1. Introduction
To one degree or another, pain syndromes accompany 90% of diseases [
1]. The International Organization for the Study of Pain (ISAP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or resembling it” [
2]. Pain provokes the development of negative effects in the body, including metabolic, vascular and vegetative disorders [
3].
The fight against pain is one of the centuries-old priorities of medicine, for which all kinds of medicinal and non-medicinal means are used. Due to the toxicity and other potential undesirable effects of pharmacological agents, the search for non-drug methods to combat pain has now been updated. Thus, photobiomodulation (PBM) using low-intensity laser radiation (LILR) is used in clinical practice to reduce patients’ level of pain [
4,
5,
6]. Experimental work of domestic and foreign researchers (in vitro and in vivo) has shown that the effect of FBM is due to a multi-link chain of consequences from the primary absorption of photons by biological tissues [
7,
8,
9]. The key primary photoacceptors are cytochrome-C oxidase and cytochrome-C oxidase, as well as hemoglobin complexes with nitric oxide (nitrosyl protein complexes), which have absorption bands in the regions of red light and near-infrared radiation [
10]. The processes induced by PBM include the photomodification of the activity of enzymes of the antioxidant defense system of living cells, photomodification of the respiratory chain of living cells, photolysis of nitrosyl complexes of proteins, changes in the synthesis of proteins and nucleic acids, and the opening of some light- and heat-dependent ion channels, which eventually lead to the synthesis of more adenosine triphosphate (ATP) by cells [
7,
8,
10,
11].
Thus, there are sufficiently convincing theoretical and experimental prerequisites for the use of PBM in the rehabilitation of patients with pain syndromes of various etiologies. It is especially effective to use LILR on acupuncture points (AP), which makes it possible to reduce the energy load on the body and simultaneously influence the correction of other interested systems if a patient has a comorbid pathology [
12,
13]. The advantages of the punctual exposure option (in terms of physiology, combinatoricity, noninvasiveness, painlessness, sterility and cost-effectiveness as well as the fact that it is holistic and evidence-based and has a lower energy load on the cell and minimal side effects) fully apply to punctual photobiomodulation (PPBM). This is due to the beneficial histomorphological and biophysical features of AP and their connection with the autonomic nervous system (ANS), which is especially important under stress [
14]. When using PPBM, there is a more pronounced induction of the adaptive reactions of the body. There are data on the identical effectiveness of laser acupuncture (LA) compared to classical acupuncture [
15]. The physiological basis of the mechanism of action of laser radiation on points is twofold: the basic component belongs to the reflex regulation of the functional state of the nervous system due to the normalization of strength, mobility and balance of the processes of inhibition and excitation in the cerebral cortex and proceeds within the framework of the general adaptation syndrome, which is confirmed in animals [
16,
17]. The second component of the body’s reaction depends on the capabilities of laser radiation and is formed by its biophysical features. Unfortunately, despite extensive successful experiences of acupuncture in clinical practice, from the point of view of evidence-based medicine, its effectiveness is not always obvious. In some published Cochrane reviews devoted to the use of laser acupuncture in the rehabilitation of patients with osteoarthritis (OA) [
18], the results are evaluated at the placebo level, which does not yet allow for the inclusion of LA in the leading guidelines for the management of OA. However, the low level of evidence may be a consequence of conducting most studies on small samples of patients with an insufficient degree of randomization.
From the point of view of rehabilitation medicine, the analgesic effect is particularly attractive for the mechanism of action of LA. It consists of the activation of endogenous analgesia systems to relieve pain and break the vicious circle. At the same time, analgesic, immunomodulatory and vegetative-regulating effects are isolated [
19]. According to many, unlike pharmacological and surgical anesthesia, acupuncture analgesia is a kind of physiological modulation. The analgesic effect develops in response to the punctual stimulation of antinociceptive brain structures, which leads to the release of specific chemicals, including the enkephalin and endorphin opioid systems, serotonin and the adrenergic system of the brain stem, as well as non-opioid neuropeptides of the hypothalamic–pituitary complex. However, its primary importance may be its non-specific response in reproducing the phenomenon of adaptation to the repeated action of mild stress and limiting the influence of hyperadrenal reactions. This fact is confirmed by the reaction of animals to the acupuncture procedure, which is close to a response to stress in the form of an increase in corticosterone content, without significant changes in the level of β-endorphin, a key link of the stress-limiting system. On the contrary, at the end of the course exposure, the absence of a reaction to stress is combined with an inversion of the ratio of these indicators [
20]. However, the lack of a clear understanding of the patterns, the mechanism of development of adaptive reactions and a broad evidence base on the possible development of undesirable effects hinders the widespread introduction of laser acupuncture into rehabilitation protocols for patients with traumatological and neuroorthopedic profiles with acute and chronic pain syndromes. Even though previous studies have been conducted [
21,
22,
23], this encourages the continuation of experimental work in order to determine the optimal energy parameters of the radiation, as well as to further study its mechanisms of influence on various systems of animal bodies under pain stress.
2. Materials and Methods
To achieve this goal, our study was conducted on 30 male Wistar rats weighing 250–300 g under conditions of the acute phase of experimental pain stress. This work was performed in accordance with ethical norms and rules of laboratory practice (GLP), the Geneva Convention for the protection of animals “International guidelines for biomedical research involving animals” (Geneva, 1990), and the approval of the Local Ethical Committee of the Volga Medical Research University (protocol No. 6 from 29 April 2022).
The animals were kept in standard vivarium conditions which entailed natural lighting and a balanced nutrition and drinking regime. A placebo-controlled study of the dynamics of metabolic, vascular and vegetative adaptation indicators using irradiation of acupuncture points (TA) was conducted. The following “general” and “local” effects were irradiated: GV.14 (in the area of the occipital protuberance, where the skin projection of the center of vegetative regulation responsible for the development of adaptive reactions of the body is located) and point BL.37, localized in the middle of the posterior surface of the thigh, where the sciatic nerve was ligated. The course of laser acupuncture (10 sessions) was conducted on TA in the near-infrared range in pulsed mode with an exposure of 10 min per session. A certified device “Elmedlife M” (Russia) was used for irradiation. Exposure parameters were as follows: the wavelength of infrared radiation—810 nm, the number of light modules with infrared LEDs—1 pc., the number of LEDs in the light module—3 pcs., the frequency of light pulses—10 Hz, the duration of the light pulse—20 ms, the power of infrared radiation in the pulse—1.5 MW, the area of the light spot from one module at contact—2 cm2, radiation intensity—55 MW/cm2 and radiation dose—6 J/cm2.
The animals were divided into three groups (10 animals in each group). Two groups of animals served as the control: intact rats (control 1) and the placebo group (control 2), in which rats with experimental pain stress received simulated irradiation. Rats of the main group were irradiated immediately after pain stress modeling operation with a point (GV.14) on the occipital tubercle in the projection of the autonomic regulation center, responsible for the development of the adaptive reactions of the organism. In addition, the point BL.37 localized in the middle of the posterior surface of the thigh above the sciatic nerve bifurcation was treated. During the procedure, the rats were fixed in special pens. Experimental pain stress was modeled under intramuscular anesthesia (Zoletil + Xyla) by double ligation of the sciatic nerve at its bifurcation. Animals were removed from the experiment at the end of the irradiation course by decapitation under anesthesia (Zoletil + Xyla).
Blood stabilized with sodium citrate (1:9) was used to assess metabolic adaptation. The intensity of lipid peroxidation (LPO) was assessed by the concentration of malonic dialdehyde (MDA), diene conjugates (DC), triene conjugates (TC) and Schiff bases (SB) [
24]. The specific activity of antioxidant enzymes was also studied, including catalase [
25], superoxide dismutase (SOD) [
26], glutathione reductase (GR) [
27] and glucose-6-phosphate dehydrogenase (Gl-6-fDH) [
27]. Lactate dehydrogenase (LDH) activity in erythrocytes was determined spectrophotometrically in forward (LDHdirect) and reverse (LDHreverse) reactions [
27]. Protein concentration was calculated according to the modified Lowry method [
28]. The content of malonic dialdehyde in blood plasma (MDA plasma) and erythrocytes (MDA erythrocytes) was determined by reaction with thiobarbituric acid using “TBK-AGAT” reagent sets (Agat-Med LLC, Moskow, Russia) on the PE-5400 spectrophotometer (EKROSCHEM LLC, St Petersburg, Russia).
The method of laser Doppler flowmetry (LDF) was used to assess the dynamics of skin microcirculation. Laser analyzer “LAKK-M” (version 2) (SPE “Lazma”, Moskow, Russia) was used. During the study, the analyzer’s probe was set perpendicularly to the studied area. The recording duration was 3 min [
29]. The integral index of microcirculation, characterizing the degree of tissue volume perfusion per unit time, was assessed. The role of active (endothelial oscillations—0.01–0.08 Hz, neurogenic oscillations—0.08–0.2 Hz, myogenic oscillations—0.2–0.7 Hz) and passive (respiratory—0.7–2 Hz, cardiac—2–5 Hz) factors of microcirculation regulation was determined with further calculation of bypass index [
29,
30].
Heart rhythm variability (HRV) was studied using Neurosoft (Russia) hardware–software complex (HSC, ver.5.2.0.0). The indices of statistical and variation heart rhythm monitoring (heart rate, stress index, variation coefficient, etc.) were studied [
31].
Statistical data processing was performed using Statistica 6.0 software (Stat Soft, Inc. Tulsa, USA). The Shapiro–Wilk criterion was used to test the hypothesis that the data were consistent with a normal distribution. The data are presented as M ± σ. Mean values of two independent groups were compared using nonparametric Mann–Whitney test. The critical value of the significance level was assumed to be 0.05.
3. Results
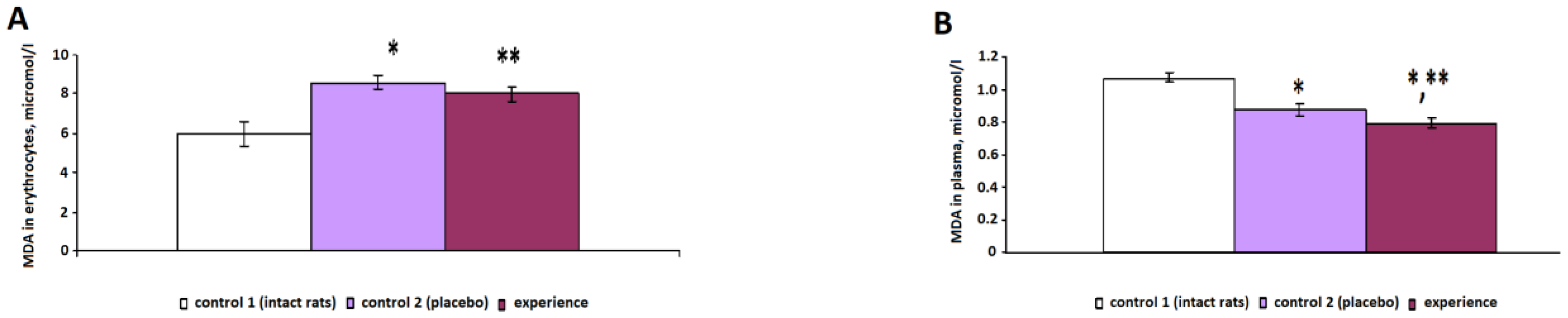
The study of the indicators of oxidative metabolism revealed the development of oxidative stress in animals with pain injuries (control 2), characterized by an increase in the concentration of LPO products against the background of a decrease in antioxidant enzymatic activity. The content of the primary products of LPO—diene conjugates—in the control 2 group increased by 13% (
p < 0.001), the concentration of the secondary products of LPO—triene conjugates (in plasma) and MDA (in erythrocytes)—increased by 17% (
p < 0.001) and 44% (
p < 0.001), respectively, and the level of the final products of LPO—SB—increased by 17% (
p = 0.002) compared with the indicators of intact rats (
Figure 1 and
Figure 2). The growth of highly toxic primary, secondary and final LPO products led to the destabilization of membranes and the degradation of cells, which is due to the ability of DC, TC and SB to damage proteins, lipoproteins and nucleic acids.
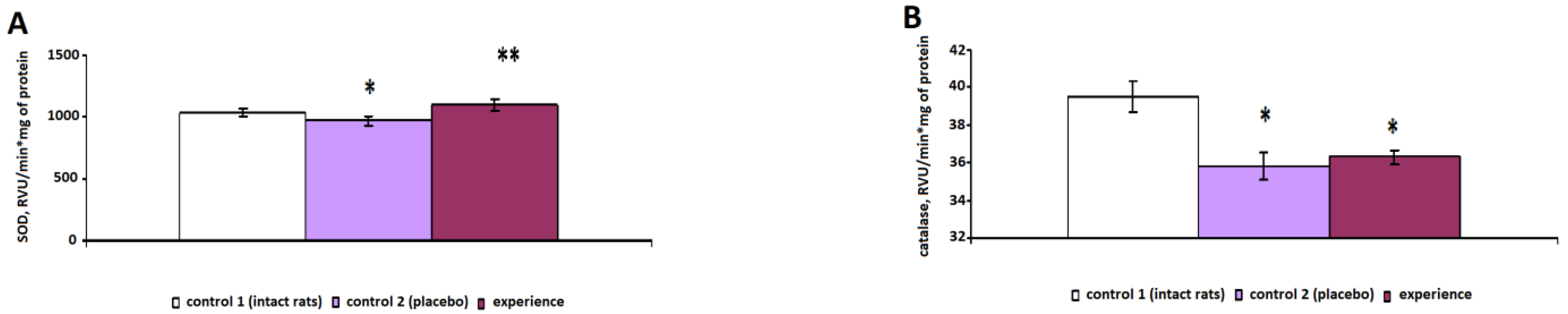
The specific activity of the antioxidant enzymes SOD (
Figure 3A) and catalase (
Figure 3B) decreased in erythrocytes by 8% (
p = 0.024) and 10% (
p < 0.001), respectively, compared with the indicators of the intact rats. In the control group 2 animals, the activity of GR (
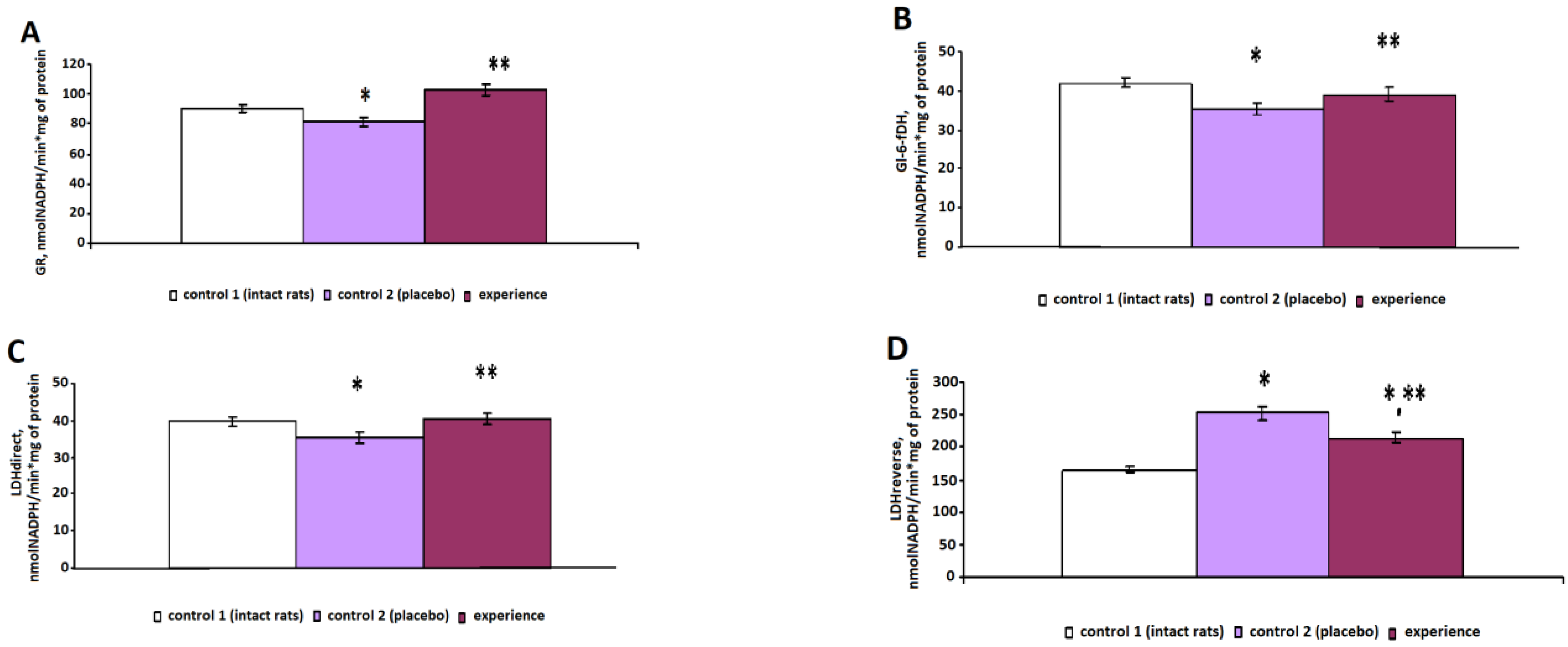
Figure 4A) and Gl-6-fDH (
Figure 4B) decreased by 11% (
p = 0.031) and 16% (
p = 0.007), respectively, in erythrocytes.
A study of the biochemical parameters of energy metabolism in the control 2 group revealed a decrease in the specific activity of LDHdirect (
Figure 4C) and an increase in LDHreverse by 54% (
p < 0.001) (
Figure 4D) compared with those of the intact animals, which led to lactic acidosis and, as a consequence, the development of hypoxia.
Thus, acute pain stress was accompanied by the inhibition of the antioxidant protection of the blood, leading to an increase in free radical processes with the accumulation of lipid peroxidation products in both plasma and erythrocytes.
After irradiation with pain stress, the specific activity of SOD statistically significantly increased by 18% (p < 0.001), that of GR increased by 26% (p < 0.001) and that of Gl-6-fDH increased by 11% (p < 0.001), compared with those of the control 2 group.
There was a decrease in the concentration of the studied LPO products in plasma and erythrocytes after irradiation with pain stress compared with that of the control 2 group: the DC concentration decreased by 5% (p = 0.026), the MDAplasma concentration decreased by 8% (p < 0.001), the MDAerythrocytes concentration decreased by 7% (p < 0.001), the TC concentration decreased by 8% (p < 0.001) and the SB concentration decreased by 9% (p < 0.001).
Near-infrared irradiation during pain stress had a normalizing effect on the energy metabolism of erythrocytes, causing an increase in the specific activity of LDH in the direct reaction by 14% (p < 0.001) and a decrease in the LDH activity in the reverse reaction by 15% (p < 0.001), which indicated a decrease in lactate levels and, as a consequence, signs of hypoxia.
The study of the dynamics of the microcirculation index showed that with sciatic nerve damage and concomitant pain syndromes, in the control 2 group, tissue perfusion decreased by 52% (
p = 0.003) compared to that of the intact animals (control 1) (
Table 1), which is natural for the pathogenesis of pain injuries.
In the experimental animal group, tissue perfusion decreased by 57% (p < 0.001) relative to that of the control 1 group. The role of shunt blood flow (the bypass index) increased only in the experimental group (by 15% (p = 0.016) from that of control 1), while in the control 2 group, it remained at the level of the control 1 group.
The variation ranges of the active regulatory factors (endothelial, neurogenic and myogenic components) decreased in the control 2 group by 23% (
p = 0.009), 17% (
p = 0.033) and 12% (
p = 0.027), in contrast to the increase in the experimental group (10% (
p = 0.018), 33% (
p = 0.004) and 15% (
p = 0.031)) relative to the control 1 group, respectively (
Table 1).
For passive regulation factors (respiratory and cardiac components), a different response was detected, namely, in the control 2 group, the respiratory component increased by 77% (
p < 0.001) and the cardiac component decreased by 6%, whereas in the experimental group, the respiratory component decreased by 6% and the cardiac component by 21% (
p = 0.016) relative to that of the control 1 group, respectively (
Table 1).
The growth of endothelial oscillations may be related to the increased release of endogenous nitric oxide.
Myogenic oscillations reflect the influence of central trophotropic mechanisms, including parasympathetic centers; their appearance in the spectrum of blood flow oscillations indicates a decrease in the ergotropic central component of regulation and a shift in central regulation in trophotropic direction.
Increased amplitude of a respiratory wave indicates decreased microcirculatory pressure and/or worsened venous outflow. The deterioration of blood outflow from the microcirculatory channel leads to an increase in the number of erythrocytes, which is accompanied by an increase in the amplitude of the respiratory wave.
A decrease in the pulse wave amplitude with increased or normal values for the mean perfusion indicates decreased arterial blood inflow into the microcirculatory bed.
An additional study of heart rate variability showed that, with sciatic nerve damage and accompanying pain syndromes, the animals’ heart rate, reflecting the total effect of heart rate regulation, decreased by 15% relative to that of the control 1 group (
p < 0.05), and the heart rate in the experimental group decreased by 37% from the control 1 values (
p < 0.05), indicating the increased tone of the parasympathetic department of the autonomic nervous system (
Table 2).
The autonomic balance index, which reflects the correlation between the activity of the sympathetic and parasympathetic sections of the autonomic nervous system, was shown to decrease in the control 2 group by 69% and in the experimental group by 42% correspondingly from the level of the control 1 group (p < 0.05). The index of the adequacy of the regulatory processes, reflecting the correspondence between the activity of the sympathetic department of the autonomic nervous system and the leading level of the sinus node functioning, increased by 46% in the control 2 group and decreased by 16% in the experimental group relative to the intact values (p < 0.05). The parameter of tension in the regulatory systems, which characterizes the state of the central regulatory circuit and has a high sensitivity to sympathetic nervous system tone increases, increased in the control 2 group by 62%, while in the experimental group, it increased only by 32% relative to that of the control 1 group (p < 0.05). The combination of these changes indicates the predominance of the parasympathetic department of the autonomic nervous system in the heart rhythm regulation of the animals in the experimental group.
4. Discussion
The paper demonstrates the pathogenetic feasibility of using laser acupuncture in the near-infrared range for the correction of systemic disorders caused by pain stress from the first hours of its development. We registered metabolic disorders in the form of the increased concentration of LPO products both in plasma and erythrocytes against the background of decreased antioxidant enzymatic activity. This led to lactic acidosis and the development of hypoxia, which is characteristic of oxidative stress.
Against this background, the impact of LA on AP stimulated the development of adaptive reactions in the body, which were expressed in the reliable positive dynamics of the energy metabolism of the animals’ red blood cells, causing an increase in the specific activity of LDH in a direct reaction and a decrease in LDH activity in a reverse reaction, which indicated a decrease in lactate levels and signs of hypoxia. We obtained similar data in previous studies [
32]. This coincides with the data of other authors who experimentally demonstrated a positive effect of acupuncture on the activity of SOD and glutathione peroxidase in the hippocampus and a reduction in oxidative stress [
33,
34].
It has been suggested that acupuncture reduces nitric oxide (NO) release and modulates the activity of the NO-synthase enzyme, which affects the production and elimination of free radicals and also leads to an increase in endothelial oscillations in microvessels [
35]. This effect was also registered in our study. Thus, a pronounced decrease in tissue perfusion (by 52%) was observed in the animals of the experimental group with pain stress. After a course of irradiation, the state of microhemodynamics in the region concerned improved significantly due to a 15% increase in the shunting index. Thus, puncture photobiomodulation promotes the normalization of perfusion indices in conditions of prolonged ischemia caused by pain stress, due to the stimulation of active and passive mechanisms of blood flow modulation.
It should be noted that the positive shifts of the metabolic and vascular disorders in the animals of the main group (experience) occurred against the background of the restoration of autonomic regulation indices, which, immediately after the application of pain stress, shifted towards the predominance of sympathetic influences and at the end of LA course, shifted towards the parasympathetic. This may be due to the effect on the “common” point, which is located in the cutaneous projection of the center of vegetative regulation of the animals. The additional irradiation of the “local” point, apparently, had a more positive effect on the state of microcirculation.
However, we cannot ignore the role of IR radiation itself, which we used as a therapeutic stimulus. This range is part of the so-called “relic” electromagnetic radiation, which contains frequencies of low-energy vibrational–rotational levels of various molecules (proteins, RNA and DNA), as well as frequencies of intermolecular interactions [
36].
The advantage of this study is the registration of reliable data on the corrective homeostatic possibilities of the combination of acupuncture points of general and local action. Exposure to infrared laser radiation on AP was a stimulus for triggering a cascade of autonomic anti-stressor reactions in the body [
37]. Thus, we can consider that acupuncture points serve as information points of peripheral autonomic regulation, correcting systemic disorders under stress.
5. Conclusions
The presented results show that the effect of low-intensity LILR on acupuncture points associated with the autonomic nervous system causes a correction of the redox balance in the body of experimental animals. It leads to the activation of antioxidant protection indicators (a statistically significant increase in the specific activity of SOD by 18%, of GR by 26.18% and of Gl-6-fDH by 10.89%) and a decrease in the concentration of the studied LPO products in plasma and erythrocytes after irradiation with pain stress compared with that of the control 2 group: the DC concentration decreased by 5% (p = 0.026), MDAplasma—by 8% (p < 0.001), MDAerythrocytes—by 7% (p < 0.001), TC—by 8% (p < 0.001) and SB—by 9% (p < 0.001).
In addition, LA contributes to the development of vegetative adaptation reactions, which are expressed as an increase in trophotropic effects, especially the index of vegetative equilibrium, which decreased in the placebo group by 69% and increased by 42% in the experimental group compared with the intact level (p < 0.05). In the end, this was reflected in the positive dynamics of vascular blood flow in the absence of negative side effects on the body.
Thus, the results of our scientific research demonstrate the prospects of using PPBM in conditions of acute pain stress, which provides a basis for using this method in the rehabilitation of patients with pain syndromes.