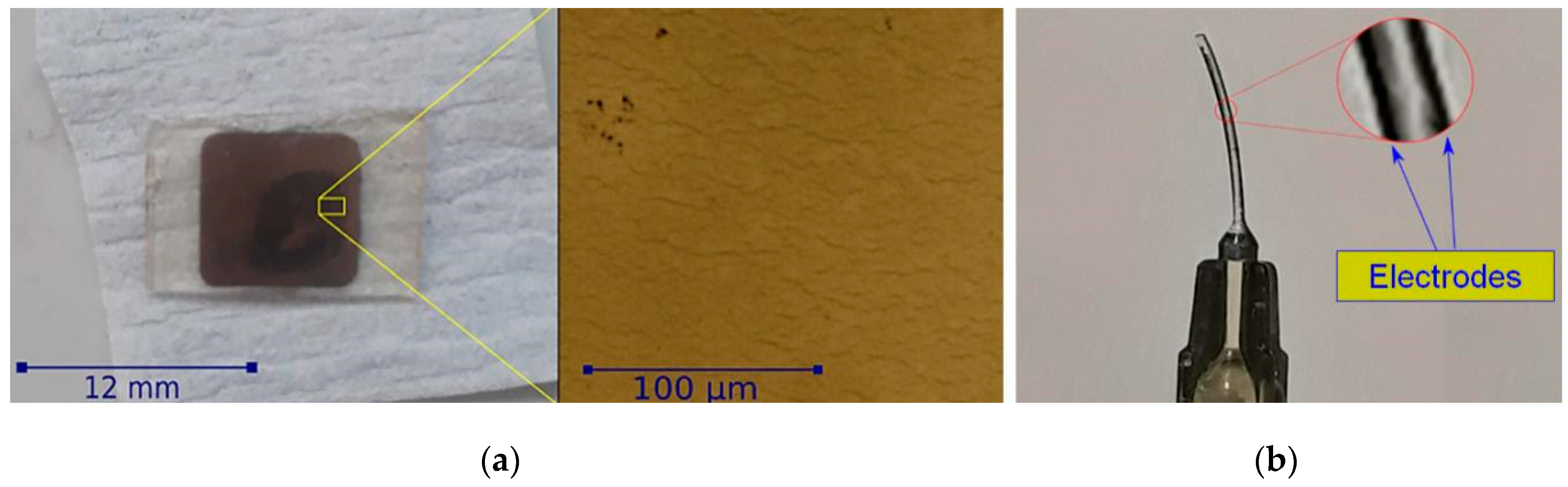
3.1. Ionogel Molding and Nanocomposite Fabrication
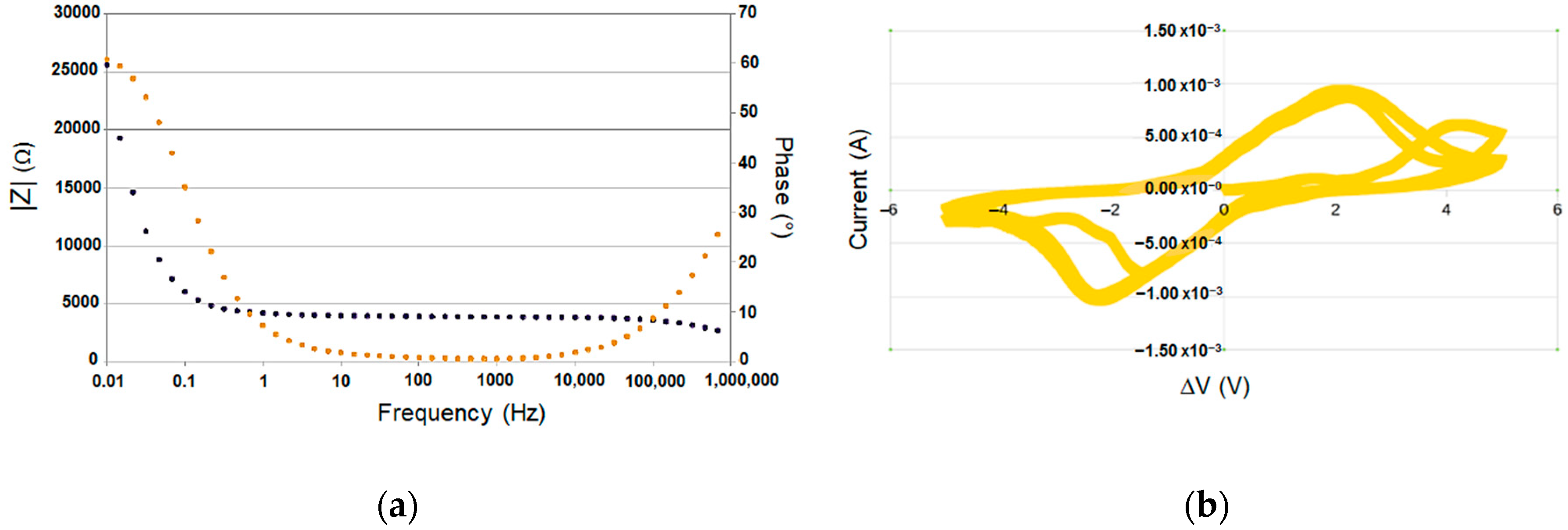
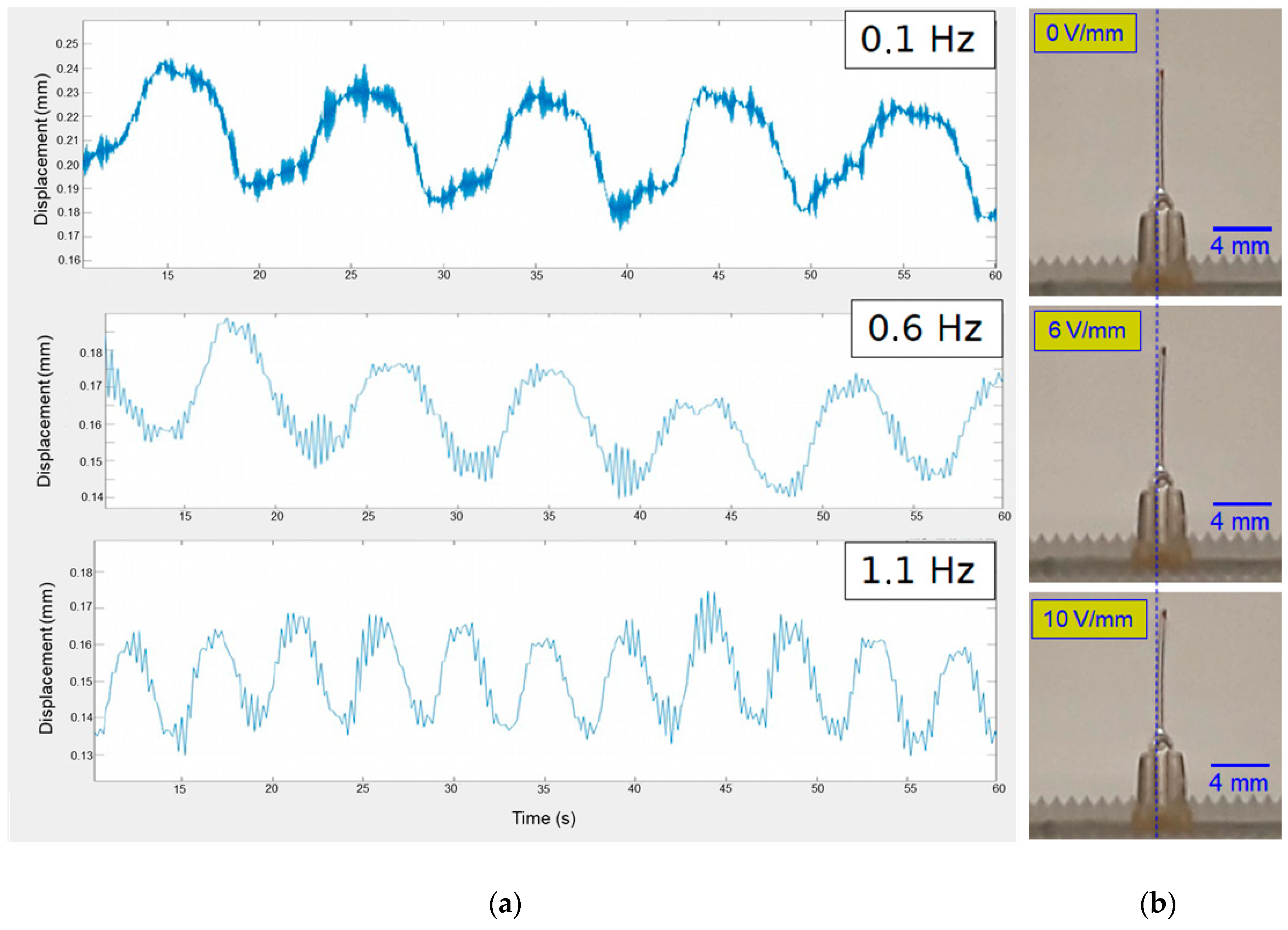
3.2. Ionogel/Gold Nanocomposites Electrochemical and Actuation Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rus, D.L.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, L.; Petersen, K.; Lum, G.Z.; Sitti, M. Soft Actuators for Small-Scale Robotics. Adv. Mater. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Carpi, F.; Kornbluh, R.; Sommer-Larsen, P.; Alici, G. Electroactive polymer actuators as artificial muscles: Are they ready for bioinspired applications? Bioinspiration Biomim. 2011, 6, 045006. [Google Scholar] [CrossRef] [PubMed]
- Nardinocchi, P.; Pezzulla, M.; Placidi, L. Thermodynamically based multiphysic modeling of ionic polymer metal composites. J. Intell. Mater. Syst. Struct. 2011, 22, 1887–1897. [Google Scholar] [CrossRef]
- Bhandari, B.; Lee, G.-Y.; Ahn, S.-H. A review on IPMC material as actuators and sensors: Fabrications, characteristics and applications. Int. J. Precis. Eng. Manuf. 2012, 13, 141–163. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Taya, M.; Kuga, Y. A Flemion-based actuator with ionic liquid as solvent. Smart Mater. Struct. 2007, 16, S214–S219. [Google Scholar] [CrossRef]
- Pugal, D.; Jung, K.; Aabloo, A.; Kim, K.J. Ionic polymer-metal composite mechanoelectrical transduction: Review and perspectives. Polym. Int. 2010, 59, 279–289. [Google Scholar] [CrossRef]
- Okazaki, H.; Sawada, S.; Kimura, M.; Tanaka, H.; Matsumoto, T.; Ohtake, T.; Inoue, S. Soft Actuator Using Ionic Polymer–Metal Composite Composed of Gold Electrodes Deposited Using Vacuum Evaporation. IEEE Electron Device Lett. 2012, 33, 1087–1089. [Google Scholar] [CrossRef]
- Yan, Y.; Santaniello, T.; Bettini, L.G.; Minnai, C.; Bellacicca, A.; Porotti, R.; Denti, I.; Faraone, G.; Merlini, M.; Lenardi, C.; et al. Electroactive Ionic Soft Actuators with Monolithically Integrated Gold Nanocomposite Electrodes. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Santaniello, T.; Migliorini, L.; Yan, Y.; Lenardi, C.; Milani, P. Supersonic cluster beam fabrication of metal–ionogel nanocomposites for soft robotics. J. Nanoparticle Res. 2018, 20, 250. [Google Scholar] [CrossRef]
- Milana, E.; Gorissen, B.; Peerlinck, S.; De Volder, M.; Reynaerts, D. Artificial Soft Cilia with Asymmetric Beating Patterns for Biomimetic Low-Reynolds-Number Fluid Propulsion. Adv. Funct. Mater. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Villa, S.M.; Mazzola, V.M.; Santaniello, T.; Locatelli, E.; Maturi, M.; Migliorini, L.; Monaco, I.; Lenardi, C.; Franchini, M.C.; Milani, P. Soft Piezoionic/Piezoelectric Nanocomposites Based on Ionogel/BaTiO3 Nanoparticles for Low Frequency and Directional Discriminative Pressure Sensing. ACS Macro Lett. 2019, 8, 414–420. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Corbelli, G.; Ghisleri, C.; Marelli, M.; Milani, P.; Ravagnan, L. Highly Deformable Nanostructured Elastomeric Electrodes With Improving Conductivity Upon Cyclical Stretching. Adv. Mater. 2011, 23, 4504–4508. [Google Scholar] [CrossRef]
- Santaniello, T.; Migliorini, L.; Borghi, F.; Yan, Y.; Rondinini, S.; Lenardi, C.; Milani, P. Spring-like electroactive actuators based on paper/ionogel/metal nanocomposites. Smart Mater. Struct. 2018, 27, 065004. [Google Scholar] [CrossRef]
- Migliorini, L.; Santaniello, T.; Rondinini, S.; Saettone, P.; Franchini, M.C.; Lenardi, C.; Milani, P. Bioplastic electromechanical actuators based on biodegradable poly(3-hydroxybutyrate) and cluster-assembled gold electrodes. Sens. Actuators B Chem. 2019, 286, 230–236. [Google Scholar] [CrossRef]
- Wegner, K.; Piseri, P.; Tafreshi, H.V.; Milani, P. Cluster beam deposition: A tool for nanoscale science and technology. J. Phys. D Appl. Phys. 2006, 39, R439–R459. [Google Scholar] [CrossRef]
- Piseri, P.; Tafreshi, H.V.; Milani, P. Manipulation of nanoparticles in supersonic beams for the production of nanostructured materials. Curr. Opin. Solid State Mater. Sci. 2004, 8, 195–202. [Google Scholar] [CrossRef]
- Borghi, F.; Podestà, A.; Piazzoni, C.; Milani, P. Growth Mechanism of Cluster-Assembled Surfaces: From Submonolayer to Thin-Film Regime. Phys. Rev. Appl. 2018, 9, 044016. [Google Scholar] [CrossRef] [Green Version]
- Mirigliano, M.; Borghi, F.; Podestá, A.; Antidormi, A.; Colombo, L.; Milani, P. Non-ohmic behavior and resistive switching of Au cluster-assembled films beyond the percolation threshold. Nanoscale Adv. 2019, 1, 3119–3130. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Guo, H.C.; Ye, E.; Li, Z.; Han, M.-Y.; Loh, X.J. Recent progress of atomic layer deposition on polymeric materials. Mater. Sci. Eng. C 2017, 70, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Weber, A.Z. Electrochemical/Mechanical Coupling in Ion-Conducting Soft Matter. J. Phys. Chem. Lett. 2015, 6, 4547–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravagnan, L.; Divitini, G.; Rebasti, S.; Marelli, M.; Piseri, P.; Milani, P. Poly(methyl methacrylate)–palladium clusters nanocomposite formation by supersonic cluster beam deposition: A method for microstructured metallization of polymer surfaces. J. Phys. D Appl. Phys. 2009, 42, 082002. [Google Scholar] [CrossRef]
- Cardia, R.; Melis, C.; Colombo, L. Neutral-cluster implantation in polymers by computer experiments. J. Appl. Phys. 2013, 113, 224307. [Google Scholar] [CrossRef] [Green Version]
- Bellacicca, A.; Santaniello, T.; Milani, P. Embedding electronics in 3D printed structures by combining fused filament fabrication and supersonic cluster beam deposition. Addit. Manuf. 2018, 24, 60–66. [Google Scholar] [CrossRef]
- Santaniello, T.; Milani, P. Additive Nano-Manufacturing of 3D Printed Electronics Using Supersonic Cluster Beam Deposition. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 15, pp. 313–333. [Google Scholar]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of High-Performance Ionogels with Excellent Transparency, Good Mechanical Strength, and High Conductivity. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kim, K.J.; Tadokoro, S. Electroactive Polymers for Robotic Applications. Artif. Muscles Sens. 2007, 23, 291. [Google Scholar] [CrossRef]
- Bian, C.; Zhu, Z.; Bai, W.; Chen, H.; Li, Y. Fast actuation properties of several typical IL-based ionic electro-active polymers under high impulse voltage. Smart Mater. Struct. 2020, 29, 035014. [Google Scholar] [CrossRef]
- Aabloo, A.; Belikov, J.; Kaparin, V.; Kotta, U. Challenges and Perspectives in Control of Ionic Polymer-Metal Composite (IPMC) Actuators: A Survey. IEEE Access 2020, 8, 121059–121073. [Google Scholar] [CrossRef]
- Marelli, M.; Divitini, G.; Collini, C.; Ravagnan, L.; Corbelli, G.; Ghisleri, C.; Gianfelice, A.; Lenardi, C.; Milani, P.; Lorenzelli, L. Flexible and biocompatible microelectrode arrays fabricated by supersonic cluster beam deposition on SU-8. J. Micromech. Microeng. 2011, 21, 45013. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milana, E.; Santaniello, T.; Azzini, P.; Migliorini, L.; Milani, P. Fabrication of High-Aspect-Ratio Cylindrical Micro-Structures Based on Electroactive Ionogel/Gold Nanocomposite. Appl. Nano 2020, 1, 59-69. https://doi.org/10.3390/applnano1010005
Milana E, Santaniello T, Azzini P, Migliorini L, Milani P. Fabrication of High-Aspect-Ratio Cylindrical Micro-Structures Based on Electroactive Ionogel/Gold Nanocomposite. Applied Nano. 2020; 1(1):59-69. https://doi.org/10.3390/applnano1010005
Chicago/Turabian StyleMilana, Edoardo, Tommaso Santaniello, Paolo Azzini, Lorenzo Migliorini, and Paolo Milani. 2020. "Fabrication of High-Aspect-Ratio Cylindrical Micro-Structures Based on Electroactive Ionogel/Gold Nanocomposite" Applied Nano 1, no. 1: 59-69. https://doi.org/10.3390/applnano1010005




