Frequency Response Analysis of FAU, LTA and MFI Zeolites Using UV-Vis and Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
Preparation of Samples
3. Scanning Electron Microscopy and X-ray Diffraction Analysis
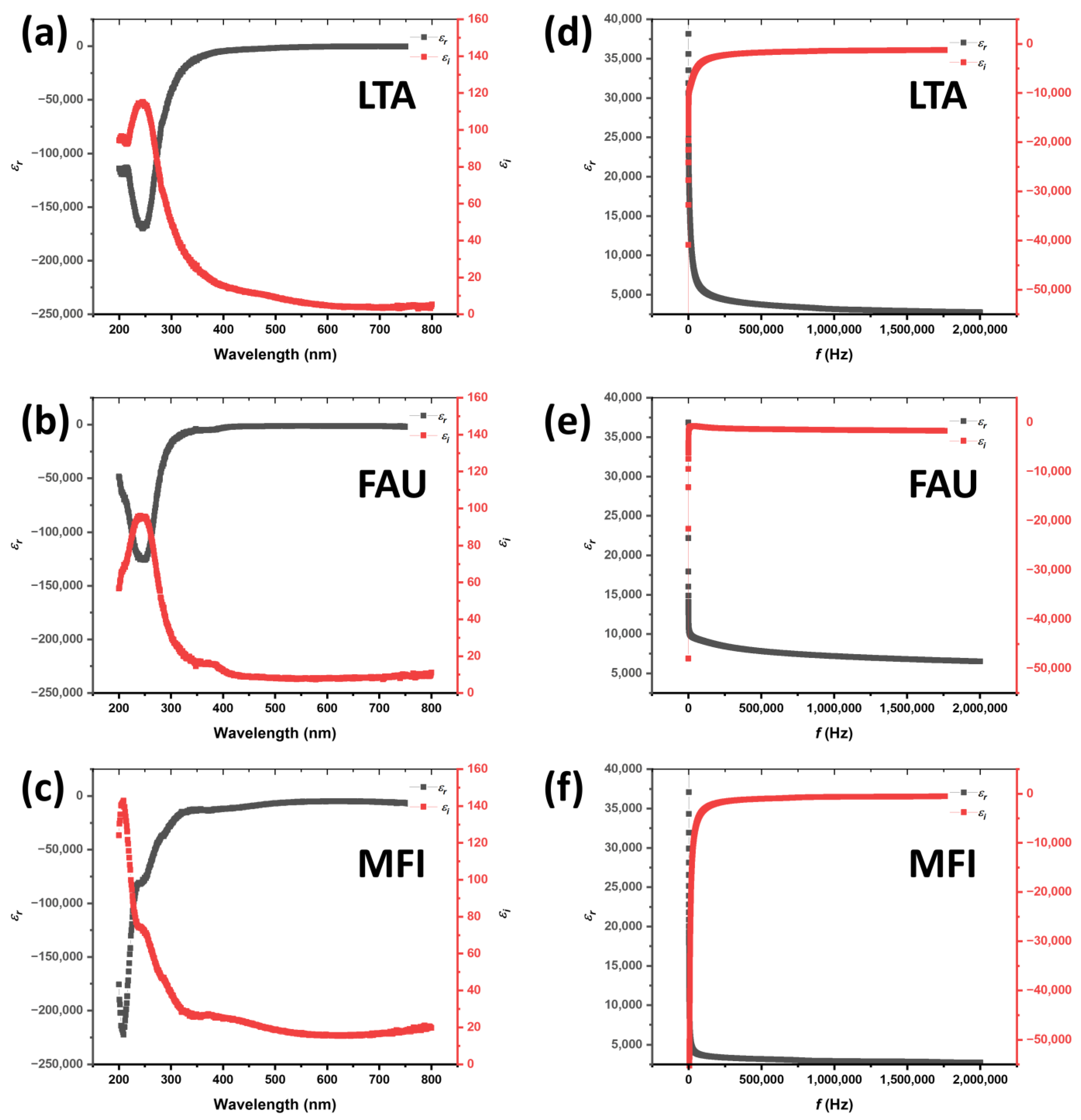
4. UV-Vis Analysis
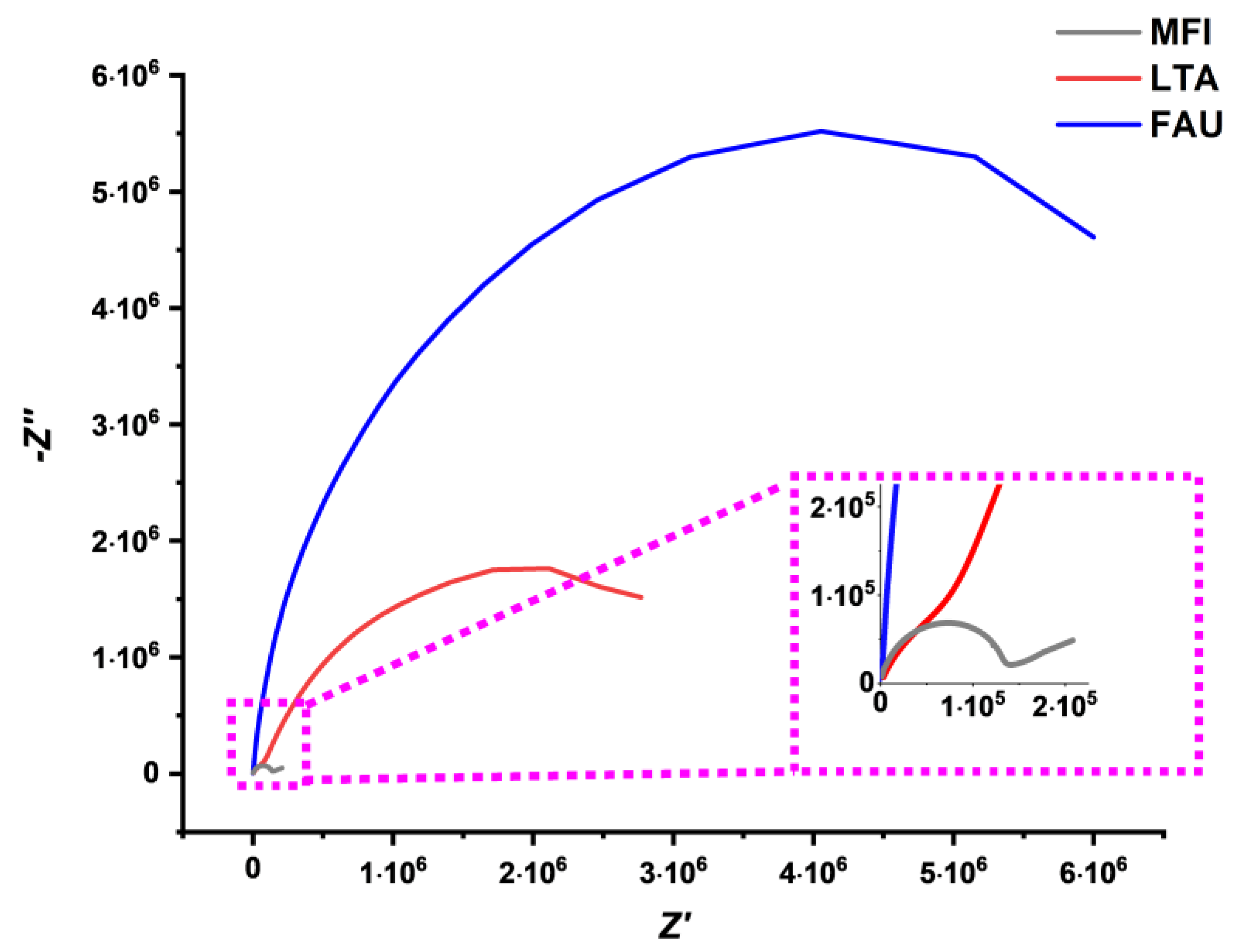
5. Electrochemical Impedance Spectroscopy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klaas, J.; Schulz-Ekloff, G.; Jaeger, N.I. UV−Visible Diffuse Reflectance Spectroscopy of Zeolite-Hosted Mononuclear Titanium Oxide Species. J. Phys. Chem. B 1997, 101, 1305–1311. [Google Scholar] [CrossRef]
- Hamidouche, F.; Ghebache, Z.; Boudieb, N.; Sanad, M.M.S.; Djelali, N.-E. Enhancing the Supercapacitive and Conductivity Properties of Polypyrrole via In-situ Polymerization with HY Zeolite Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 31, 704–715. [Google Scholar] [CrossRef]
- Saragi, I.R.; Krisnandi, Y.K.; Sihombing, R. Synthesis and Characterization HY Zeolite from Natural Aluminosilicate for n-Hexadecane Cracking. Mater. Today: Proc. 2019, 13, 76–81. [Google Scholar] [CrossRef]
- Fois, E.; Gamba, A.; Tabacchi, G. Bathochromic Effects in Electronic Excitation Spectra of Hydrated Ti Zeolites: A Theoretical Characterization. ChemPhysChem 2008, 9, 538–543. [Google Scholar] [CrossRef]
- Conner, W.C.; Tompsett, G.; Lee, K.H.; Yngvesson, K.S. Microwave synthesis of zeolites: 1. Reactor engineering. J. Phys. Chem. B 2004, 108, 13913–13920. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Yu, R.; Shui, J. Porous CNTs/Co Composite Derived from Zeolitic Imidazolate Framework: A Lightweight, Ultrathin, and Highly Efficient Electromagnetic Wave Absorber. ACS Appl. Mater. Interfaces 2016, 8, 34686–34698. [Google Scholar] [CrossRef] [PubMed]
- Kurzweil, P.; Maunz, W.; Plog, C. Impedance of zeolite-based gas sensors. Sens. Actuators B Chem. 1995, 25, 653–656. [Google Scholar] [CrossRef]
- Urbiztondo, M.; Pellejero, I.; Rodriguez, A.; Pina, M.; Santamaria, J. Zeolite-coated interdigital capacitors for humidity sensing. Sens. Actuators B Chem. 2011, 157, 450–459. [Google Scholar] [CrossRef]
- İzci, E.; Izci, A. Dielectric behavior of the catalyst zeolite NaY. Turk. J. Chem. 2007, 31, 523–530. [Google Scholar]
- Saad, I.B.; Hannachi, N.; Roisnel, T.; Hlel, F. Optical, UV-Vis spectroscopy studies, electrical and dielectric properties of transition metal-based of the novel organic–inorganic hybrid (C6H10N2)(Hg2Cl5)2·3H2O. J. Adv. Dielectr. 2019, 9, 1950040. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.izastructure.org/databases (accessed on 29 November 2022).
- Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Antúnez-García, J.; Reyes-Serrato, A.; Sánchez, P.; Petranovskii, V. Textile Functionalization Using LTA and FAU Zeolitic Materials. Polymers 2022, 15, 99. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Murrieta-Rico, F.N.; Antúnez-García, J.; Yocupicio-Gaxiola, R.I.; Zamora, J.; Reyes-Serrato, A.; Pestryakov, A.; Petranovskii, V. Study of Electric and Magnetic Properties of Iron-Modified MFI Zeolite Prepared by a Mechanochemical Method. Materials 2022, 15, 7968. [Google Scholar] [CrossRef]
- Sharma, P.; Han, M.H.; Cho, C.-H. Synthesis of Zeolite Nanomolecular Sieves of Different Si/Al Ratios. J. Nanomater. 2015, 2015, 912575. [Google Scholar] [CrossRef]
- Hanbury, A.; Serra, J. Mathematical morphology in the cielab space. Image Anal. Ster. 2002, 21, 201–206. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Galván, D.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. The effect of chemical composition on the properties of LTA zeolite: A theoretical study. Comput. Mater. Sci. 2021, 196, 110557. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Galván, D.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. Aluminum distribution in mordenite-zeolite framework: A new outlook based on density functional theory calculations. J. Solid State Chem. 2021, 306, 122725. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Fuentes-Moyado, S. Theoretical study of the effect of isomorphous substitution by Al3+ and/or Fe3+ cations to tetrahedral positions in the framework of a zeolite with erionite topology. J. Mater. Sci. 2019, 54, 13190–13199. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Yocupicio-Gaxiola, R.I.; Serrato, A.R.; Petranovskii, V.; Murrieta-Rico, F.N.; Shelyapina, M.G.; Fuentes-Moyado, S. A theoretical study of the effect of exchange cations in surface of ZSM-5 lamellar zeolites. J. Solid State Chem. 2023, 317, 123725. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Silva, M.A.S.; Sombra, A.S.B. Measurement of Microwave Dielectric Properties and Factors Affecting Them. In Microwave Materials and Applications 2V Set; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–51. [Google Scholar]
- Yang, X.; Liu, X.; Yu, S.; Gan, L.; Zhou, J.; Zeng, Y. Permittivity of Undoped Silicon in the Millimeter Wave Range. Electronics 2019, 8, 886. [Google Scholar] [CrossRef]
- Martínez-Rosas, M.E.; Garrafa-Gálvez, H.E.; Nava, O.; Murrieta-Rico, F.N.; Chinchillas-Chinchillas, M.J.; Carrillo-Castillo, A.; Luque, P.A. Electrochemical impedance characterization of ZnO semiconductor nanoparticles biosynthesized with Verbascum thapsus. J. Mater. Sci. Mater. Electron. 2021, 32, 10510–10519. [Google Scholar] [CrossRef]
- Garrafa-Gálvez, H.E.; Cardoza-Avendaño, L.; López-Gutiérrez, R.M.; Martínez-Rosas, M.E.; Murrieta-Rico, F.N.; Luque, P.A. Use of Tilia extract to improve the optical and electrochemical properties of ZnO semiconductor nanoparticles. J. Mater. Sci. Mater. Electron. 2023, 34, 14. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, L.; Li, Y.C.; Wycisk, R.J.; Pintauro, P.N.; Hickner, M.A.; Mallouk, T.E. The balance of electric field and interfacial catalysis in promoting water dissociation in bipolar membranes. Energy Environ. Sci. 2018, 11, 2235–2245. [Google Scholar] [CrossRef]
- Luque, P.A.; Nava, O.; Romo-Cardenas, G.; Nieto-Hipolito, J.I.; Vilchis-Nestor, A.R.; Valdez, K.; Sanchez-Lopez, J.D.D.; Murrieta-Rico, F.N. Facile Zinc Oxide Nanoparticle Green Synthesis Using Citrus reticulata Extract for Use in Optoelectronic Sensors. IEEE Sens. J. 2020, 21, 11275–11282. [Google Scholar] [CrossRef]






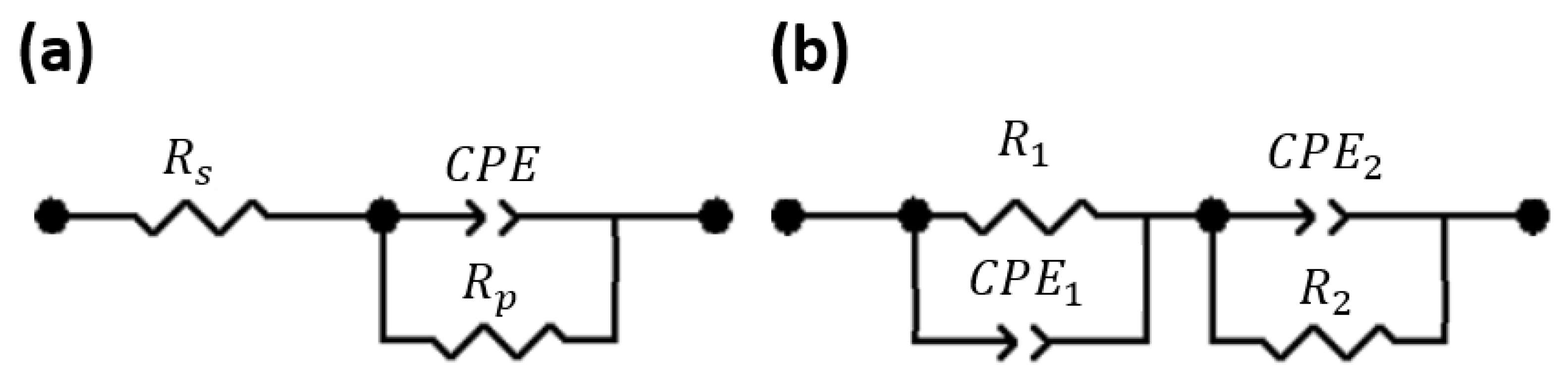

| Zeolite | (Ω) | (Ω) | (F) | |
|---|---|---|---|---|
| FAU | 1462 | 0.95398 | ||
| MFI | 0.90562 |
| R1 (Ω) | CPE1T | CPE1P | R2 (Ω) | CPE2T (F) | CPE2P |
|---|---|---|---|---|---|
| 57,776 | 1.023 × 10−10 | 0.90556 | 3.7769 × 106 | 5.68 × 10−10 | 0.81504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murrieta-Rico, F.N.; Antúnez-García, J.; Yocupicio-Gaxiola, R.I.; Reyes Serrato, A.; Petranovskii, V.; Xiao, M.; Sergiyenko, O.; Flores-Fuentes, W.; Rodríguez-Quiñonez, J.C. Frequency Response Analysis of FAU, LTA and MFI Zeolites Using UV-Vis and Electrochemical Impedance Spectroscopy. Optics 2023, 4, 459-472. https://doi.org/10.3390/opt4030033
Murrieta-Rico FN, Antúnez-García J, Yocupicio-Gaxiola RI, Reyes Serrato A, Petranovskii V, Xiao M, Sergiyenko O, Flores-Fuentes W, Rodríguez-Quiñonez JC. Frequency Response Analysis of FAU, LTA and MFI Zeolites Using UV-Vis and Electrochemical Impedance Spectroscopy. Optics. 2023; 4(3):459-472. https://doi.org/10.3390/opt4030033
Chicago/Turabian StyleMurrieta-Rico, Fabian N., Joel Antúnez-García, Rosario I. Yocupicio-Gaxiola, Armando Reyes Serrato, Vitalii Petranovskii, Mufei Xiao, Oleg Sergiyenko, Wendy Flores-Fuentes, and Julio C. Rodríguez-Quiñonez. 2023. "Frequency Response Analysis of FAU, LTA and MFI Zeolites Using UV-Vis and Electrochemical Impedance Spectroscopy" Optics 4, no. 3: 459-472. https://doi.org/10.3390/opt4030033
APA StyleMurrieta-Rico, F. N., Antúnez-García, J., Yocupicio-Gaxiola, R. I., Reyes Serrato, A., Petranovskii, V., Xiao, M., Sergiyenko, O., Flores-Fuentes, W., & Rodríguez-Quiñonez, J. C. (2023). Frequency Response Analysis of FAU, LTA and MFI Zeolites Using UV-Vis and Electrochemical Impedance Spectroscopy. Optics, 4(3), 459-472. https://doi.org/10.3390/opt4030033










