Epidermal Protein C Levels Correspond to Local Injury Severity and Increased Clinical Support in Burn Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Histology
2.3. Immunohistochemistry
2.4. Image Analysis
2.5. Statistal Analyses
3. Results
3.1. Clinical Details
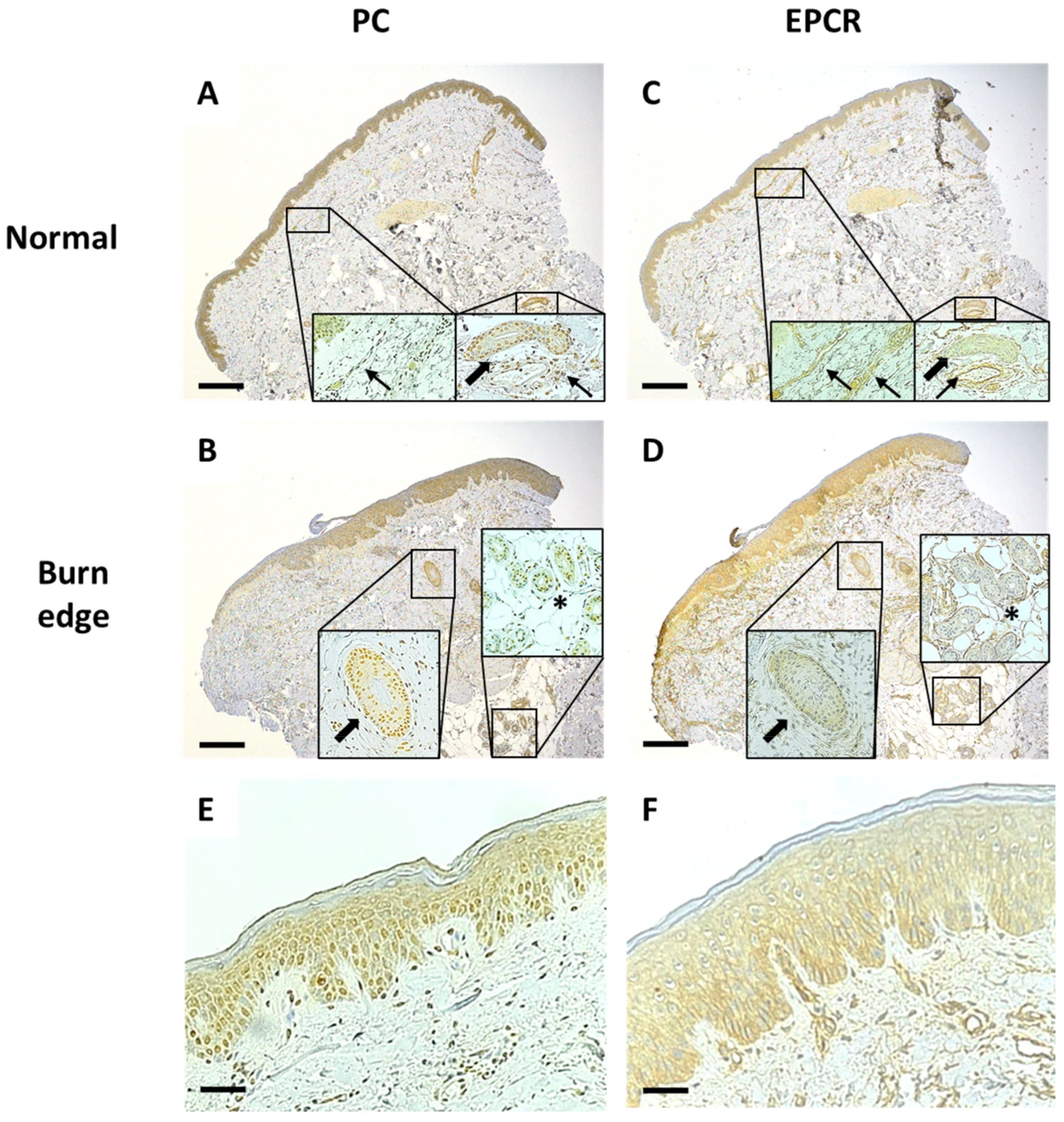
3.2. Expression of PC Is Decreased and EPCR Increased in Burn-Damaged Epidermis
3.3. Progressive Burn Damage Is Correlated with Decreased PC and Increased EPCR Expression
3.4. Macrophage Infiltration Is Greater in Burn Edge Dermis and Is Associated with Epidermal PC Expression
3.5. PC and EPCR Levels Are Not Associated with Their Levels in Plasma
3.6. PC Levels in Burn Tissue Are Directly Associated with Patients Who Require Increased Support
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffin, J.H.; Fernandez, J.A.; Gale, A.J.; Mosnier, L.O. Activated protein C. J. Thromb. Haemost. 2007, 5 (Suppl. 1), 73–80. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Kurosawa, D.J.; Kurosawa, S.; Mollica, J.S.; Ferrell, G.L.; Esmon, C.T. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc. Natl. Acad. Sci. USA 1996, 93, 10212–10216. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, K.; Esmon, C.T. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J. Biol. Chem. 1994, 269, 26486–26491. [Google Scholar] [CrossRef]
- Esmon, C.T. Molecular Events that Control the Protein C Anticoagulant Pathway. Thromb. Haemost. 1993, 70, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, E.; Cooper, P.; Forman, K.; Grimley, C.; Khair, K.; Minford, A.; Morgan, M.; Mumford, A.D. Purpura fulminans: Recognition, diagnosis and management. Arch. Dis. Child. 2011, 96, 1066–1071. [Google Scholar] [CrossRef]
- RRiewald, M.; Petrovan, R.J.; Donner, A.; Mueller, B.M.; Ruf, W. Activation of Endothelial Cell Protease Activated Receptor 1 by the Protein C Pathway. Science 2002, 296, 1880–1882. [Google Scholar] [CrossRef]
- Fisher, C.J., Jr.; Yan, S.B. Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Crit. Care Med. 2000, 28 (Suppl. 9), S49–S56. [Google Scholar] [CrossRef]
- Lang, T.C.; Zhao, R.; Kim, A.; Wijewardena, A.; Vandervord, J.; McGrath, R.; Fitzpatrick, S.; Fulcher, G.; Jackson, C.J. Plasma protein C levels are directly associated with better outcomes in patients with severe burns. Burn. J. Int. Soc. Burn Inj. 2019, 45, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Liaw, P.C.; Esmon, C.T.; Kahnamoui, K.; Schmidt, S.; Kahnamoui, S.; Ferrell, G.; Beaudin, S.; Julian, J.A.; Weitz, J.I.; Crowther, M.; et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood 2004, 104, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Bernard, G.R.; Dhainaut, J.-F.; Russell, J.R.; Macias, W.L.; Nelson, D.R.; Sundin, D.P. Protein C concentrations in severe sepsis: An early directional change in plasma levels predicts outcome. Crit. Care 2006, 10, R92. [Google Scholar] [CrossRef]
- Lindstrom, O.; Kylanpaa, L.; Mentula, P.; Puolakkainen, P.; Kemppainen, E.; Haapiainen, R.; Fernandez, J.A.; Griffin, J.H.; Repo, H.; Petaja, J. Upregulated but insufficient generation of activated protein C is associated with development of multiorgan failure in severe acute pancreatitis. Crit. Care 2006, 10, R16. [Google Scholar] [CrossRef]
- Cohen, M.J.; Call, M.; Nelson, M.; Calfee, C.S.; Esmon, C.T.; Brohi, K.; Pittet, J.F. Critical Role of Activated Protein C in Early Coagulopathy and Later Organ Failure, Infection and Death in Trauma Patients. Ann. Surg. 2012, 255, 379–385. [Google Scholar] [CrossRef]
- Zhao, R.; Lang, T.C.; Kim, A.; Wijewardena, A.; Vandervord, J.; McGrath, R.; Fitzpatrick, G.; Xue, M.; Jackson, C. Early protein C activation is reflective of burn injury severity and plays a critical role in inflammatory burden and patient outcomes. Burn. J. Int. Soc. Burn. Inj. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Vern, A.; Gamelli, R.L.; Walenga, J.M.; Hoppensteadt, D.; Sharp-Pucci, M.; Schumacher, H.R. The effect of burn wound size on hemostasis: A correlation of the hemostatic changes to the clinical state. J. Trauma 1992, 33, 50–56; discussion 56–57. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Avello, A.; Lorente, J.A.; Cesar-Perez, J.; Garcia-Frade, L.J.; Alvarado, R.; Arevalo, J.M.; Navarro, J.L.; Esteban, A. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb. Res. 1998, 89, 59–64. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kontakiotis, T.; Bitzani, M.; Papaioannou-Gaki, G.; Parlapani, A.; Thomareis, O.; Tsotsolis, N.; Giala, M.-A. Early coagulation disorders after severe burn injury: Impact on mortality. Intensive Care Med. 2008, 34, 700–706. [Google Scholar] [CrossRef]
- Van Haren, R.M.; Thorson, C.M.; Valle, E.J.; Busko, A.M.; Guarch, G.A.; Andrews, D.M.; Pizano, L.R.; Schulman, C.I.; Namias, N.; Proctor, K.G. Hypercoagulability after burn injury. J. Trauma Acute Care Surg. 2013, 75, 37–43; discussion 43. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Campbell, D.; Jackson, C. Protein C Is an Autocrine Growth Factor for Human Skin Keratinocytes. J. Biol. Chem. 2007, 282, 13610–13616. [Google Scholar] [CrossRef]
- Xue, M.; Campbell, D.; Sambrook, P.N.; Fukudome, K.; Jackson, C. Endothelial Protein C Receptor and Protease-Activated Receptor-1 Mediate Induction of a Wound-Healing Phenotype in Human Keratinocytes by Activated Protein C. J. Investig. Dermatol. 2005, 125, 1279–1285. [Google Scholar] [CrossRef]
- Brandstrup, B.; Tønnesen, H.; Beier-Holgersen, R.; Hjortsø, E.; Ørding, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641. [Google Scholar] [CrossRef] [PubMed]
- Minne, L.; Abu-Hanna, A.; De Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit. Care 2009, 12, R161. [Google Scholar] [CrossRef]
- Abbott, T.; Ahmad, T.; Phull, M.; Fowler, A.; Hewson, R.; Biccard, B.; Chew, M.; Gillies, M.; Pearse, R.; Beattie, S.; et al. The surgical safety checklist and patient outcomes after surgery: A prospective observational cohort study, systematic review and meta-analysis. Br. J. Anaesth. 2018, 120, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, E.N.; Gregori, D.; Berchialla, P.; Zingarelli, E.; Cairo, M.; Bollero, D.; Ganem, J.; Capocelli, R.; Cuccuru, F.; Cassano, P.; et al. Epidemiology and Risk Factors for Pathologic Scarring After Burn Wounds. Arch. Facial Plast. Surg. 2008, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.E.; Noel-Storr, A.; Ritchie, C.W. The Impact of General and Regional Anesthesia on the Incidence of Post-Operative Cognitive Dysfunction and Post-Operative Delirium: A Systematic Review with Meta-Analysis. J. Alzheimer’s Dis. 2010, 22, S67–S79. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, J.S. User Guide for the MH Program (Version 1.1). Available online: http://ourworld.compuserve.com/homepages/jsuebersax/mh.htm (accessed on 12 January 2019).
- Moritz, A.R. Studies of Thermal Injury: III. The Pathology and Pathogenesis of Cutaneous Burns. An Experimental Study. Am. J. Pathol. 1947, 23, 915–941. [Google Scholar] [PubMed]
- Raivio, P.; Fernandez, J.; Kuitunen, A.; Griffin, J.H.; Lassila, R.; Petäjä, J. Activation of protein C and hemodynamic recovery after coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2007, 133, 44–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ilmakunnas, M.; Petäjä, J.; Höckerstedt, K.; Mäkisalo, H.; Fernandez, J.A.; Griffin, J.H.; Jansson, S.-E.; Repo, H.; Pesonen, E.J. Activation of protein C during reperfusion in clinical liver transplantation. Transplantation 2003, 75, 467–472. [Google Scholar] [CrossRef]
- Taylor, F.B., Jr.; Stearns-Kurosawa, D.J.; Kurosawa, S.; Ferrell, G.; Chang, A.C.K.; Laszik, Z.; Kosanke, S.; Peer, G.; Esmon, C.T. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood 2000, 95, 1680–1686. [Google Scholar] [CrossRef]
- Iwaki, T.; Cruz, D.T.; Martin, J.A.; Castellino, F.J. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood 2005, 105, 2364–2371. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.; Gu, J.; Hunter, J.; Ferrell, G.L.; Lupu, F.; Esmon, N.L.; Esmon, C.T. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J. Thromb. Haemost. 2005, 3, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Farina, J.A.; Rosique, M.J.; Rosique, R.G. Curbing Inflammation in Burn Patients. Int. J. Inflamm. 2013, 2013, 715645. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.P.; Letson, H.L.; Sharma, R.; Sheppard, F.R.; Cap, A.P. Mechanisms of early trauma-induced coagulopathy: The clot thickens or not? J. Trauma Acute Care Surg. 2015, 79, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, J.; Satterwhite, T.; Allo, M. The use of drotrecogin alfa recombinant activated protein C for severe sepsis in the critically burned patient: A new treatment approach. Burns 2006, 32, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Uchiba, M.; Okajima, K.; Oike, Y.; Ito, Y.; Fukudome, K.; Isobe, H.; Suda, T. Activated Protein C Induces Endothelial Cell Proliferation by Mitogen-Activated Protein Kinase Activation In Vitro and Angiogenesis In Vivo. Circ. Res. 2004, 95, 34–41. [Google Scholar] [CrossRef]
- Jackson, C.J.; Xue, M.; Thompson, P.; Davey, R.A.; Whitmont, K.; Smith, S.; Buisson-Legendre, N.; Sztynda, T.; Furphy, L.J.; Cooper, A.; et al. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 2005, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Whitmont, K.; Reid, I.; Tritton, S.; March, L.; Xue, M.; Lee, M.; Fulcher, G.; Sambrook, P.; Slobedman, E.; Cooper, A.; et al. Treatment of Chronic Leg Ulcers With Topical Activated Protein C. Arch. Dermatol. 2008, 144, 1479–1483. [Google Scholar] [CrossRef]
- Wijewardena, A.; Vandervord, E.; Lajevardi, S.S.; Vandervord, J.; Jackson, C.J. Combination of Activated Protein C and Topical Negative Pressure Rapidly Regenerates Granulation Tissue Over Exposed Bone to Heal Recalcitrant Orthopedic Wounds. Int. J. Low. Extrem. Wounds 2011, 10, 146–151. [Google Scholar] [CrossRef]
- Kapila, S.; Reid, I.; Dixit, S.; Fulcher, G.; March, L.; Jackson, C.; Cooper, A. Use of dermal injection of activated protein C for treatment of large chronic wounds secondary to pyoderma gangrenosum. Clin. Exp. Dermatol. 2014, 39, 785–790. [Google Scholar] [CrossRef]
- Zhao, R.; Jackson, C.J.; Xue, M. Extracellular Matrix and Other Factors that Impact on Cutaneous Scarring. In Chronic Wounds, Wound Dressings and Wound Healing; Shiffman, M.A., Low, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 135–178. [Google Scholar]
- Rae, L.; Fidler, P.; Gibran, N. The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation. Crit. Care Clin. 2016, 32, 491–505. [Google Scholar] [CrossRef]
- Lopez, O.N.; Cambiaso-Daniel, J.; Branski, L.K.; Norbury, W.B.; Herndon, D.N. Predicting and managing sepsis in burn patients: Current perspectives. Ther. Clin. Risk Manag. 2017, 13, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Brueckmann, M.; Hoffmann, U.; de Rossi, L.; Weiler, H.M.; Liebe, V.; Lang, S.; Kaden, J.J.; Borggrefe, M.; Haase, K.K.; Huhle, G. Activated protein C inhibits the release of macrophage inflammatory protein-1-alpha from THP-1 cells and from human monocytes. Cytokine 2004, 26, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, M.; Okajima, K.; Uchiba, M.; Horiuchi, S.; Okabe, H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb. Haemost. 2002, 88, 267–273. [Google Scholar] [CrossRef]
- Franscini, N.; Bachli, E.B.; Blau, N.; Leikauf, M.-S.; Schaffner, A.; Schoedon, G. Gene Expression Profiling of Inflamed Human Endothelial Cells and Influence of Activated Protein C. Circulation 2004, 110, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Xue, M. Anti-inflammatory actions of the anticoagulant, activated protein C. In Inflammatory Diseases–A Modern Perspective; Nagal, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 42–74. [Google Scholar]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential Roles of Macrophages in Diverse Phases of Skin Repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- Brant, J.; Yoon, J.H.; Polvadore, T.; Barbazuk, W.; Maden, M. Cellular events during scar-free skin regeneration in the spiny mouse, Acomys. Wound Repair Regen. 2016, 24, 75–88. [Google Scholar] [CrossRef] [PubMed]


| Biopsied Patients | All Patients | |
|---|---|---|
| Total patients | 34 | 86 |
| Female (%) | 8 (24) | 22 (26) |
| Male (%) | 26 (76) | 64 (74) |
| Age, mean ± SD | 43 ± 16 | 44 ± 19 |
| Burn size, mean ± SD | 22 ± 16 | 21 ± 13 |
| Burn depth | ||
| Partial (%) | 24 (71) | 59 (69) |
| Full (%) | 10 (29) | 27 (31) |
| Burn Biopsy PC | Total | |||||
|---|---|---|---|---|---|---|
| Negative | Low Positive | Positive | High Positive | |||
| Normal biopsy PC | Negative | 0 | 0 | 0 | 0 | 0 (0%) |
| Low positive | 0 | 1 | 3 | 0 | 4 (12%) | |
| Positive | 2 | 6 | 6 | 2 | 16 (47%) | |
| High positive | 0 | 1 | 11 | 2 | 14 (41%) | |
| Total | 2 (6%) | 8 (24%) | 20 (59%) | 4 (12%) | 34 | |
| Burn Biopsy EPCR | Total | |||||
|---|---|---|---|---|---|---|
| Negative | Low Positive | Positive | High Positive | |||
| Normal biopsy EPCR | Negative | 0 | 0 | 0 | 1 | 1 (3%) |
| Low positive | 1 | 6 | 11 | 0 | 19 (56%) | |
| Positive | 0 | 3 | 8 | 2 | 13 (38%) | |
| High positive | 0 | 0 | 1 | 0 | 1 (3%) | |
| Total | 1 (3%) | 9 (27%) | 20 (59%) | 4 (12%) | 34 | |
| Standard Admission | Increased Support | |
|---|---|---|
| Total patients | 24 | 10 |
| Female (%) | 6 (67) | 3 (33) |
| Male (%) | 18 (72) | 7 (28) |
| Age, mean ± SD | 43 ± 17 | 41 ± 14 |
| Burn size, mean ± SD | 19 ± 12 | 30 ± 21 |
| Burn depth | ||
| Partial (%) | 20 (83) | 4 (17) |
| Full (%) | 4 (4) | 6 (60) |
| Length of stay, mean ± SD | 19 ± 31 | 60 ± 56 |
| ICU length of stay, mean ± SD | 0 ± 1 | 21 ± 30 |
| Number of surgeries, mean ± SD | 2 ± 1 | 8 ± 5 |
| Mean IV fluids per day over first 72 h, mean ± SD | 2 ± 1 | 5 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wang, D.; Lang, T.C.; Kim, A.; Wijewardana, A.; Vandervord, J.; McGrath, R.; Fulcher, G.; Lin, H.; Xue, M.; et al. Epidermal Protein C Levels Correspond to Local Injury Severity and Increased Clinical Support in Burn Patients. Eur. Burn J. 2021, 2, 226-237. https://doi.org/10.3390/ebj2040017
Zhao R, Wang D, Lang TC, Kim A, Wijewardana A, Vandervord J, McGrath R, Fulcher G, Lin H, Xue M, et al. Epidermal Protein C Levels Correspond to Local Injury Severity and Increased Clinical Support in Burn Patients. European Burn Journal. 2021; 2(4):226-237. https://doi.org/10.3390/ebj2040017
Chicago/Turabian StyleZhao, Ruilong, Duo Wang, Thomas Charles Lang, Albert Kim, Aruna Wijewardana, John Vandervord, Rachel McGrath, Gregory Fulcher, Haiyan Lin, Meilang Xue, and et al. 2021. "Epidermal Protein C Levels Correspond to Local Injury Severity and Increased Clinical Support in Burn Patients" European Burn Journal 2, no. 4: 226-237. https://doi.org/10.3390/ebj2040017
APA StyleZhao, R., Wang, D., Lang, T. C., Kim, A., Wijewardana, A., Vandervord, J., McGrath, R., Fulcher, G., Lin, H., Xue, M., & Jackson, C. J. (2021). Epidermal Protein C Levels Correspond to Local Injury Severity and Increased Clinical Support in Burn Patients. European Burn Journal, 2(4), 226-237. https://doi.org/10.3390/ebj2040017








