The Compressive Strength and Microstructure of Alkali-Activated Mortars Utilizing By-Product-Based Binary-Blended Precursors
Abstract
1. Introduction
1.1. Research Background
1.2. Literature Review
1.3. Motivation for the Research
1.4. Research Objective
2. Materials and Methods
2.1. Materials
2.1.1. Palm Oil Fuel Ash
2.1.2. Fly Ash
2.1.3. Ground Blast-Furnace Slag
2.1.4. Aggregates
2.1.5. Alkaline Activator
2.1.6. Methods for Determining Material Properties
2.2. Design of the Mixtures
2.2.1. Alkali
2.2.2. Analysis and Testing
3. Results and Discussion
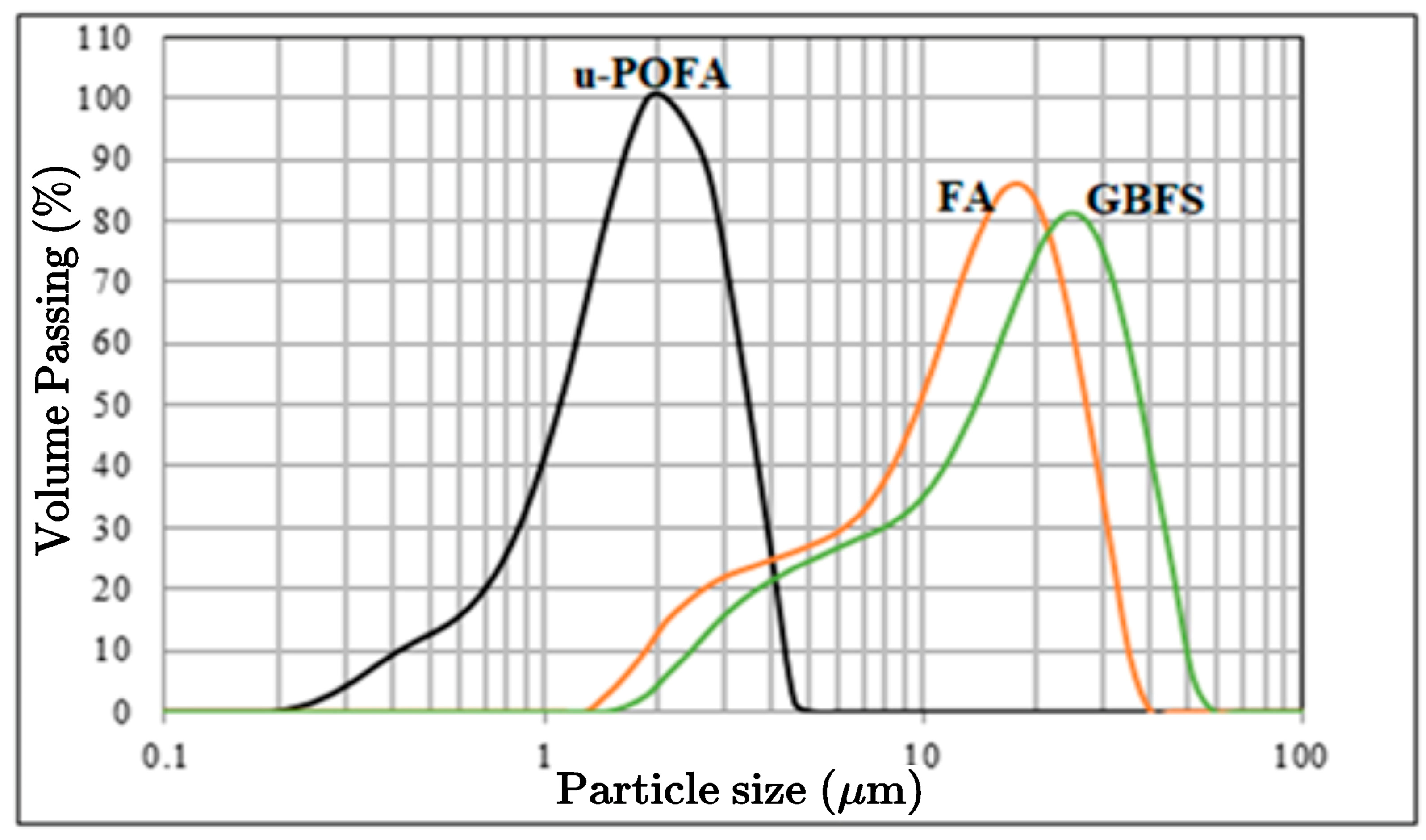
3.1. Characteristics of the Raw Materials
3.1.1. Physical and Chemical Properties of the Raw Materials
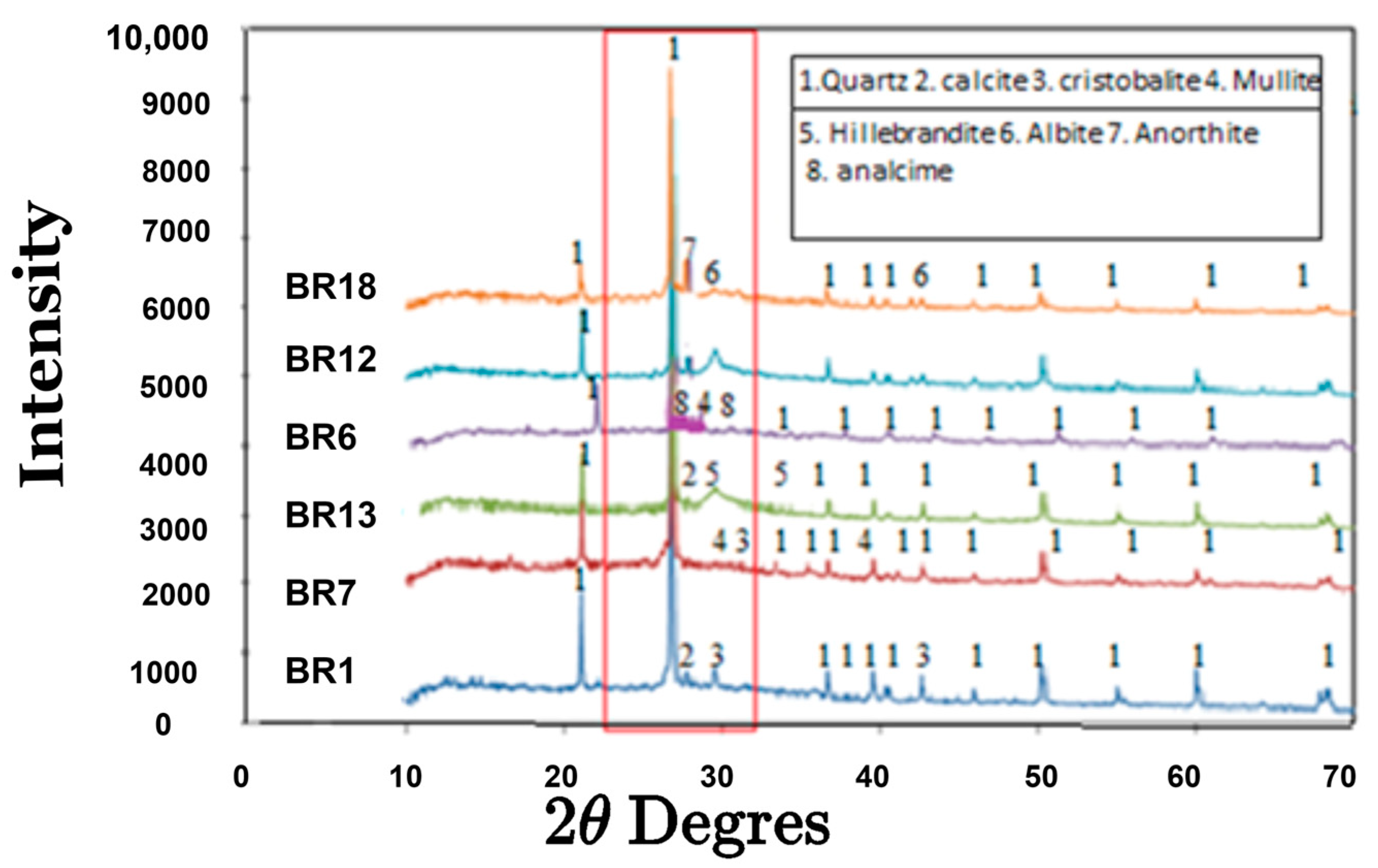
3.1.2. Mineralogy of the Raw Materials
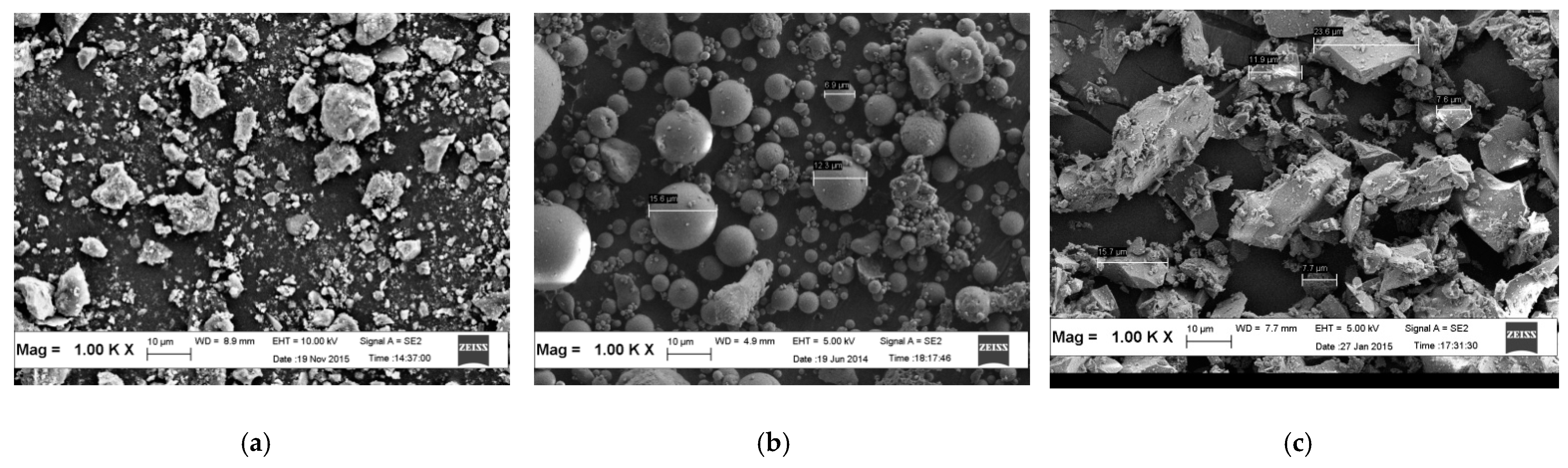
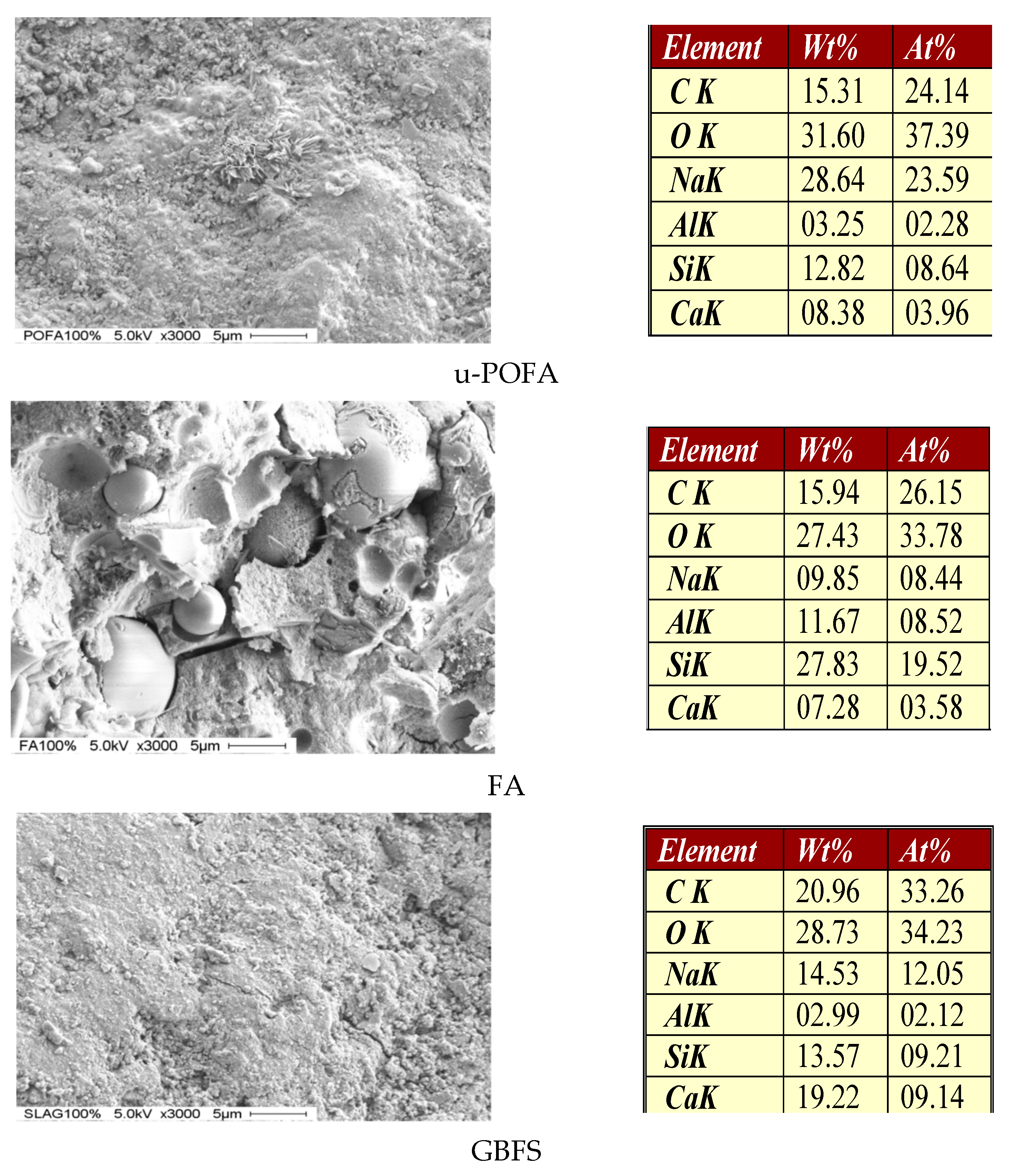
3.1.3. Particle Morphology of the Raw Materials
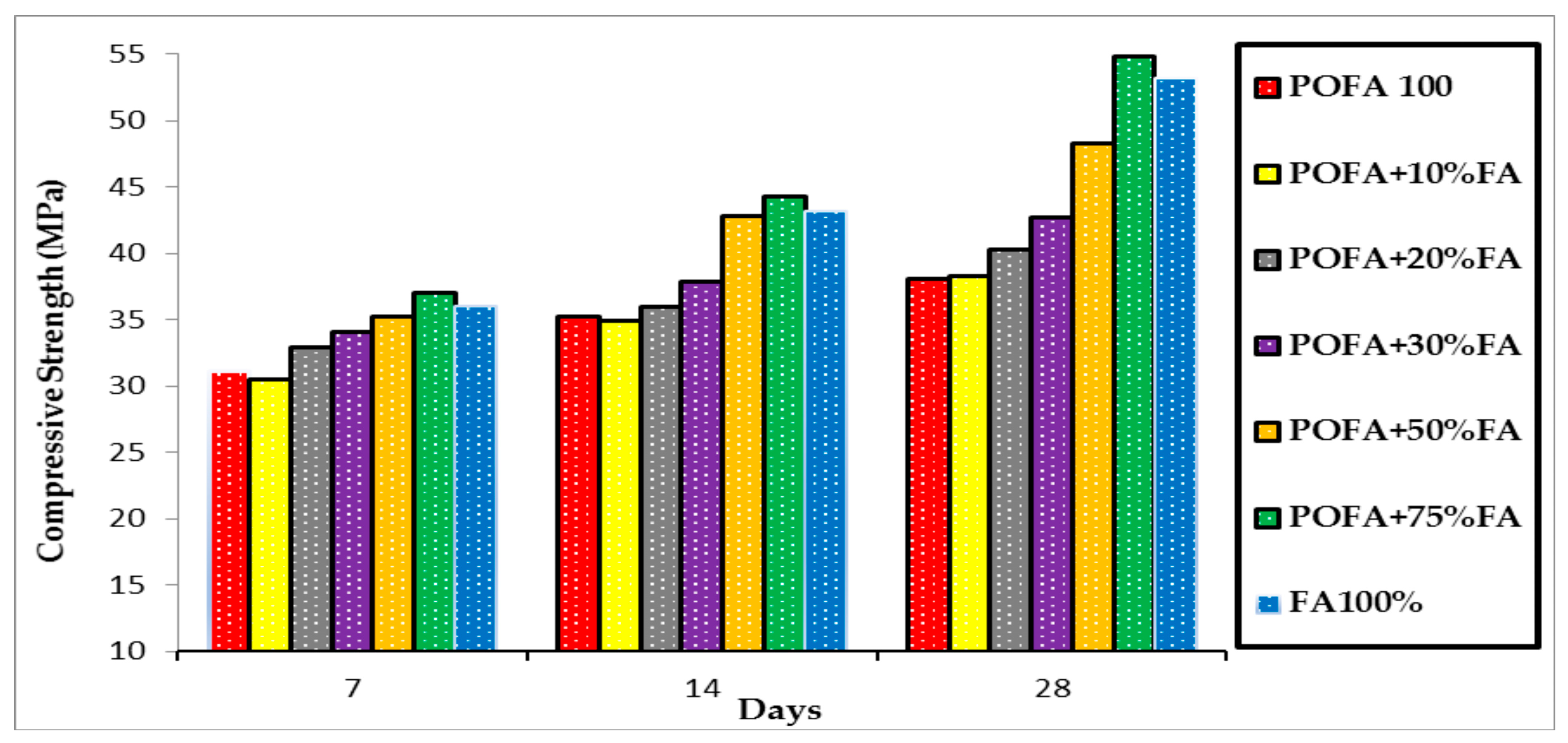
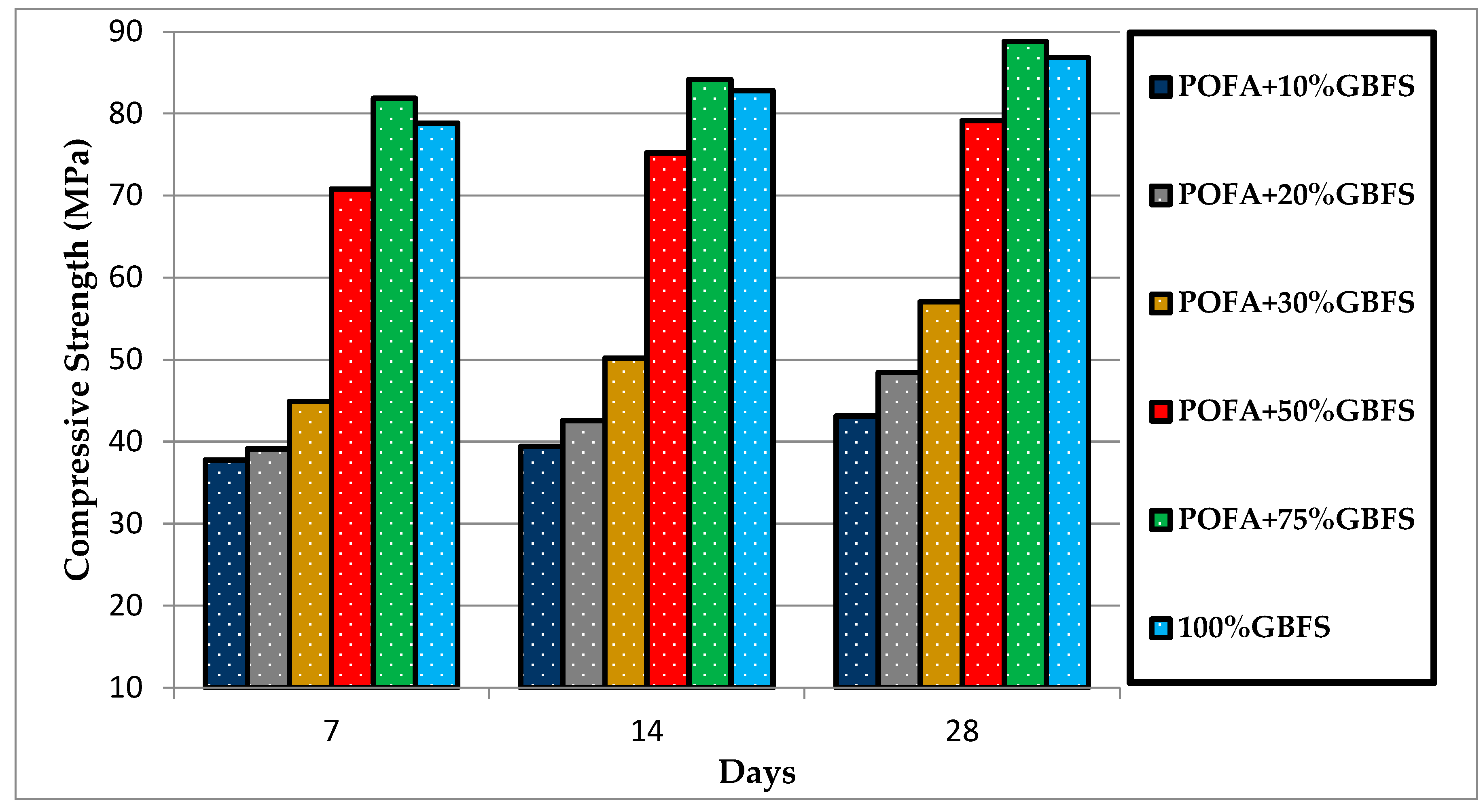
3.2. Compressive Strength
3.2.1. The Effects of FA on the u-POFA-based Mortars
3.2.2. The Effects of GBFS on u-POFA-Based Mortars
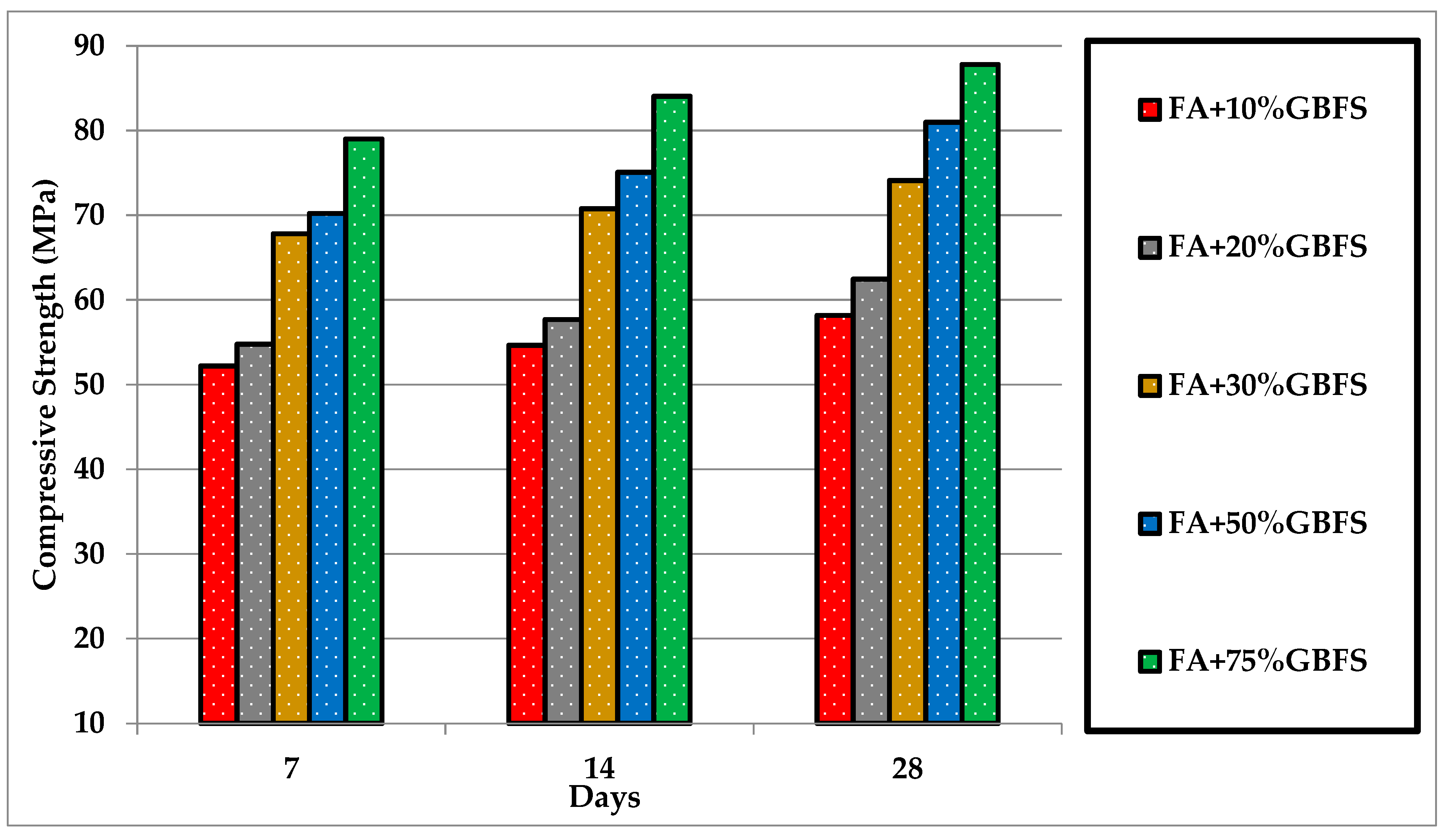
3.2.3. The Effects of GBFS on FA-Based Mortars
4. Characteristics of the Binary-Blended, Alkali-Activated Mortar Mixtures
4.1. Mineralogical Analysis
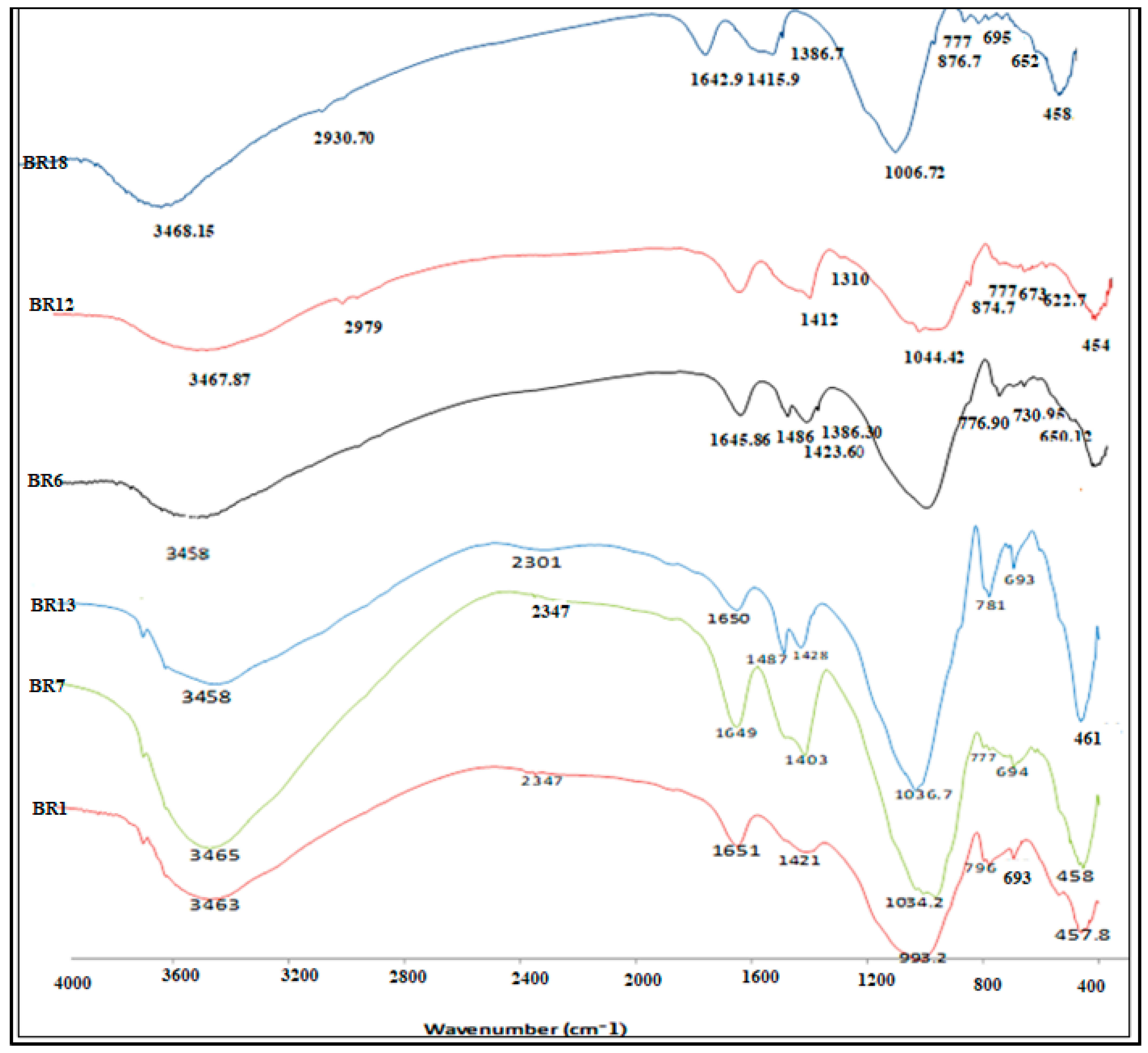
4.2. Fourier-Transform Infrared (FTIR) Spectroscopy
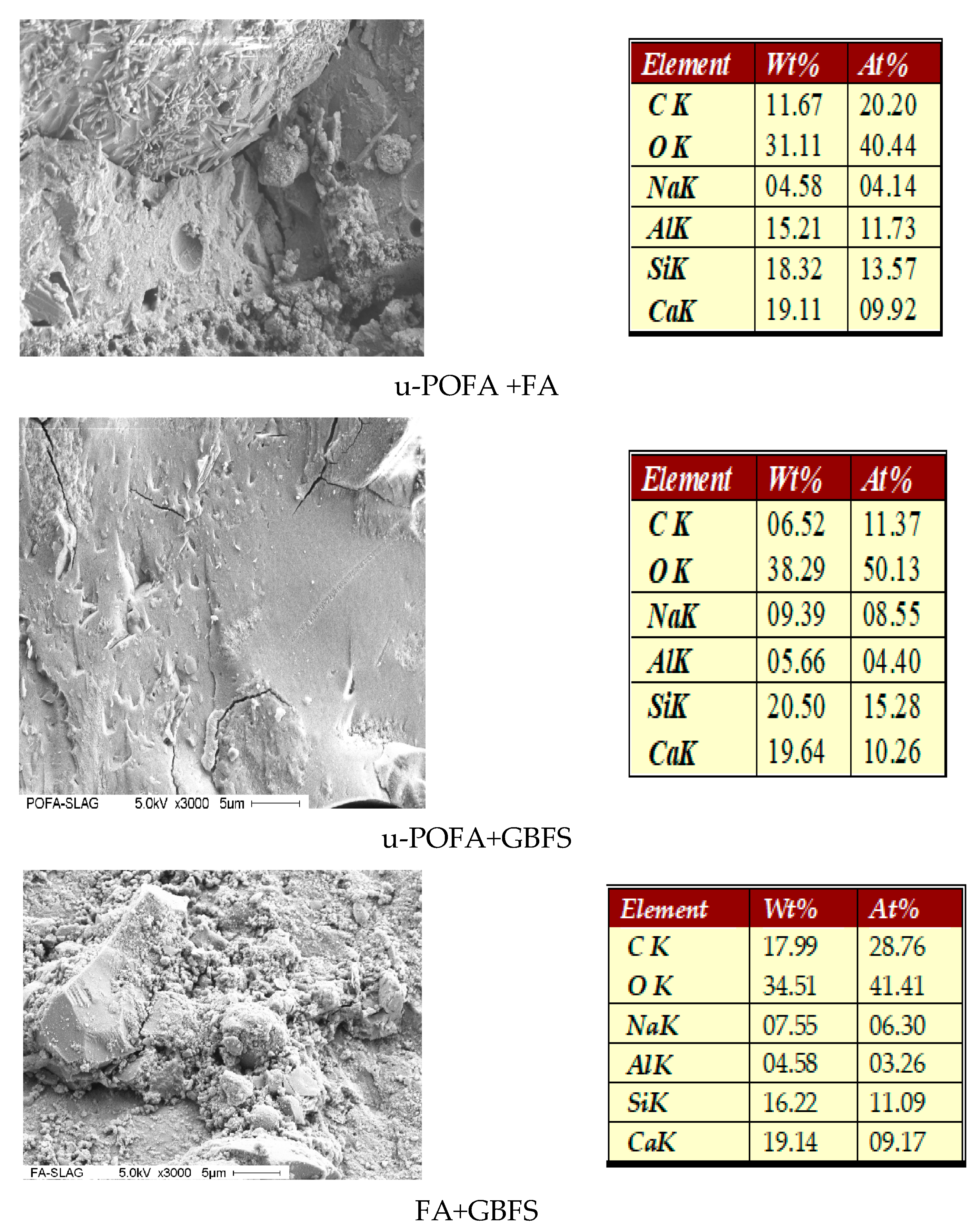
4.3. Field Emission Scanning Electron Microscopy (FESEM)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Matějková, P.; Matějka, V.; Sabovčík, T.; Gryžbon, L.; Vlček, J. Alkali Activation of Ground Granulated Blast Furnace Slag and Low Calcium Fly Ash Using “One-Part” Approach. J. Sustain. Metall. 2022, 8, 511–521. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Ahmed, H.U.; Al Dughaishi, H.; Al Lawati, J.; Sabri, M.M.; Alkhatib, F.; Milad, A. Fly Ash-Based Geopolymer Composites: A Review of the Compressive Strength and Microstructure Analysis. Materials 2022, 15, 7098. [Google Scholar] [CrossRef] [PubMed]
- Alawi, A.; Milad, A.; Barbieri, D.; Alosta, M.; Alaneme, G.U.; Imran Latif, Q.B.A. Eco-Friendly Geopolymer Composites Prepared from Agro-Industrial Wastes: A State-of-the-Art Review. CivilEng 2023, 4, 433–453. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Des. 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Arafa, S.; Milad, A.; Yusoff, N.I.M.; Al-Ansari, N.; Yaseen, Z.M. Investigation into the permeability and strength of pervious geopolymer concrete containing coated biomass aggregate material. J. Mater. Res. Technol. 2021, 15, 2075–2087. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The development of compressive strength of ground granulated blast furnace slag-palm oil fuel ash-fly ash based geopolymer mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of blast furnace slag grades on fly ash based geopolymer waste forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Alabduljabbar, H.; Huseien, G.F.; Sam, A.R.M.; Alyouef, R.; Algaifi, H.A.; Alaskar, A. Engineering Properties of Waste Sawdust-Based Lightweight Alkali-Activated Concrete: Experimental Assessment and Numerical Prediction. Materials 2020, 13, 5490. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Shrinkage and strength of alkaline activated ground steel slag/ultrafine palm oil fuel ash pastes and mortars. Mater. Des. 2014, 63, 710–718. [Google Scholar] [CrossRef]
- 109/C 109M-99; Standard Test Method for the Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens), Annual Book of ASTM, Standards, 4. ASTM: West Conshohocken, PN, USA, 1999.
- Yusuf, M.O.; Megat Johari, M.A.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, T.; Zhao, Y.; Bao, S. Preparation of eco-friendly construction bricks from hematite tailings. Constr. Build. Mater. 2011, 25, 2107–2111. [Google Scholar] [CrossRef]
- Elbasir, O.M.M.; Johari, M.A.M.; Ahmad, Z.A. Effect of fineness of palm oil fuel ash on compressive strength and microstructure of alkaline activated mortar. Eur. J. Environ. Civ. Eng. 2017, 23, 136–152. [Google Scholar] [CrossRef]
- Mehmannavaz, T.; Ismail, M.; Sumadi, S.R.; Bhutta, M.A.R.; Samadi, M.; Sajjadi, S.M. Binary Effect of Fly Ash and Palm Oil Fuel Ash on Heat of Hydration Aerated Concrete. Sci. World J. 2014, 2014, 461241. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Shen, J.; Wang, C.; Wu, H. Characterization of Sustainable Mortar Containing High-Quality Recycled Manufactured Sand Crushed from Recycled Coarse Aggregate. Cem. Concr. Compos. 2022, 132, 104629. [Google Scholar] [CrossRef]
- Megat Megat Johari, M.A.; Zeyad, A.M.; Muhamad Bunnori, N.; Ariffin, K.S. Engineering and transport properties of high-strength green concrete containing high volume of ultrafine palm oil fuel ash. Constr. Build. Mater. 2012, 30, 281–288. [Google Scholar] [CrossRef]
- Chandara, C.; Azizli, K.A.M.; Ahmad, Z.A.; Hashim, S.F.S.; Sakai, E. Analysis of mineralogical component of palm oil fuel ash with or without unburned carbon. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2011; Volume 173, pp. 7–11. [Google Scholar]
- ASTM C 2005 618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PN, USA, 2023.
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Supraja, V.; Rao, M.K. Experimental study on Geo-Polymer concrete incorporating GGBS. Int. J. Electron. Commun. Soft Comput. Sci. Eng. 2012, 2, 11. [Google Scholar]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A.; Macphee, D.E. Effect of calcium addtiton in N–A–S–H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar]
- Guneyisi, E.; Gesoglu, M. A study on durability properties of high-performance concretes incorporating high replacement levels of slag. Mater. Struct. 2008, 41, 479–493. [Google Scholar] [CrossRef]
- Amran, M.; Lee, Y.H.; Fediuk, R.; Murali, G.; Mosaberpanah, M.A.; Ozbakkaloglu, T.; Yong Lee, Y.; Vatin, N.; Klyuev, S.; Karelia, M. Palm Oil Fuel Ash-Based Eco-Friendly Concrete Composite: A Critical Review of the Long-Term Properties. Materials 2021, 14, 7074. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Influence of curing methods and concentration of NaOH on strength of the synthesized alkaline activated ground slag-ultrafine palm oil fuel ash mortar/concrete. Constr. Build. Mater. 2014, 66, 541–548. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Performance of different grades of palm oil fuel ash with ground slag as base materials in the synthesis of alkaline activated mortar. J. Adv. Concr. Technol. 2014, 12, 378–387. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S. Effect of sodium carbonate/sodium silicate activator on the rheology, geopolymerization and strength of fly ash/slag geopolymer pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar]
- Mijarsh, M.J.; Johari, M.M.; Ahmad, Z.A. Effect of delay time and Na2SiO3 concentrations on compressive strength development of geopolymer mortar synthesized from TPOFA. Constr. Build Mater. 2015, 1, 64–74. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Mijarsh, M.J.A.; Johari, M.A.M.; Ahmad, Z.A. Compressive strength of treated palm oil fuel ash based geopolymer mortar containing calcium hydroxide, aluminum hydroxide and silica fume as mineral additives. Cem. Concr. Compos. 2015, 60, 65–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative study of the reactivity of fly ash in geopolymerization by FTIR. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]

| Mix | Solid Material (kg) | Alkaline Activator | |||||||
|---|---|---|---|---|---|---|---|---|---|
| u-POFA | FA | GBFS | Sand | Na2SiO3 (kg) | 10 M NaOH (kg) | Water (kg) | Added Water (kg) | ||
| Mixture (u-POFA+FA) | |||||||||
| BR1 | POFA % | 0.856 | 0.00 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR 2 | 10% FA | 0.770 | 0.0856 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR 3 | 20% FA | 0.685 | 0.1712 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR 4 | 30% FA | 0.599 | 0.2567 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR 5 | 50% FA | 0.428 | 0.428 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR 6 | 75% FA | 0.214 | 0.6418 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| BR7 | 100% FA | 0.00 | 0.856 | 0.00 | 1.280 | 0.293 | 0.040 | 0.08 | 0.06 |
| Mixture (u-POFA+GBFS) | |||||||||
| BR8 | 10% GBFS | 0.755 | 0.00 | 0.0839 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR9 | 20% GBFS | 0.671 | 0.00 | 0.1678 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR10 | 30% GBFS | 0.587 | 0.00 | 0.2517 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR11 | 50% GBFS | 0.420 | 0.00 | 0.420 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR12 | 75% GBFS | 0.210 | 0.00 | 0.6294 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR13 | 100% GBFS | 0.00 | 0.00 | 0.8391 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| Mixture (FA+GBFS) | |||||||||
| BR14 | 10% GBFS | 0.00 | 0.755 | 0.0839 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR15 | 20% GBFS | 0.00 | 0.671 | 0.1678 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR16 | 30% GBFS | 0.00 | 0.587 | 0.2517 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR17 | 50% GBFS | 0.00 | 0.420 | 0.420 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| BR18 | 75% GBFS | 0.00 | 0.210 | 0.6294 | 1.260 | 0.312 | 0.04 | 0.09 | 0.06 |
| Oxides (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | P2O5 | K2O | SO3 | TiO2 | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| u-POFA | 64.595 | 5.851 | 4.737 | 9.293 | 3.130 | 5.198 | 5.219 | 0.471 | 0.216 | 0.054 | 2.50 |
| FA | 49.053 | 23.516 | 6.422 | 5.080 | 0.698 | 1.018 | 1.309 | 0.475 | 1.121 | 0.2102 | 2.130 |
| GBFS | 36.83 | 14.44 | 0.396 | 39.35 | 3.592 | 0.0191 | 0.3761 | 4.207 | 0.402 | 0.0593 | 0.601 |
| Mix No. | Mix Ratio (%) | SiO2/Al2O3 | SiO2/CaO | |
|---|---|---|---|---|
| BR1 | 100% POFA | 100:00 | 11.03 | 6.95 |
| BR6 | 25% POFA, 75% FA | 25:75 | 2.77 | 8.62 |
| BR12 | 25% POFA, 75% GBFS | 25:75 | 3.56 | 1.376 |
| BR7 | 100% FA | 100:00 | 2.08 | 9.65 |
| BR18 | 25% FA, 75% GBFS | 25:75 | 2.5 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbasir, O.M.M.; Johari, M.A.M.; Ahmad, Z.A.; Mashaan, N.S.; Milad, A. The Compressive Strength and Microstructure of Alkali-Activated Mortars Utilizing By-Product-Based Binary-Blended Precursors. Appl. Mech. 2023, 4, 885-898. https://doi.org/10.3390/applmech4030046
Elbasir OMM, Johari MAM, Ahmad ZA, Mashaan NS, Milad A. The Compressive Strength and Microstructure of Alkali-Activated Mortars Utilizing By-Product-Based Binary-Blended Precursors. Applied Mechanics. 2023; 4(3):885-898. https://doi.org/10.3390/applmech4030046
Chicago/Turabian StyleElbasir, Otman M. M., Megat Azmi Megat Johari, Zainal Arifin Ahmad, Nuha S. Mashaan, and Abdalrhman Milad. 2023. "The Compressive Strength and Microstructure of Alkali-Activated Mortars Utilizing By-Product-Based Binary-Blended Precursors" Applied Mechanics 4, no. 3: 885-898. https://doi.org/10.3390/applmech4030046
APA StyleElbasir, O. M. M., Johari, M. A. M., Ahmad, Z. A., Mashaan, N. S., & Milad, A. (2023). The Compressive Strength and Microstructure of Alkali-Activated Mortars Utilizing By-Product-Based Binary-Blended Precursors. Applied Mechanics, 4(3), 885-898. https://doi.org/10.3390/applmech4030046








