Enhancing Burn Recovery: A Systematic Review on the Benefits of Electrical Stimulation in Accelerating Healing
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Assessment of Methodological Quality
2.3. Data Extraction
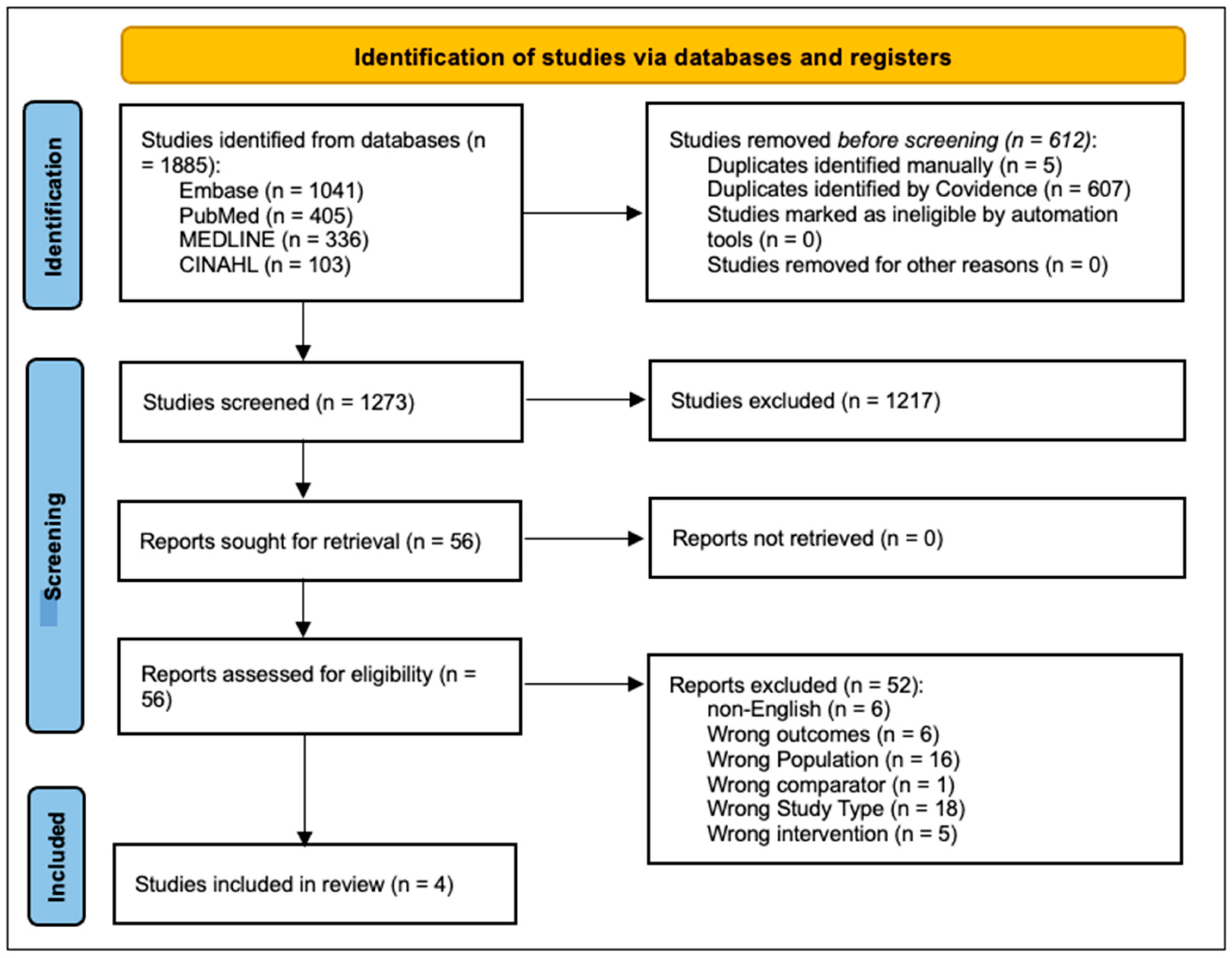
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Effects of Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy
- exp Burns/
- (burns OR burn OR burnt OR burned). ab or (burns OR burn OR burnt OR burned). Ti. Or (burns OR burn OR burnt OR burned).kw.
- 1 OR 2
- exp Electric Stimulation/
- exp Electric Stimulation Therapy/
- therapeutic electrical stimulation af.
- functional electrical stimulation.af.
- (electric* stimulation or e-stim).af.
- electrotherapy.af.
- electric stimulation therapy.af.
- Neuromuscular electrical stimulation.af.
- Transcutaneous electrical nerve stimulation.af.
- Electrophysical agent*.af.
- 4 or 5 or 6 or 7 or 8 or 9 or 10 or 10 or 11 or 12 or 13
- 3 and 14
- S14. S3 and S13
- S13. S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12
- S12. Electrophysical agent*
- S11. Transcutaneous electrical nerve stimulation
- S10. Neuromuscular electrical stimulation
- S9. Electric stimulation therapy
- S8. Electrotherapy
- S7. Electric* stimulation OR e-stim
- S6. Functional electrical stimulation
- S5. TX therapeutic electrical stimulation
- S4. (MH “Electric Stimulation+”)
- S3. S1 or S2
- S2. TI (burns OR burn OR burnt OR burned) OR AB (burns OR burn OR burnt OR burned)
- S1. (MH “Burns+”)
References
- Datta, P.K.; Roy Chowdhury, S.; Aravindan, A.; Saha, S.; Rapaka, S. Medical and Surgical Care of Critical Burn Patients: A Comprehensive Review of Current Evidence and Practice. Cureus 2022, 14, e31550. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Kornhaber, R.; Rickard, G.; McLean, L.; Wiechula, R.; Lopez, V.; Cleary, M. Burn care and rehabilitation in Australia: Health professionals’ perspectives. Disabil. Rehabil. 2019, 41, 714–719. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- Spronk, I.; Wood, F.M.; Fear, M.W.; Lansdorp, C.A.; Edgar, D.W. The Short- and Long-Term Outcome Priorities of a Western Australian Adult Burn Population. J. Burn Care Res. 2024, 45, 451–458. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Jackson, D.M. The diagnosis of the depth of burning. J. Br. Surg. 1953, 40, 588–596. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, F.; Xie, H.; Chi, Z.; Liu, C. Conductive Hyaluronic Acid/Deep Eutectic Solvent Composite Hydrogel as a Wound Dressing for Promoting Skin Burn Healing Under Electrical Stimulation. Adv. Healthc. Mater. 2024, 13, 2304117. [Google Scholar] [CrossRef]
- Wurzer, P.; Culnan, D.; Cancio, L.C.; Kramer, G.C. Pathophysiology of Burn Shock and Burn Edema. In Total Burn Care; Elsevier: Philadelphia, PA, USA, 2018; pp. 66–76.e3. [Google Scholar]
- Finlay, V.; Burrows, S.; Burmaz, M.; Yawary, H.; Lee, J.; Edgar, D.W.; Wood, F.M. Increased burn healing time is associated with higher Vancouver Scar Scale score. Scars Burn. Heal. 2017, 3, 2059513117696324. [Google Scholar] [CrossRef]
- Kohlhauser, M.; Tuca, A.; Kamolz, L.-P. The efficacy of adipose-derived stem cells in burn injuries: A systematic review. Cell. Mol. Biol. Lett. 2024, 29, 10. [Google Scholar] [CrossRef]
- Almodumeegh, A.S.; AlKhudair, M.R.; Altammami, A.F.; Alsuhaim, R.H.; Alhumaidan, A.I.; Alothman, A.M. Patient Satisfaction After Conservative Treatment for Burn Scars in Saudi Arabia. Cureus 2022, 14, e21896. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.G.; Cramer, M.N.; Kowalske, K.J. Edward F. Adolph Distinguished Lecture. It’s more than skin deep: Thermoregulatory and cardiovascular consequences of severe burn injuries in humans. J. Appl. Physiol. 2021, 131, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.B.; Geenen, R.; Egberts, M.R.; Wanders, H.; Van Loey, N.E. Patients’ perspectives on quality of life after burn. Burns 2017, 43, 747–756. [Google Scholar] [CrossRef]
- Kurz, P.; Danner, G.; Lembelembe, J.P.; Nair, H.K.R.; Martin, R. Activation of healing and reduction of pain by single-use automated microcurrent electrical stimulation therapy in patients with hard-to-heal wounds. Int. Wound J. 2023, 20, 2053–2061. [Google Scholar] [CrossRef]
- Wiechman, S.; Saxe, G.; Fauerbach, J.A. Psychological Outcomes Following Burn Injuries. J. Burn Care Res. 2017, 38, e629–e631. [Google Scholar] [CrossRef]
- Meirte, J.; Van Loey, N.E.E.; Maertens, K.; Moortgat, P.; Hubens, G.; Van Daele, U. Classification of quality of life subscales within the ICF framework in burn research: Identifying overlaps and gaps. Burns 2014, 40, 1353–1359. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn wound healing: Clinical complications, medical care, treatment, and dressing types: The current state of knowledge for clinical practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Braddock, M.; Campbell, C.J.; Zuder, D. Current therapies for wound healing: Electrical stimulation, biological therapeutics, and the potential for gene therapy. Int. J. Dermatol. 1999, 38, 808–817. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef]
- Jaffe, L.F.; Vanable, J.W. Electric fields and wound healing. Clin. Dermatol. 1984, 2, 34–44. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Yang, J.; Liu, J.; Zhang, J. Electric Field: A Key Signal in Wound Healing. Chin. J. Plast. Reconstr. Surg. 2021, 3, 95–102. [Google Scholar] [CrossRef]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Farber, P.L.; Isoldi, F.C.; Ferreira, L.M. Electric Factors in Wound Healing. Adv. Wound Care 2021, 10, 461–476. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef]
- Avendaño-Coy, J.; López-Muñoz, P.; Serrano-Muñoz, D.; Comino-Suárez, N.; Avendaño-López, C.; Martin-Espinosa, N. Electrical microcurrent stimulation therapy for wound healing: A meta-analysis of randomized clinical trials. J. Tissue Viability 2022, 31, 268–277. [Google Scholar] [CrossRef]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081. [Google Scholar] [CrossRef]
- Johnson, M.I. Resolving long-standing uncertainty about the clinical efficacy of transcutaneous electrical nerve stimulation (TENS) to relieve pain: A comprehensive review of factors influencing outcome. Medicina 2021, 57, 378. [Google Scholar] [CrossRef]
- Wood, F.M. The evolution of burn care research since the Bali bombing in 2002. Australas. J. Plast. Surg. 2023, 6, 1–5. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis Is Induced and Wound Size Is Reduced by Electrical Stimulation in an Acute Wound Healing Model in Human Skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G. Bacterial Inhibition by Electrical Stimulation. Adv. Wound Care 2014, 3, 91–97. [Google Scholar] [CrossRef]
- Wang, K.; Parekh, U.; Ting, J.K.; Yamamoto, N.A.; Zhu, J.; Costantini, T.; Arias, A.C.; Eliceiri, B.P.; Ng, T.N. A platform to study the effects of electrical stimulation on immune cell activation during wound healing. Adv. Biosyst. 2019, 3, 1900106. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Buyong, M.R.; Mohd Yunus, M.H. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Urabe, H.; Akimoto, R.; Kamiya, S.; Hosoki, K.; Ichikawa, H.; Nishiyama, T. Effects of pulsed electrical stimulation on growth factor gene expression and proliferation in human dermal fibroblasts. Mol. Cell. Biochem. 2021, 476, 361–368. [Google Scholar] [CrossRef]
- Reid, B.; Zhao, M. The electrical response to injury: Molecular mechanisms and wound healing. Adv. Wound Care 2014, 3, 184–201. [Google Scholar] [CrossRef]
- Foulds, I.; Barker, A. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef]
- Chang, H.-F.; Lee, Y.-S.; Tang, T.K.; Cheng, J.-Y. Pulsed DC electric field–induced differentiation of cortical neural precursor cells. PLoS ONE 2016, 11, e0158133. [Google Scholar] [CrossRef]
- Hammerick, K.E.; Longaker, M.T.; Prinz, F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010, 397, 12–17. [Google Scholar] [CrossRef]
- Banerjee, J.; Das Ghatak, P.; Roy, S.; Khanna, S.; Sequin, E.K.; Bellman, K.; Dickinson, B.C.; Suri, P.; Subramaniam, V.V.; Chang, C.J.; et al. Improvement of Human Keratinocyte Migration by a Redox Active Bioelectric Dressing. PLoS ONE 2014, 9, e89239. [Google Scholar] [CrossRef] [PubMed]
- Poltawski, L.; Watson, T. Bioelectricity and microcurrent therapy for tissue healing—A narrative review. Phys. Ther. Rev. 2009, 14, 104–114. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21(3), 494–506. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.K.; Nuutila, K.; Mathew-Steiner, S.S.; Diaz, V.; Anselmo, K.; Batchinsky, M.; Carlsson, A.; Ghosh, N.; Sen, C.K.; Roy, S. A Prospective, Randomized, Controlled Study to Evaluate the Effectiveness of a Fabric-Based Wireless Electroceutical Dressing Compared to Standard-of-Care Treatment Against Acute Trauma and Burn Wound Biofilm Infection. Adv. Wound Care 2024, 13, 1–13. [Google Scholar] [CrossRef]
- Edwick, D.O.; Hince, D.A.; Rawlins, J.M.; Wood, F.M.; Edgar, D.W. Does electrical stimulation improve healing in acute minor burn injury, as measured by bioimpedance spectroscopy? A single center, randomized, controlled trial. Burn. Open 2022, 6, 42–50. [Google Scholar] [CrossRef]
- Huckfeldt, R.; Flick, A.B.; Mikkelson, D.; Lowe, C.; Finley, P.J. Wound Closure After Split-Thickness Skin Grafting Is Accelerated with the Use of Continuous Direct Anodal Microcurrent Applied to Silver Nylon Wound Contact Dressings. J. Burn Care Res. 2007, 28, 703–707. [Google Scholar] [CrossRef]
- Ibrahim, Z.M.; Waked, I.S.; Ibrahim, O. Negative pressure wound therapy versus microcurrent electrical stimulation in wound healing in burns. J. Wound Care 2019, 28, 214–219. [Google Scholar] [CrossRef]
- Onuh, O.C.; Brydges, H.T.; Nasr, H.; Savage, E.; Gorenstein, S.; Chiu, E. Capturing essentials in wound photography past, present, and future: A proposed algorithm for standardization. Adv. Skin Wound Care 2022, 35, 483–492. [Google Scholar] [CrossRef]
- Antoszewska, M.; Spychalski, P.; Kekonen, A.; Viik, J.; Barańska-Rybak, W. Bioimpedance sensor array for monitoring chronic wounds: Validation of method feasibility. Int. Wound J. 2024, 21, e14899. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Moore, M. Bioelectrical impedance assessment of wound healing. J. Diabetes Sci. Technol. 2012, 6, 209–212. [Google Scholar] [CrossRef]
- Zhao, S.; Mehta, A.S.; Zhao, M. Biomedical applications of electrical stimulation. Cell. Mol. Life Sci. 2020, 77, 2681–2699. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Rahman, E.; Powner, M.B.; Triantis, I.F. Making Sense of Electrical Stimulation: A Meta-analysis for Wound Healing. Ann. Biomed. Eng. 2024, 52, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Sinha, M.; Kapoor, A.; Kumar, M.; Singh, K.; Mathew-Steiner, S.S.; Sen, C.K. Deficient functional wound closure as measured by elevated trans-epidermal water loss predicts chronic wound recurrence: An exploratory observational study. Sci. Rep. 2024, 14, 23593. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, C.L.; Buro, B.L.; Hopkins, K.M.; Eiland, J.E. The impact of continuous electrical microcurrent on acute and hard-to-heal wounds: A systematic review. J. Wound Care 2020, 29 (Suppl. S7), S6–S15. [Google Scholar] [CrossRef]
- Tsolakidis, S.; Rosenauer, R.; Schmidhammer, R.; Pallua, N.; Rennekampff, H.O. Wireless microcurrent stimulation improves blood flow in burn wounds. Burns 2022, 48, 1230–1235. [Google Scholar] [CrossRef]
- Koh, K.; Lim, G.J.S.; Por, Y.C.; Mok, W.L.J. Wireless Micro Current Stimulation (WMCS) therapy to enhance burn wound healing: A randomized clinical trial. Burns 2025, 51, 107286. [Google Scholar] [CrossRef]

| Author/Year Country | Study Design | N | Age (Mean + Range) | M/F Ratio (%) | Baseline Burn Size | Burn Type | Aim | Treatment Previous to Intervention | Routine Care |
|---|---|---|---|---|---|---|---|---|---|
| Chan et al. (2024) U.S.A. [42] | RCT | 38 | Mean 45 23–84 | 76.32/23.68 | 1 × Burns wounds ≥ 300 cm2, or 2 × Burns wounds ≥ 150 cm2 | 29 thermal 5 electrical 1 chemical 1 explosion/blast 1 degloving | To evaluate the effect of wireless electroceutical dressing (WED) compared with routine dressings to reduce biofilm on burn wounds. | Treatments deemed necessary which included debridement, negative pressure wound therapy (NPWT), and skin grafting. | Standard care included but was not limited to silver nylon, SSD ointment, bacitracin, xeroform, 5% sulfamylon solution, and Manuka honey. |
| Edwick et al. (2022) Australia [43] | RCT | 30 | Median 32.5 18–72 | 80/20 | 0.1–5.5% TBSA | 5 scalds 5 contact 14 flash/flame 2 friction 3 chemical 1 radiation | To measure wound healing in acute minor burn injuries using Bioimpedance Spectroscopy (BIS), where electrical stimulation was used as a treatment to reduce the presence of edema. | Surgical intervention was required in 20 of the patients, including dermabrasion and ReCell (n =11), and a combination of split-thickness skin graft ±ReCell (n =9). All wounds were debrided and equivalent surgery technique was applied to each wound of the paired study wounds. | Patients received proactive oedema treatments from their therapists, which including exercise and compression therapies, and education and assistance with positioning for elevation of affected limbs, in addition to routine dressing changes. |
| Huckfeldt et al. (2007) U.S.A. [44] | RCT | 30 | Mean 40 18–68 | Not Specified | Not Specified | 30 thermal | To test the effect of continuous direct anodal microcurrent on wound closure time when applied to silver nylon wound contact dressings after split-thickness skin grafting. | Split-thickness skin grafting. | The control group were dressed at the graft site using silver nylon fabric moistened with sterile water in direct contact with the wound surface and covered with gauze and an elastic bandage. |
| Ibrahim et al. (2019) Egypt [45] | RCT | 45 | Mean 26.63 20–40 | 53.33/46.67 | 31.26% TBSA | 25 flame 20 scalds | To compare the efficacy of negative pressure wound therapy (NPWT) with that of microcurrent electrical stimulation (MES) on wound surface area, length of stay (LoS) and colony count of wounds in patients with burns. | N/A | The control group (as well as the NPWT and MES groups) received standard medical wound care (wound dressing, nursing care and pain relief medication) and a routine rehabilitation programme (range of motion exercises, ambulation training, and positioning and stretching exercises) throughout the study period. |
| Author | Type of Electric Stimulation | Intensity Current (µA) OR Voltage (V) | Frequency (Hz) | Pulse Duration (μs) | Duration (Per Session) | Time (Days) | Outcome Measure | Key Results |
|---|---|---|---|---|---|---|---|---|
| Chan et al. (2024) [42] | Wireless Electrical Dressing (WED) Polyester dressing with a matrix of elemental silver and zinc nano particles that generate a weak electrical field on contact with a conductive medium (e.g., hydrogel) | ~1 V | N/A | N/A | 24 h/day | 7 days |
| WED did not significantly impact the long-term outcome of wound healing. |
| Edwick et al. (2022) [43] | Electrical stimulation delivered via Bio-Flex stimulation electrodes (ActivMed stimulation device) on intact skin—one inferior and one superior to wound | 12–30 V | 6–12 Hz | 200 μs | >20 h/day (recommended by device manufacturer) | 10–14 days |
| No significant difference (p = 0.371) in time to heal (days) between control group and stimulation group. |
| Huckfeldt et al. (2007) [44] | Moistened silver nylon fabric covered with gauze with addition of continuous direct anodal microcurrent application | 5.0 V 50–100 μA | direct anodic microcurrent | constant | 24 h/day | until 95% wound closure | Time to 95% wound closure was measured using digital photography. | The study group experienced a 36% reduction in time to wound closure (mean of 4.6 days) as compared to the control group (mean of 7.2 days), (p < 0.05) |
| Ibrahim et al. (2019) [45] | Microcurrent Electrical Stimulation (MES) delivered through a modified square biphasic pulsed waveform | 300 μA | 10 Hz | constant | 1 h/day | 3 days a week for 3 weeks (21 days), or until wound closure |
|
|
| Author | Outcome | Outcome Measure | Key Results |
|---|---|---|---|
| Chan et al. (2024) [42] | Biofilm and bacterial infection | Scanning electron microscopy (SEM) quantification imaging. SEM biofilm grading used a 0–3 scale. | WED significantly decreased biofilm by 72% compared with 48% decrease from SoC treatment. WED decreased infection by 62% compared with 52% from standard of care treatment. Non-grafted burn wounds had significantly lower levels of biofilm detected in WED treated wounds than SoC. |
| Edwick et al. (2022) [43] | Evaluate BIS raw variables as a valid measure for edema and wound responses. | Repeated serial BIS was used to measure wound healing using the following 4 measures.
| BIS can be interpreted as a direct physiological measure of cellular architecture and function (tissue and cellular health)
|
| Huckfeldt et al. (2007) [44] | N/A | N/A | N/A |
| Ibrahim et al. (2019) [45] | Infection | Bacterial Count (Colonies) | At day 21, both the NPWT and MES groups had a statistically significant lower mean bacterial count while the control group revealed a statistically significant higher mean bacterial count. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwick, D.O.; Burns, K.L.; Buonvecchi, L.N.; Wang, X.; Lim, A.M.; Edgar, D.W. Enhancing Burn Recovery: A Systematic Review on the Benefits of Electrical Stimulation in Accelerating Healing. Eur. Burn J. 2025, 6, 21. https://doi.org/10.3390/ebj6020021
Edwick DO, Burns KL, Buonvecchi LN, Wang X, Lim AM, Edgar DW. Enhancing Burn Recovery: A Systematic Review on the Benefits of Electrical Stimulation in Accelerating Healing. European Burn Journal. 2025; 6(2):21. https://doi.org/10.3390/ebj6020021
Chicago/Turabian StyleEdwick, Dale O., Kerry L. Burns, Lara N. Buonvecchi, Xiaolu Wang, Audrey M. Lim, and Dale W. Edgar. 2025. "Enhancing Burn Recovery: A Systematic Review on the Benefits of Electrical Stimulation in Accelerating Healing" European Burn Journal 6, no. 2: 21. https://doi.org/10.3390/ebj6020021
APA StyleEdwick, D. O., Burns, K. L., Buonvecchi, L. N., Wang, X., Lim, A. M., & Edgar, D. W. (2025). Enhancing Burn Recovery: A Systematic Review on the Benefits of Electrical Stimulation in Accelerating Healing. European Burn Journal, 6(2), 21. https://doi.org/10.3390/ebj6020021








