Administration Methods of Mesenchymal Stem Cells in the Treatment of Burn Wounds
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Searches
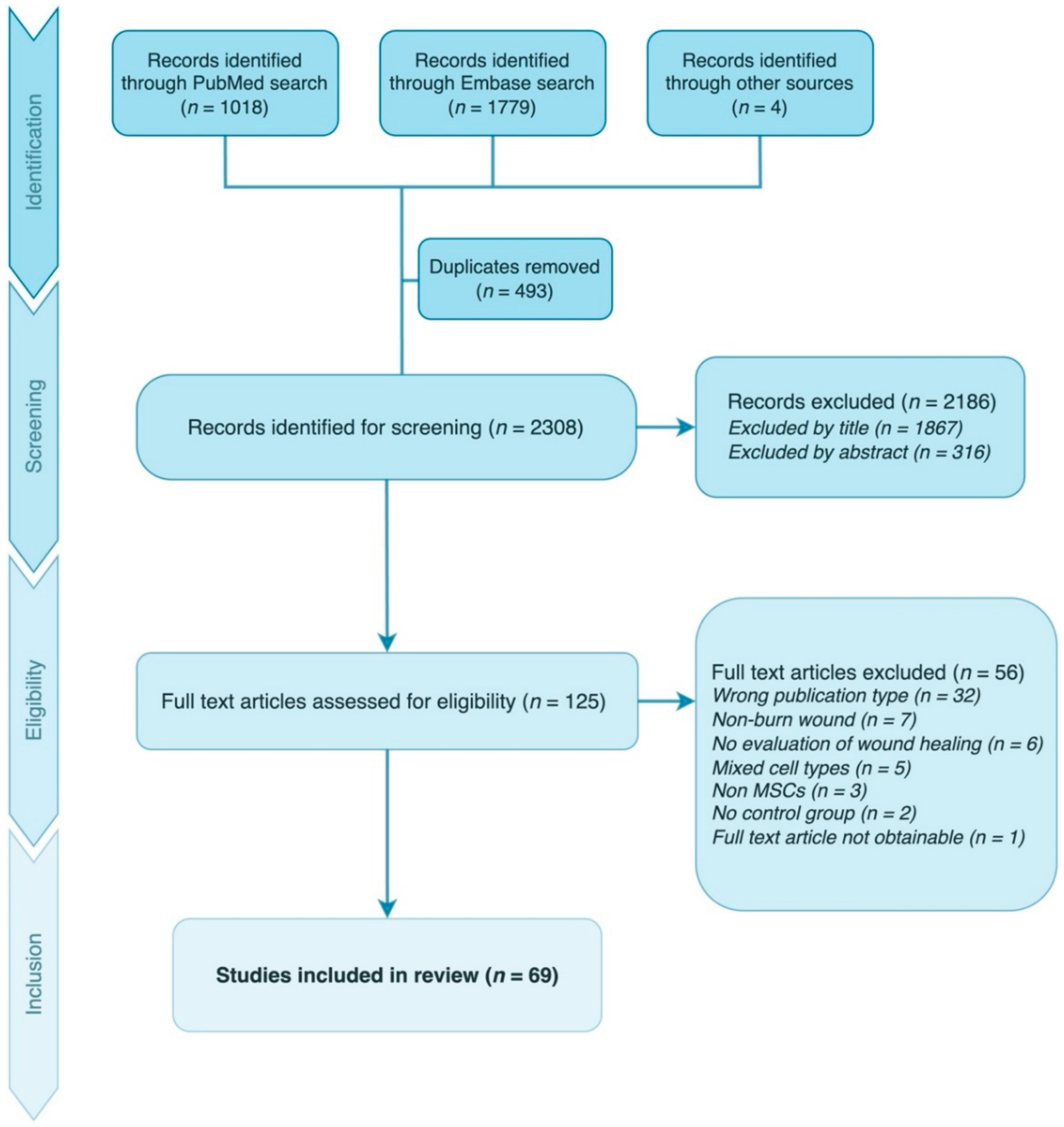
2.2. Study Selection
3. Results
3.1. Study Characteristics
3.2. Clinical Studies
3.3. Preclinical Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esselman, P.C.; Thombs, B.D.; Magyar-Russell, G.; Fauerbach, J.A. Burn Rehabilitation: State of the Science. Am. J. Phys. Med. Rehabil. 2006, 85, 383–413. [Google Scholar] [CrossRef]
- Onarheim, H.; Jensen, S.A.; Rosenberg, B.E.; Guttormsen, A.B. The Epidemiology of Patients with Burn Injuries Admitted to Norwegian Hospitals in 2007. Burns J. Int. Soc. Burn Inj. 2009, 35, 1142–1146. [Google Scholar] [CrossRef]
- Hop, M.J.; Polinder, S.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Costs of Burn Care: A Systematic Review. Wound Repair Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef]
- Williams, E.E.; Griffiths, T.A. Psychological Consequences of Burn Injury. Burns 1991, 17, 478–480. [Google Scholar] [CrossRef]
- Goverman, J.; Mathews, K.; Nadler, D.; Henderson, E.; McMullen, K.; Herndon, D.; Meyer, W.; Fauerbach, J.A.; Wiechman, S.; Carrougher, G.; et al. Satisfaction with Life after Burn: A Burn Model System National Database Study. Burns 2016, 42, 1067–1073. [Google Scholar] [CrossRef]
- Capek, K.D.; Sousse, L.E.; Hundeshagen, G.; Voigt, C.D.; Suman, O.E.; Finnerty, C.C.; Jennings, K.; Herndon, D.N. Contemporary Burn Survival. J. Am. Coll. Surg. 2018, 226, 453–463. [Google Scholar] [CrossRef]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef]
- Holmes IV, J.H.; Molnar, J.A.; Carter, J.E.; Hwang, J.; Cairns, B.A.; King, B.T.; Smith, D.J.; Cruse, C.W.; Foster, K.N.; Peck, M.D.; et al. A Comparative Study of the ReCell® Device and Autologous Split-Thickness Meshed Skin Graft in the Treatment of Acute Burn Injuries. J. Burn Care Res. 2018, 39, 694–702. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in Creating Skin Substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472. [Google Scholar] [CrossRef]
- Rangatchew, F.; Vester-Glowinski, P.; Rasmussen, B.S.; Haastrup, E.; Munthe-Fog, L.; Talman, M.-L.; Bonde, C.; Drzewiecki, K.T.; Fischer-Nielsen, A.; Holmgaard, R. Mesenchymal Stem Cell Therapy of Acute Thermal Burns: A Systematic Review of the Effect on Inflammation and Wound Healing. Burns J. Int. Soc. Burn Inj. 2020, 47, 270–294. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, E.; Spretz, R.; Larsen, G.; Nuñez, L.; Drago, H.; Sturla, F.; Marin, G.H.; Roque, G.; Martire, K.; Díaz Aquino, V.; et al. Outstanding Survival and Regeneration Process by the Use of Intelligent Acellular Dermal Matrices and Mesenchymal Stem Cells in a Burn Pig Model. Transplant. Proc. 2010, 42, 4275–4278. [Google Scholar] [CrossRef] [PubMed]
- Wittig, O.; Diaz-Solano, D.; Chacín, T.; Rodriguez, Y.; Ramos, G.; Acurero, G.; Leal, F.; Cardier, J.E. Healing of Deep Dermal Burns by Allogeneic Mesenchymal Stromal Cell Transplantation. Int. J. Dermatol. 2020, 59, 941–950. [Google Scholar] [CrossRef] [PubMed]
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 650664. [Google Scholar] [CrossRef]
- Isakson, M.; de Blacam, C.; Whelan, D.; McArdle, A.; Clover, A.J.P. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int. 2015, 2015, 831095. [Google Scholar] [CrossRef]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of Cord Blood and Bone Marrow Mesenchymal Stem Cells in Recent Deep Burn: A Case-Control Prospective Study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar]
- Jeschke, M.G.; Rehou, S.; McCann, M.R.; Shahrokhi, S. Allogeneic Mesenchymal Stem Cells for Treatment of Severe Burn Injury. Stem Cell Res. Ther. 2019, 10, 337. [Google Scholar] [CrossRef]
- Mansilla, E.; Marín, G.H.; Berges, M.; Scafatti, S.; Rivas, J.; Núñez, A.; Menvielle, M.; Lamonega, R.; Gardiner, C.; Drago, H.; et al. Cadaveric Bone Marrow Mesenchymal Stem Cells: First Experience Treating a Patient with Large Severe Burns. Burns Trauma 2015, 3, 17. [Google Scholar] [CrossRef]
- Rasulov, M.F.; Vasilchenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First Experience of the Use Bone Marrow Mesenchymal Stem Cells for the Treatment of a Patient with Deep Skin Burns. Bull. Exp. Biol. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef]
- Thanusha, A.V.; Mohanty, S.; Dinda, A.K.; Koul, V. Fabrication and Evaluation of Gelatin/Hyaluronic Acid/Chondroitin Sulfate/Asiatic Acid Based Biopolymeric Scaffold for the Treatment of Second-Degree Burn Wounds—Wistar Rat Model Study. Biomed. Mater. 2020, 15, 055016. [Google Scholar] [CrossRef]
- Abdel-Gawad, D.R.I.; Moselhy, W.A.; Ahmed, R.R.; Al-Muzafar, H.M.; Amin, K.A.; Amin, M.M.; El-Nahass, E.-S.; Abdou, K.A.H. Therapeutic Effect of Mesenchymal Stem Cells on Histopathological, Immunohistochemical, and Molecular Analysis in Second-Grade Burn Model. Stem Cell Res. Ther. 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Alapure, B.V.; Lu, Y.; He, M.; Chu, C.-C.; Peng, H.; Muhale, F.; Brewerton, Y.-L.; Bunnell, B.; Hong, S. Accelerate Healing of Severe Burn Wounds by Mouse Bone Marrow Mesenchymal Stem Cell-Seeded Biodegradable Hydrogel Scaffold Synthesized from Arginine-Based Poly(Ester Amide) and Chitosan. Stem Cells Dev. 2018, 27, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Caliari-Oliveira, C.; Yaochite, J.N.U.; Ramalho, L.N.Z.; Palma, P.V.B.; Carlos, D.; de Queiróz Cunha, F.; De Souza, D.A.; Frade, M.A.C.; Covas, D.T.; Malmegrim, K.C.R.; et al. Xenogeneic Mesenchymal Stromal Cells Improve Wound Healing and Modulate the Immune Response in an Extensive Burn Model. Cell Transplant. 2016, 25, 201–215. [Google Scholar] [CrossRef]
- Clover, A.J.P.; Kumar, A.H.S.; Isakson, M.; Whelan, D.; Stocca, A.; Gleeson, B.M.; Caplice, N.M. Allogeneic Mesenchymal Stem Cells, but Not Culture Modified Monocytes, Improve Burn Wound Healing. Burns 2015, 41, 548–557. [Google Scholar] [CrossRef]
- Fu, X.; Fang, L.; Li, X.; Cheng, B.; Sheng, Z. Enhanced Wound-Healing Quality with Bone Marrow Mesenchymal Stem Cells Autografting after Skin Injury: Enhancing Wound Healing with Stem Cells. Wound Repair Regen. 2006, 14, 325–335. [Google Scholar] [CrossRef]
- Guo, X.; Xia, B.; Lu, X.-B.; Zhang, Z.-J.; Li, Z.; Li, W.-L.; Xiong, A.-B.; Deng, L.; Tan, M.-Y.; Huang, Y.-C. Grafting of Mesenchymal Stem Cell-Seeded Small Intestinal Submucosa to Repair the Deep Partial-Thickness Burns. Connect. Tissue Res. 2016, 57, 388–397. [Google Scholar] [CrossRef]
- Ha, X.; Lü, T.; Hui, L.; Dong, F. Effects of Mesenchymal Stem Cells Transfected with Human Hepatocyte Growth Factor Gene on Healing of Burn Wounds. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2010, 13, 349–355. [Google Scholar]
- Hosni Ahmed, H.; Rashed, L.A.; Mahfouz, S.; Elsayed Hussein, R.; Alkaffas, M.; Mostafa, S.; Abusree, A. Can Mesenchymal Stem Cells Pretreated with Platelet-Rich Plasma Modulate Tissue Remodeling in a Rat with Burned Skin? Biochem. Cell Biol. 2017, 95, 537–548. [Google Scholar] [CrossRef]
- Imam, R.A.; Rizk, A.A.-E. Efficacy of Erythropoietin-Pretreated Mesenchymal Stem Cells in Murine Burn Wound Healing: Possible in Vivo Transdifferentiation into Keratinocytes. Folia Morphol. 2019, 78, 11. [Google Scholar] [CrossRef]
- Imbarak, N.; Abdel-Aziz, H.I.; Farghaly, L.M.; Hosny, S. Effect of Mesenchymal Stem Cells versus Aloe Vera on Healing of Deep Second-Degree Burn. Stem Cell Investig. 2021, 8, 12. [Google Scholar] [CrossRef]
- Liu, P.; Deng, Z.; Han, S.; Liu, T.; Wen, N.; Lu, W.; Geng, X.; Huang, S.; Jin, Y. Tissue-Engineered Skin Containing Mesenchymal Stem Cells Improves Burn Wounds. Artif. Organs 2008, 32, 925–931. [Google Scholar] [CrossRef]
- Lykov, A.P.; Bondarenko, N.A.; Poveshchenko, O.V.; Miller, T.V.; Poveshchenko, A.F.; Surovtseva, M.A.; Bgatova, N.P.; Konenkov, V.I. Prospect of Using Cell Product for the Therapy of Skin Defects in Diabetes Mellitus. Bull. Exp. Biol. Med. 2017, 164, 266–268. [Google Scholar] [CrossRef]
- Mohajer Ansari, J.; Ramhormozi, P.; Shabani, R.; Pazoki-toroudi, H.; Yari, A.; Barati, M.; Babakhani, A.; Nobakht, M. Simvastatin Combined with Bone Marrow Mesenchymal Stromal Cells (BMSCs) Improve Burn Wound Healing by Ameliorating Angiogenesis through SDF-1α/CXCR4 Pathway. Iran. J. Basic Med. Sci. 2020, 23, 751–759. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.H.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In Vivo Migration of Mesenchymal Stem Cells to Burn Injury Sites and Their Therapeutic Effects in a Living Mouse Model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Palakkara, S.; Maiti, S.K.; Mohan, D.; Shivaraju, S.; Raguvaran, R.; Kalaiselvan, E.; Kumar, N. Healing Potential of Chitosan and Decellularized Intestinal Matrix with Mesenchymal Stem Cells and Growth Factor in Burn Wound in Rat. Wound Med. 2020, 30, 100192. [Google Scholar] [CrossRef]
- Paramasivam, T.; Maiti, S.K.; Palakkara, S.; Rashmi; Mohan, D.; Manjunthaachar, H.V.; Karthik, K.; Kumar, N. Effect of PDGF-B Gene-Activated Acellular Matrix and Mesenchymal Stem Cell Transplantation on Full Thickness Skin Burn Wound in Rat Model. Tissue Eng. Regen. Med. 2021, 18, 235–251. [Google Scholar] [CrossRef]
- Rasulov, M.F.; Vasilenko, V.T.; Zaidenov, V.A.; Onishchenko, N.A. Cell Transplantation Inhibits Inflammatory Reaction and Stimulates Repair Processes in Burn Wound. Bull. Exp. Biol. Med. 2006, 142, 112–115. [Google Scholar] [CrossRef]
- Revilla, G.; Darwin, E.Y.; Rantam, F.A. Effect of Allogeneic Bone Marrow-Mesenchymal Stem Cells (BM-MSCs) to Accelerate Burn Healing of Rat on the Expression of Collagen Type I and Integrin A2β1. Pak. J. Biol. Sci. 2016, 19, 345–351. [Google Scholar] [CrossRef][Green Version]
- Revilla, G.; Afriani, N.; Rusnita, D. Effects of Bone Marrow Mesenchymal Stem Cell to Transforming Grow Factor-Β3 and Matrix Metalloproteinase-9 Expression in Burns. J. Med. Sci. 2018, 18, 164–171. [Google Scholar] [CrossRef][Green Version]
- Revilla, G.; Mulyani, H. The Effect of Human Bone Marrow Mesenchymal Stem Cells on Epidermal Growth Factor and Epidermal Growth Factor Receptor Expression in Re-Epithelialization Process in the Healing of Burns on Experimental Rats. Open Access Maced. J. Med. Sci. 2020, 8, 508–511. [Google Scholar] [CrossRef]
- Rodriguez-Menocal, L.; Davis, S.C.; Guzman, W.; Gil, J.; Valdes, J.; Solis, M.; Higa, A.; Natesan, S.; Schulman, C.I.; Christy, R.J.; et al. Model to Inhibit Contraction in Third-Degree Burns Employing Split-Thickness Skin Graft and Administered Bone Marrow-Derived Stem Cells. J. Burn Care Res. 2022, irac119. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, E.; Chehelgerdi, M.; Fatahian-Kelishadrokhi, A.; Yazdani-Nafchi, F.; Ashrafi-Dehkordi, K. Comparison of Therapeutic Effects of Encapsulated Mesenchymal Stem Cells in Aloe Vera Gel and Chitosan-Based Gel in Healing of Grade-II Burn Injuries. Regen. Ther. 2021, 18, 30–37. [Google Scholar] [CrossRef]
- Shumakov, V.I.; Onishchenko, N.A.; Rasulov, M.F.; Krasheninnikov, M.E.; Zaidenov, V.A. Mesenchymal Bone Marrow Stem Cells More Effectively Stimulate Regeneration of Deep Burn Wounds than Embryonic Fibroblasts. Bull. Exp. Biol. Med. 2003, 136, 192–195. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, M.; Mou, X.; Ye, L. Overexpressing of Caveolin-1 in Mesenchymal Stem Cells Promotes Deep Second-Degree Burn Wound Healing. J. Biosci. Bioeng. 2021, 131, 341–347. [Google Scholar] [CrossRef]
- Xue, L.; Xu, Y.-B.; Xie, J.-L.; Tang, J.-M.; Shu, B.; Chen, L.; Qi, S.-H.; Liu, X.-S. Effects of Human Bone Marrow Mesenchymal Stem Cells on Burn Injury Healing in a Mouse Model. Int. J. Clin. Exp. Pathol. 2013, 6, 1327–1336. [Google Scholar]
- Alemzadeh, E.; Oryan, A.; Mohammadi, A.A. Hyaluronic Acid Hydrogel Loaded by Adipose Stem Cells Enhances Wound Healing by Modulating IL-1β, TGF-β1, and BFGF in Burn Wound Model in Rat. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 555–567. [Google Scholar] [CrossRef]
- Andrade, A.L.M.; Brassolatti, P.; Luna, G.F.; Parisi, J.R.; Oliveira Leal, Â.M.; Frade, M.A.C.; Parizotto, N.A. Effect of Photobiomodulation Associated with Cell Therapy in the Process of Cutaneous Regeneration in Third Degree Burns in Rats. J. Tissue Eng. Regen. Med. 2020, 14, 673–683. [Google Scholar] [CrossRef]
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, C.J.; Mittal, S.; et al. Adipose-Derived Stromal Cells Seeded in Pullulan-Collagen Hydrogels Improve Healing in Murine Burns. Tissue Eng. Part A 2021, 27, 844–856. [Google Scholar] [CrossRef]
- Bliley, J.M.; Argenta, A.; Satish, L.; McLaughlin, M.M.; Dees, A.; Tompkins-Rhoades, C.; Marra, K.G.; Rubin, J.P. Administration of Adipose-Derived Stem Cells Enhances Vascularity, Induces Collagen Deposition, and Dermal Adipogenesis in Burn Wounds. Burns 2016, 42, 1212–1222. [Google Scholar] [CrossRef]
- Boukani, L.M.; Kheirjou, R.; Khosroshahi, R.F.; Khosroshahi, A.F. Experimental Repairing of the Defect of Rat Full-Thickness Burn with Cell-Engineered Structure. Regen. Eng. Transl. Med. 2022. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Stone, R.; Wrice, N.; Laborde, A.; Becerra, S.C.; Natesan, S.; Christy, R.J. Delivery of Allogeneic Adipose Stem Cells in Polyethylene Glycol-Fibrin Hydrogels as an Adjunct to Meshed Autografts After Sharp Debridement of Deep Partial Thickness Burns. Stem Cells Transl. Med. 2018, 7, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Arista, B.; Melgarejo-Ramírez, Y.; Retana-Flores, A.; Martínez-López, V.; Márquez-Gutiérrez, E.; Almanza-Pérez, J.; Lecona, H.; Reyes-Frías, M.L.; Ibarra, C.; Martínez-Pardo, M.E.; et al. Effects of Mesenchymal Stem Cell Culture on Radio Sterilized Human Amnion or Radio Sterilized Pig Skin in Burn Wound Healing. Cell Tissue Bank. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Scutaru, T.T.; Ghetu, N.; Carasevici, E.; Lupascu, C.D.; Ferariu, D.; Pieptu, D.; Coman, C.-G.; Danciu, M. The Effects of Adipose-Derived Stem Cell–Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J. Burn Care Res. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Chung, E.; Rybalko, V.Y.; Hsieh, P.; Leal, S.L.; Samano, M.A.; Willauer, A.N.; Stowers, R.S.; Natesan, S.; Zamora, D.O.; Christy, R.J.; et al. Fibrin-based Stem Cell Containing Scaffold Improves the Dynamics of Burn Wound Healing. Wound Repair Regen. 2016, 24, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Costa de Oliveira Souza, C.M.; de Souza, C.F.; Mogharbel, B.F.; Irioda, A.C.; Cavichiolo Franco, C.R.; Sierakowski, M.R.; Athayde Teixeira de Carvalho, K. Nanostructured Cellulose–Gellan–Xyloglucan–Lysozyme Dressing Seeded with Mesenchymal Stem Cells for Deep Second-Degree Burn Treatment. Int. J. Nanomed. 2021, 16, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable Hyaluronic Acid Hydrogel Delivers Adipose-Derived Stem Cells and Promotes Regeneration of Burn Injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Feng, C.-J.; Lin, C.-H.; Tsai, C.-H.; Yang, I.-C.; Ma, H. Adipose-Derived Stem Cells-Induced Burn Wound Healing and Regeneration of Skin Appendages in a Novel Skin Island Rat Model. J. Chin. Med. Assoc. 2019, 82, 635–642. [Google Scholar] [CrossRef]
- Franck, C.L.; Senegaglia, A.C.; Leite, L.M.B.; de Moura, S.A.B.; Francisco, N.F.; Ribas Filho, J.M. Influence of Adipose Tissue-Derived Stem Cells on the Burn Wound Healing Process. Stem Cells Int. 2019, 2019, 2340725. [Google Scholar] [CrossRef]
- Fujiwara, O.; Prasai, A.; Perez-Bello, D.; El Ayadi, A.; Petrov, I.Y.; Esenaliev, R.O.; Petrov, Y.; Herndon, D.N.; Finnerty, C.C.; Prough, D.S.; et al. Adipose-Derived Stem Cells Improve Grafted Burn Wound Healing by Promoting Wound Bed Blood Flow. Burns Trauma 2020, 8, tkaa009. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Seifalian, A.M.; Urbanska, A.M.; Omrani, M.D.; Hardy, J.G.; Madjd, Z.; Hashemi, S.M.; Ghanbarian, H.; Brouki Milan, P.; Mozafari, M.; et al. 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules 2018, 19, 2409–2422. [Google Scholar] [CrossRef]
- Karimi, H.; Soudmand, A.; Orouji, Z.; Taghiabadi, E.; Mousavi, S.J. Burn Wound Healing with Injection of Adipose-Derived Stem Cells: A Mouse Model Study. Ann. Burns Fire Disasters 2014, 27, 44–49. [Google Scholar] [PubMed]
- Karina, K.; Rosadi, I.; Sobariah, S.; Afini, I.; Widyastuti, T.; Rosliana, I. Comparable Effect of Adipose-Derived Stromal Vascular Fraction and Mesenchymal Stem Cells for Wound Healing: An in Vivo Study. Biomed. Res. Ther. 2019, 6, 3412–3421. [Google Scholar] [CrossRef]
- Karina, K.; Biben, J.A.; Ekaputri, K.; Rosadi, I.; Rosliana, I.; Afini, I.; Widyastuti, T.; Sobariah, S.; Subroto, W.R. In Vivo Study of Wound Healing Processes in Sprague-Dawley Model Using Human Mesenchymal Stem Cells and Platelet-Rich Plasma. Biomed. Res. Ther. 2021, 8, 4316–4324. [Google Scholar] [CrossRef]
- Loder, S.; Peterson, J.R.; Agarwal, S.; Eboda, O.; Brownley, C.; DeLaRosa, S.; Ranganathan, K.; Cederna, P.; Wang, S.C.; Levi, B. Wound Healing after Thermal Injury Is Improved by Fat and Adipose-Derived Stem Cell Isografts. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2015, 36, 70–76. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Yu, K.-F.; Kuo, S.-H.; Cheng, N.-C.; Chuang, E.-Y.; Yu, J.-S. Enzyme-Crosslinked Gelatin Hydrogel with Adipose-Derived Stem Cell Spheroid Facilitating Wound Repair in the Murine Burn Model. Polymers 2020, 12, 2997. [Google Scholar] [CrossRef]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-Based Skin Substitutes Accelerate Regeneration of Extensive Burn Wounds in Rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine Gellan Gum-Collagen Interpenetrating Network Hydrogels as Mechanically Enhanced Anti-Inflammatory Biologic Wound Dressings for Burn Wound Therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing Potential of Injectable Aloe Vera Hydrogel Loaded by Adipose-Derived Stem Cell in Skin Tissue-Engineering in a Rat Burn Wound Model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A. Application of Honey as a Protective Material in Maintaining the Viability of Adipose Stem Cells in Burn Wound Healing: A Histological, Molecular and Biochemical Study. Tissue Cell 2019, 61, 89–97. [Google Scholar] [CrossRef]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Khosroshahi, A.F. Using 3D-Bioprinting Scaffold Loaded with Adipose-Derived Stem Cells to Burns Wound Healing. J. Tissue Eng. Regen. Med. 2021, 15, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Shokrgozar, M.A.; Fattahi, M.; Bonakdar, S.; Kashani, I.R.; Majidi, M.; Haghighipour, N.; Bayati, V.; Sanati, H.; Saeedi, S.N. Healing Potential of Mesenchymal Stem Cells Cultured on a Collagen-Based Scaffold for Skin Regeneration. Iran. Biomed. J. 2012, 16, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, T.; Hu, Y.; Jiang, S.; Luo, Y.; Liu, C.; Wang, G.; Zhang, J.; Xu, T.; Zhu, L. 3D Bioprinting of Integral ADSCs-NO Hydrogel Scaffolds to Promote Severe Burn Wound Healing. Regen. Biomater. 2021, 8, rbab014. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019, 28, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Afzali, L.; Mirahmadi-Babaheydari, F.; Shojaei-Ghahrizjani, F.; Rahmati, S.; Shahmoradi, B.; Banitalebi-Dehkordi, M. The Effect of Encapsulated Umbilical Cord-Derived Mesenchymal Stem Cells in PRPCryogel on Regeneration of Grade-II Burn Wounds. Regen. Eng. Transl. Med. 2022, 8, 75–85. [Google Scholar] [CrossRef]
- Cheng, R.Y.; Eylert, G.; Gariepy, J.-M.; He, S.; Ahmad, H.; Gao, Y.; Priore, S.; Hakimi, N.; Jeschke, M.G.; Günther, A. Handheld Instrument for Wound-Conformal Delivery of Skin Precursor Sheets Improves Healing in Full-Thickness Burns. Biofabrication 2020, 12, 025002. [Google Scholar] [CrossRef] [PubMed]
- Gholipour-Kanani, A.; Bahrami, S.H.; Samadi-Kochaksaraie, A.; Ahmadi-Tafti, H.; Rabbani, S.; Kororian, A.; Erfani, E. Effect of Tissue-Engineered Chitosan-Poly(Vinyl Alcohol) Nanofibrous Scaffolds on Healing of Burn Wounds of Rat Skin. IET Nanobiotechnol. 2012, 6, 129–135. [Google Scholar] [CrossRef]
- Gholipour-Kanani, A.; Bahrami, S.H.; Joghataie, M.T.; Samadikuchaksaraei, A.; Ahmadi-Taftie, H.; Rabbani, S.; Kororian, A.; Erfani, E. Tissue Engineered Poly(Caprolactone)-Chitosan-Poly(Vinyl Alcohol) Nanofibrous Scaffolds for Burn and Cutting Wound Healing. IET Nanobiotechnol. 2014, 8, 123–131. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Pourfath, M.R.; Derakhshanfar, A.; Behzad-Behbahani, A.; Moayedi, J. The Role of Labeled Cell Therapy with and without Scaffold in Early Excision Burn Wounds in a Rat Animal Model. Iran. J. Basic Med. Sci. 2020, 23, 673–679. [Google Scholar] [CrossRef]
- Jehangir, S.; Ramesh, S.; Thomas, M.; Madhuri, V. Wharton’s Jelly Mesenchymal Stem Cells on a Novel Aloe Vera-Polycaprolactone (A-PCL) Composite Scaffold in Burns. Regen. Eng. Transl. Med. 2022. [Google Scholar] [CrossRef]
- Nazempour, M.; Mehrabani, D.; Mehdinavaz-Aghdam, R.; Hashemi, S.-S.; Derakhshanfar, A.; Zare, S.; Zardosht, M.; Moayedi, J.; Vahedi, M. The Effect of Allogenic Human Wharton’s Jelly Stem Cells Seeded onto Acellular Dermal Matrix in Healing of Rat Burn Wounds. J. Cosmet. Dermatol. 2020, 19, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Pourfath, M.R.; Behbahani, A.B.; Hashemi, S.S.; Derakhsahnfar, A.; Taheri, M.N.; Salehi, S. Monitoring Wound Healing of Burn in Rat Model Using Human Wharton’s Jelly Mesenchymal Stem Cells Containing CGFP Integrated by Lentiviral Vectors. Iran. J. Basic Med. Sci. 2018, 21, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; La, X.; Fan, L.; Li, P.; Yu, Y.; Huang, Y.; Ding, J.; Xing, Y. Immunosuppressive Effects of Mesenchymal Stem Cell Transplantation in Rat Burn Models. Int. J. Clin. Exp. Pathol. 2015, 8, 5129–5136. [Google Scholar] [PubMed]
- Lim, J.; Liew, S.; Chan, H.; Jackson, T.; Burrows, S.; Edgar, D.W.; Wood, F.M. Is the Length of Time in Acute Burn Surgery Associated with Poorer Outcomes? Burns 2014, 40, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Beltrán, P.; Gálvez-Martín, P.; Nieto-García, D.; Marchal, J.A.; López-Ruiz, E. Advances in Spray Products for Skin Regeneration. Bioact. Mater. 2022, 16, 187–203. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Rubin, J.P.; Pfeifer, M.E.; Moore, L.R.; Donnenberg, V.S.; Donnenberg, A.D. Human Adipose Stromal Vascular Cell Delivery in a Fibrin Spray. Cytotherapy 2013, 15, 102–108. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous Bone Marrow–Derived Cultured Mesenchymal Stem Cells Delivered in a Fibrin Spray Accelerate Healing in Murine and Human Cutaneous Wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Cohen, M.; Bahoric, A.; Clarke, H.M. Aerosolization of Epidermal Cells with Fibrin Glue for the Epithelialization of Porcine Wounds with Unfavorable Topography. Plast. Reconstr. Surg. 2001, 107, 1208–1215. [Google Scholar] [CrossRef]
- Joch, C. The Safety of Fibrin Sealants. Cardiovasc. Surg. 2003, 11, 23–28. [Google Scholar] [CrossRef]
- Ho, W.; Tawil, B.; Dunn, J.C.Y.; Wu, B.M. The Behavior of Human Mesenchymal Stem Cells in 3D Fibrin Clots: Dependence on Fibrinogen Concentration and Clot Structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2016, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, F.P.; Munthe-Fog, L.; Kring, M.K.M.; Duscher, D.; Taudorf, M.; Katz, A.J.; Kølle, S.-F.T. Differences of Embedding Adipose-Derived Stromal Cells in Natural and Synthetic Scaffolds for Dermal and Subcutaneous Delivery. Stem Cell Res. Ther. 2021, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The Safety of MSC Therapy over the Past 15 Years: A Meta-Analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental Models and Methods for Cutaneous Wound Healing Assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Rodriguez-Menocal, L.; Salgado, M.; Ford, D.; Van Badiavas, E. Stimulation of Skin and Wound Fibroblast Migration by Mesenchymal Stem Cells Derived from Normal Donors and Chronic Wound Patients. Stem Cells Transl. Med. 2012, 1, 221–229. [Google Scholar] [CrossRef]
- Hamrahi, V.F.; Goverman, J.; Jung, W.; Wu, J.C.; Fischman, A.J.; Tompkins, R.G.; Yu, Y.-M.; Fagan, S.P.; Carter, E.A. In Vivo Molecular Imaging of Murine Embryonic Stem Cells Delivered To A Burn Wound Surface via Integra® Scaffolding. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2012, 33, e49–e54. [Google Scholar] [CrossRef]
- Raposio, E.; Bertozzi, N. How to Isolate a Ready-to-Use Adipose-Derived Stem Cells Pellet for Clinical Application. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4252–4260. [Google Scholar] [PubMed]
- Abbas, O.L.; Özatik, O.; Gönen, Z.B.; Öğüt, S.; Özatik, F.Y.; Salkın, H.; Musmul, A. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Dental Pulp as Sources of Cell Therapy for Zone of Stasis Burns. J. Investig. Surg. 2019, 32, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, R.; Slezak, P.; Haffner, N.; Smolen, D.; Hartinger, J.; Hofmann, A.; Schense, J.; Spazierer, D.; Gampfer, J.; Goppelt, A.; et al. Controlled Release of Fibrin Matrix-Conjugated Platelet Derived Growth Factor Improves Ischemic Tissue Regeneration by Functional Angiogenesis. Acta Biomater. 2016, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Zeisberger, S.M.; Raeber, G.P.; Hubbell, J.A.; Schnell, C.; Zisch, A.H. The Role of Actively Released Fibrin-Conjugated VEGF for VEGF Receptor 2 Gene Activation and the Enhancement of Angiogenesis. Biomaterials 2008, 29, 1720–1729. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| English language Original scientific studies In vivo studies Bone-marrow-, umbilical-cord-, or adipose-tissue-derived stem cells Cutaneous burn wounds | Not in English Review articles In vitro studies Radiation or chemical burn studies Other types of MSCs Non-MSC cells included in the treatment group Use of further differentiated MSCs Genetic alteration of MSCs beyond genetic marking |
| Authors | Study Model | Patient Characteristics (n, Age, Sex, TBSA) | Burn Depth | Cell Species | Groups | Cell Delivery, Medium | Cell Dosage | Results (End Points) | |
|---|---|---|---|---|---|---|---|---|---|
| Dose (Passage) | Cells/ cm2 1 | ||||||||
| Abo-Elkheir et al. (2017) [17] | Prospective case–control | n = 60, 18–35 y, male and female, 10–25% TBSA IC: Both sexes, age 15–50 y, TBSA 10–25% EC: Comorbidity, superficial or old burns, chemical, radiation or electric burns | Full thickness | Al BM-MSC, Al UC-MSC | Excision and STSG, excision and BM-MSC, excision and UC-MSC | Local injection, n/a | 1 × 105 cells/cm2 (n/a) | 1 × 105 | Increased rate of wound healing compared to STSG group, in both MSC groups, and shorter length of stay. Less early complications in BM-MSC group; infection was seen in 25% of the patients, but higher early complication rates in UC-MSC group; infection in 70%. Early complication rate was 50% in excision + STSG group. A total of 95% of patients in STSG group had late complications, compared to 45% in BM-MSC group and 30% in UC-MSC group. (Rate of burn healing, early and late complications, hospital stay length, costs) |
| Jeschke et al. (2019) [18] | Case report | n = 1, male, mid-twenties, >70% TBSA 18 months after injury | Full thickness | Al UC-MSC and commercial Al Ch-MSC | Topical application and injection, fibrin sealant and Ringer’s lactate | 3 × 106 cells/mL in topical solution (n/a) | n/a | Rapid re-epithelialization. Reduction in wound percentage and healing of infections. Limited scarring over 6 years and no adverse effects. (Effect on burn wounds with delayed healing) | |
| Mansilla et al. (2015) [19] | Case report | n = 1, 26 y, male 60% TBSA, 30% full thickness | Full thickness | Al cadaveric BM-MSC | n/a | Topical application, fibrinogen and thrombin spray | 1 × 104 cells/cm2 (P2) | 1 × 104 | Rapid epithelialization, more normal skin appearance compared to previous experiences in the burn unit. No adverse effects. (Safety) |
| Rasulov et al. (2005) [20] | Case report | n = 1, 45 y, female 40% TBSA, 30% full thickness | Deep partial and full thickness | Al BM-MSC | n/a | Topical application, n/a | 2–3 × 104 cells/cm2 (n/a) | 2.5 × 104 | Rapid epithelialization. Increased angiogenesis and granulation. Pain relief. Good graft-take of STSGs. (Neo-angiogenesis and graft take) |
| Wittig et al. (2020) [13] | Case series | n = 5, 2–58 y, male TBSA 12–55% IC: Age >= 2 y, full thickness burns, not healed within >= 21 days EC: Infection | Deep partial and full thickness | Al BM-MSC | n/a | Cell scaffold, pre-clotted PRP and thrombin | 1–3 × 107 cells per patient (n/a) | n/a | Early granulation tissue, rapid re-epithelialization. Full healing in 1–5 months. Recovery of pigmentation. Slight discoloration of healed skin, less hypertrophy, and contractures. (Effect on burn wounds with delayed healing) |
| Authors | Animal Model (n) | Burn Depth | Cell Species | Administration Method, Medium | Cell Dosage | Results in MSC Group | |
|---|---|---|---|---|---|---|---|
| Dose (Passage) | Cells/cm2 1 | ||||||
| A V et al. (2020) [21] | Rat (n/a) | Partial thickness | Xe BM-MSC, human | Cell scaffold and topical application (2 groups), hydrogel and DMEM | 1 × 106 cells (P3–5) | n/a | Increased wound contraction. Earlier wound closure, but only in scaffold group. No effect in topical MSC group. |
| Abdel-Gawad et al. (2021) [22] | Rat (90) | Partial thickness | Al BM-MSC | Subcutaneous injection, DMEM | 2 × 106 cells/mL (n/a) | n/a | Increased wound healing. Reduced scar formation. |
| Alapure et al. (2018) [23] | Mice (n/a) | Full thickness | Al BM-MSC | Cell scaffold, ACgel scaffold | 1 × 105 cells/scaffold (P3–5) | 5.1 × 105 | Increased wound closure rate, re-epithelialization, blood vessel growth and granulation. |
| Caliari-Oliveira et al. (2016) [24] | Rat (134) | Full thickness | Xe BM-MSC, Mice | Intradermal injection, PBS | 5 × 106 cells/wound (P3–4) | 1.1 × 105 | Increased epithelialization after 60 days. |
| Clover et al. (2015) [25] | Porcine (3) | Deep partial thickness | Al BM-MSC | Topical application, fibrin sealant (Tisseel™) | 4.5 × 106 cells/wound (P4) | 1 × 106 | Increased wound healing. Increased collagen density, increased epidermal area and dermal thickness. |
| Fu et al. (2006) [26] | Porcine (6) | Deep partial thickness | Au BM-MSC | Local injection, n/a | 2 × 106 cells/wound (n/a) | n/a | Faster re-epithelialization, increased vascularization and collagen. |
| Guo et al. (2016) [27] | Rat (49) | Deep partial thickness | Al BM-MSC | Cell scaffold, small intestinal submucosa | 5 × 105 cells/cm2 (P3) | 5 × 105 | Accelerated wound closure and granulation, vascularization and neo-epidermal cells. |
| Ha et al. (2010) [28] | Rat (32) | Partial thickness | Al BM-MSC | Intradermal injection, saline solution | n/a (n/a) | n/a | Earlier wound closure. |
| Hosni Ahmed et al. (2017) [29] | Rat (72) | n/a | Al BM-MSC | Local injection, PBS | 1 × 106 cells/mL (P3) | n/a | Accelerated wound healing. |
| Imam et al. (2019) [30] | Rat (40) | Full thickness | Al BM-MSC | Local injection, PBS | 1 × 106 cells/cm2 (P3) | 1 × 106 | Increased wound healing and epithelialization. |
| Imbarak et al. (2021) [31] | Rat (60) | Deep partial thickness | Al BM-MSC | Intradermal injection, PBS | 1 × 106 cells/wound (n/a) | n/a | Accelerated wound healing, increased epidermal thickness. Regenerated hair follicles. |
| Liu et al. (2008) [32] | Porcine (24) | Deep partial thickness | Au BM-MSC | Cell scaffold, collagen-GAG | 2 × 106 cells/mL (P2–5) | n/a | Better healing and keratinization, less wound contraction. Increased vascularization. No adverse effects. |
| Lykov et al. (2017) [33] | Rat (25) | Partial thickness | Al BM-MSC | Local injection, n/a | 2 × 105 cells/wound (P2–4) | n/a | Decrease in defect skin area, increased re-epithelialization and wound closure rate. |
| Mansilla et al. (2010) [12] | Porcine (1) | Full thickness | Xe BM-MSC, rabbit | Topical application, fibrin sealant | 2 × 106 cells/mL/cm2 (n/a) | n/a | Increased granulation, vascularization, healing of wound with skin appendages. |
| Mohajer Ansari et al. (2020) [34] | Rat (48) | Deep partial thickness | Al BM-MSC | Intradermal injection, PBS | 1 × 106 cells (n/a) | 4.4 × 105 | Increased biomechanical strength of wound, increased wound closure rate, increased epithelialization, increased remodeled collagen content, increased angiogenesis. |
| Oh et al. (2018) [35] | Mice (30) | Full thickness | Al BM-MSC | Systemic injection, n/a | 5 × 105 cells/mouse (n/a) | n/a | MSC migration to burn wound and increased wound healing. |
| Palakkara et al. (2020) [36] | Rat (105) | Full thickness | Al BM-MSC | Cell scaffold and local injection, Chitosan powder and decellularized porcine SIS (two groups) | 1 × 106 cells/wound (P3) | n/a | Increased angiogenesis and re-epithelialization. Best results in scaffold group. |
| Paramasivam et al. (2021) [37] | Rat (75) | Full thickness | Al BM-MSC | Cell scaffold, acellular porcine bladder | 2.5 × 106 cells/scaffold (P3) | n/a | Increased rate of healing. Increased granulation and early angiogenesis. Increased and more regular collagen deposition. |
| Rasulov et al. (2006) [38] | Rat (30) | Deep partial thickness | Al BM-MSC | Topical application, n/a | 2 × 104 cells/wound (n/a) | n/a | Increased angiogenesis and granulation. |
| Revilla et al. (2016) [39] | Rat (12) | Full thickness | Al BM-MSC | Local injection, n/a | 2 × 106 cells/wound (n/a) | n/a | Faster wound healing, increased collagen type 1. No infection in MSC group. |
| Revilla et al. (2018) [40] | Rat (10) | Full thickness | Al BM-MSC | Local injection, n/a | 2 × 106 cells/wound (n/a) | 8.9 × 105 | Accelerated wound closure, good healing quality. |
| Revilla et al. (2020) [41] | Rat (30) | Full thickness | Xe BM-MSC, human | Subcutaneous injection, n/a | 2 × 106 cells/mL (n/a) | n/a | Accelerated wound healing, increased re-epithelialization. |
| Rodriguez-Menocal et al. (2022) [42] | Porcine (4) | Full thickness | Al BM-MSC | Local injection, n/a | n/a (P1) | n/a | Reduced wound contraction, less collagen type I/III deposition. Reduced scarring. |
| Sharifi et al. (2021) [43] | Rat (48) | Partial thickness | Al BM-MSC | Cell scaffold and local injection (3 groups), Aloe vera gel, chitosan-based gel and n/a | 2 × 106 cells/wound (n/a) | n/a | Earlier wound closure. Increased angiogenesis and granulation. |
| Shumakov et al. (2003) [44] | Rat (40) | Full thickness | Au and Al BM-MSC | Topical application, n/a | 2 × 106 cells/wound (n/a) | n/a | Increased wound closure rate, most in Au group. Increased angiogenesis and granulation. |
| Wu et al. (2021) [45] | Rat (n/a) | Deep partial thickness | Al BM-MSC | Intradermal injection, n/a | 1 × 106 cells/wound (P5–7) | n/a | Increased wound closure rate and healing. |
| Xue et al. (2013) [46] | Mice (60) | Full thickness | Xe BM-MSC, human | Intradermal injection and topical application, PBS and growth factor reduced matrigel | 1 × 106 cells/wound (n/a) | n/a | Increased wound healing and angiogenesis. Faster wound closure. Found MSCs in other tissues than treated. |
| Authors | Animal Model (n) | Burn Depth | Cell Species | Administration Method, Medium | Cell Dosage | Results in MSC Group | |
|---|---|---|---|---|---|---|---|
| Dose (Passage) | Cells/cm2 1 | ||||||
| Alemzadeh et al. (2020) [47] | Rat (12) | Full thickness | Al ASC | Topical application and local injection around wound, hyaluronic acid hydrogel, covered with ADM | 1 × 106 cells/wound (P3–5) | 1.3 × 106 | Increased wound closure rate. Reduced inflammation, increased angiogenesis and granulation. |
| Andrade et al. (2020) [48] | Rat (96) | Full thickness | Xe ASC | Intradermal injection, n/a | 1.5 × 106 cells/wound (P4–5) | 2.1 × 105 | Increased wound closure rate. |
| Barrera et al. (2021) [49] | Mice (32) | Partial thickness | Al ASC | Cell scaffold and injection (2 groups), collagen–pullulan hydrogel and n/a | 2.5 × 105 cells/wound (P0–2) | n/a | Accelerated wound healing in scaffold group. Increased vascularization. |
| Bliley et al. (2016) [50] | Mice (24) | Full thickness | Xe ASC, human | Subcutaneous injection, PBS | 6.8 × 106 cells/wound (P3) | n/a | No statistical difference in wound closure times. ASC enhanced vascularization, collagen deposition and adipocyte differentiation. Increased hair follicle regeneration. |
| Boukani et al. (2022) [51] | Rat (36) | Full thickness | Al ASC | Cell scaffold, decellularized dOSIS | n/a (P3) | n/a | Increased wound closure rate, increased angiogenesis and collagen deposition. Multi-layer epidermis in MSC group. |
| Burmeister et al. (2018) [52] | Porcine (6) | Deep partial thickness | Al ASC | Topical application, FPEG hydrogel (fibrin-based) | 1 × 105, 5 × 105 and 1 × 106 cells/wound, 3 groups (n/a) | 7.6 × 104 | Increased size of blood vessels and collagen deposition dose-related to ASC. |
| Cabello-Arista et al. (2022) [53] | Mice (25) | Full thickness | Xe ASC, human | Cell scaffold, radiosterilized human amnion and pig skin | 6 × 104 cells/cm2 (n/a) | 6 × 104 | No effect on wound closure. Increased collagen deposition. |
| Chen et al. (2017) [54] | Rat (15) | n/a | Al ASC | Subcutaneous injection, PBS | 1 × 106 cells/wound (n/a) | 1.4 × 105 | Accelerated wound healing rate. |
| Chung et al. (2016) [55] | Rat (n/a) | Full thickness | Al ASC | Cell scaffold, PEGylated fibrin gel | 4 × 105 cells/gel (P3–5) | 7.6 × 104 | Earlier neovascularization. Better tissue organization. |
| Costa de Oliveira Souza et al. (2021) [56] | Rat (70) | Deep partial thickness | Al ASC | Cell scaffold, nanostructured cellulose–gellan–xyloglucan–lysozyme dressing | 1 × 103 cells/cm2 (n/a) | 1 × 103 | Increased wound healing |
| Dong et al. (2020) [57] | Mice (15) | Deep partial thickness | Al ASC | Topical application, conformable hydrogel | 3 × 105 cells/wound (P3–5) | n/a | Significantly increased healing rate and accelerated wound closure. Enhanced neovascularization, reduction in scar formation. |
| Feng et al. (2019) [58] | Rat (12) | Deep partial thickness | Al ASC | Intradermal injection, PBS | 5 × 105 cells/wound (P3) | 5 × 105 | Increased healing at all time points, vascular density and percentage of live follicles. |
| Franck et al. (2019) [59] | Rat (23) | Full thickness | Al ASC | Intradermal injection, n/a | 3.2 × 106 cells/wound (n/a) | 6.6 × 105 | Increased wound healing and collagen deposition. Decreased lymphatic vessels. No significant difference in vascular amt. |
| Fujiwara et al. (2020) [60] | Ovine (7) | Full thickness | Al ASC | Topical application, PBS | 7 × 106 cells/wound (P4) | 2.8 × 105 | Improved graft-take and graft size. Increased blood flow and epithelialization. |
| Gholipourmalekabadi et al. (2018) [61] | Mice (75) | Full thickness | Al ASC | Cell scaffold, decellularized human amniotic membrane | 1 × 104 cells/scaffold (P2) | 1.3 × 104 | Accelerated wound healing, reduced scarring, increased neo-vascularization and re-epithelialization. |
| Karimi et al. (2014) [62] | Mice (40) | Full thickness | Al ASC | Local injection, n/a | 1 × 106 cells/mL (n/a) | n/a | Not statistically significant improvements. |
| Karina et al. (2019) [63] | Rat (28) | Partial thickness | Xe ASC, human | Intradermal injection, saline | 4 × 105 cells (P1) | n/a | Increased wound closure rate, but delayed wound closure at end of study compared to the control. Increased re-epithelialization, larger and more prominent skin appendages, increased angiogenesis. |
| Karina et al. (2021) [64] | Rat (30) | Deep partial thickness | Xe ASC, human | Intradermal injection, n/a | 4 × 105 cells/rat (P1) | n/a | Increased wound healing rate. Increased differentiation of healed skin. Increased vascularization. Not accelerated epithelialization. |
| Loder et al. (2014) [65] | Mice (20) | Partial thickness | Al ASC | Subcutaneous injection, PBS | 1 × 106 cells/wound (P3+) | n/a | Decreased wound depth, decreased apoptosis, increase in vascularization (not significant). |
| Lu et al. (2020) [66] | Rat (25) | Partial thickness | Xe ASC, human | Topical application, gelatin hydrogel and suspension | n/a (n/a) | n/a | Increased wound closure rate, most in group using hydrogel compared to cell suspension. Increased epidermal thickness. |
| Motamed et al. (2017) [67] | Rat (32) | Full thickness | Xe ASC, human | Cell scaffold, human amniotic membrane | 5 × 105 cells/cm2 (P3) | 5 × 105 | Increased wound closure rate, lower inflammatory cell infiltration. Most healing in the first 14 days. |
| Ng et al. (2021) [68] | Mice (42) | Full thickness | ASC, n/a | Topical application, gellan gum-collagen hydrogel | 6 × 104 cells/wound (P3–5) | n/a | Increased wound healing and closure rate. |
| Oryan et al. (2019) [69] | Rat (48) | Full thickness | Al ASC | Intradermal injection and topical application, Aloe vera hydrogel | 1 × 106 cells/wound (P3–5) | 1.3 × 106 | Increased rate of healing, less inflammation. |
| Oryan et al. (2019) [70] | Rat (48) | Full thickness | Al ASC | Intradermal injection and topical application, honey | 1 × 106 cells/wound(P3–5) | n/a | Increased angiogenesis, re-epithelialization and granulation. |
| Roshangar et al. (2021) [71] | Rat (36) | Full thickness | Al ASC | Cell scaffold, 3D-printed collagen and alginate scaffold | n/a (n/a) | n/a | Accelerated wound contraction and healing. Increased re-epithelialization, and multi-layer epidermis. |
| Shokrgozar et al. (2012) [72] | Rat (10) | Full thickness | Al ASC | Cell scaffold, collagen–chitosan | n/a (n/a) | n/a | Increased wound healing rate, increased epithelialization. |
| Wu et al. (2021) [73] | Mice (32) | Full thickness | Al ASC | Cell scaffold, 3D GS alginate hydrogel | 2 × 106 cells/scaffold (P3–5) | 8.9 × 105 | Faster epithelialization. Increased angiogenesis and collagen deposition. |
| Zhou et al. (2019) [74] | Rat (27) | Full thickness | Au ASC | Subcutaneous injection, n/a | 2 × 106 cells/wound P3) | 1 × 106 | Increased wound healing and angiogenesis. |
| Authors | Animal Model (n) | Burn Depth | Cell Species | Administration Method, Medium | Cell Dosage | Results in MSC Group | |
|---|---|---|---|---|---|---|---|
| Dose (Passage) | Cells/cm2 1 | ||||||
| Afzali et al. (2022) [75] | Rat (40) | Superficial partial thickness | Xe UC-MSC, human | Cell scaffold and local injection, PRP cryogel and cell culture medium (two groups) | 2 × 106 cells (n/a) | n/a | Improved wound healing, increased wound closure rate, best results in scaffold group. Increased re-epithelialization and increased early neo-angiogenesis. |
| Cheng et al. (2020) [76] | Porcine (4) | Full thickness | Xe WJ-MSC, human | Topical application, in situ fibrin–HA bioink | 1 × 106 cells/mL (P1) | n/a | Better healing with less inflammation, scarring and contraction. Increased re-epithelialization, better archeology. No infection. |
| Gholipour-Kanani et al. (2012) [77] | Rat (12) | Full thickness | Xe WJ-MSC, human | Cell scaffold, Cs:PVA nanofibrous web | 4 × 104 cells/scaffold (P1) | 1.8 × 104 | Accelerated wound healing and wound closure rate. Less inflammation. Increased re-epithelialization and granulation, regular pattern of regenerated collagen. |
| Gholipour-Kanani et al. (2014) [78] | Rat (12) | Full thickness | Xe WJ-MSC, human | Cell scaffold, PCL:Cs:PVA nanofibrous web | 4 × 104 cells/scaffold (P1) | 4.2 × 104 | Accelerated healing process, but longer than non-burn wound group. Increased collagen deposition, granulation, and re-epithelialization. No complications reported. |
| Hashemi et al. (2020) [79] | Rat (32) | Full thickness | Xe WJ-MSC, human | Cell scaffold, HAM | 1 × 106 cells/scaffold (P3) | n/a | Increased rate of healing, re-epithelialization, granulation. Mature and organized scar tissue, less hemorrhage and inflammation. |
| Jehangir et al. (2022) [80] | Rat (35) | Partial thickness | Xe WJ-MSC, human | Cell scaffold, A-PCL composite scaffold and collagen (two groups) | 1 × 105 cells/cm (P1) | 1 × 105 | Increased wound healing and complete epithelialization in both MSC groups, best in A-PCL-WJ-MSC group with complete epidermal restoration and near normal skin appendage regeneration. Wound infection in one animal in the collagen-WJ-MSC group. |
| Nazempour et al. (2020) [81] | Rat (40) | Full thickness | Xe WJ-MSC, human | Cell scaffold, ADM | 2 × 106 cells/scaffold (n/a) | n/a | Increased wound closure rate, angiogenesis, granulation, and epithelialization. |
| Pourfath et al. (2018) [82] | Rat (24) | Full thickness | Xe WJ-MSC, human | Topical application, cell spray + sterile gauze Vaseline covering | 5 × 105 cells/wound (P3) | n/a | Increased re-epithelialization and granulation, decreased hemorrhage and inflammation. |
| Zhang et al. (2015) [83] | Rat (84) | Full thickness | Xe WJ-MSC, human | Subcutaneous injection, saline | 2 × 106 cells/rat (P2–4) | n/a | Significantly higher wound healing rate, shorter wound healing time. Lower increase in inflammatory cytokines. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenssen, A.B.; Mohamed-Ahmed, S.; Kankuri, E.; Brekke, R.L.; Guttormsen, A.B.; Gjertsen, B.T.; Mustafa, K.; Almeland, S.K. Administration Methods of Mesenchymal Stem Cells in the Treatment of Burn Wounds. Eur. Burn J. 2022, 3, 493-516. https://doi.org/10.3390/ebj3040043
Jenssen AB, Mohamed-Ahmed S, Kankuri E, Brekke RL, Guttormsen AB, Gjertsen BT, Mustafa K, Almeland SK. Administration Methods of Mesenchymal Stem Cells in the Treatment of Burn Wounds. European Burn Journal. 2022; 3(4):493-516. https://doi.org/10.3390/ebj3040043
Chicago/Turabian StyleJenssen, Astrid Bjørke, Samih Mohamed-Ahmed, Esko Kankuri, Ragnvald Ljones Brekke, Anne Berit Guttormsen, Bjørn Tore Gjertsen, Kamal Mustafa, and Stian Kreken Almeland. 2022. "Administration Methods of Mesenchymal Stem Cells in the Treatment of Burn Wounds" European Burn Journal 3, no. 4: 493-516. https://doi.org/10.3390/ebj3040043
APA StyleJenssen, A. B., Mohamed-Ahmed, S., Kankuri, E., Brekke, R. L., Guttormsen, A. B., Gjertsen, B. T., Mustafa, K., & Almeland, S. K. (2022). Administration Methods of Mesenchymal Stem Cells in the Treatment of Burn Wounds. European Burn Journal, 3(4), 493-516. https://doi.org/10.3390/ebj3040043






