Imaging Techniques Used for Wound Healing Assessment: A Systematic Review Part 1 Chronic Wounds
Abstract
:1. Introduction
Aim
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
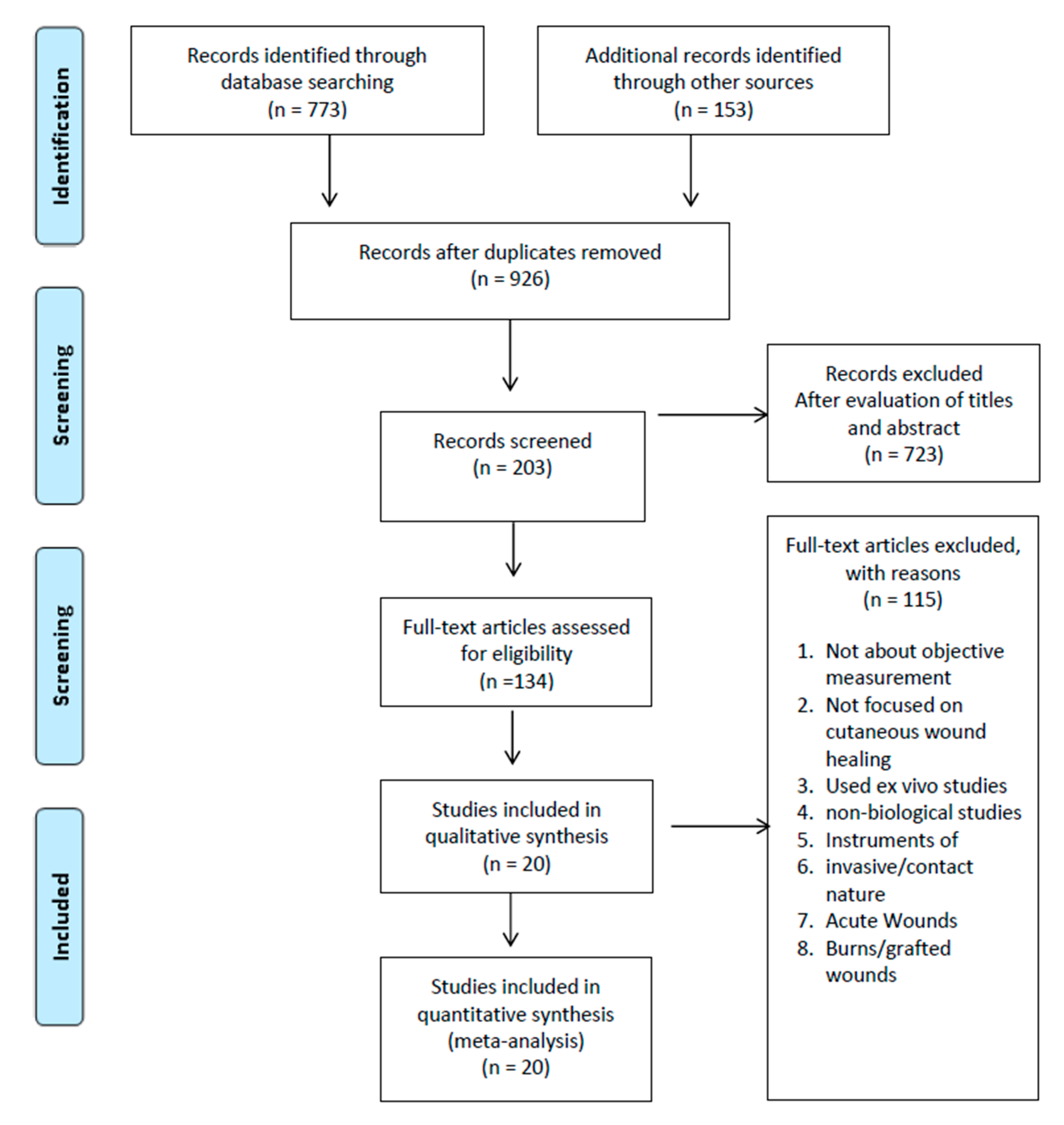
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
3.1. Morphological Assessment
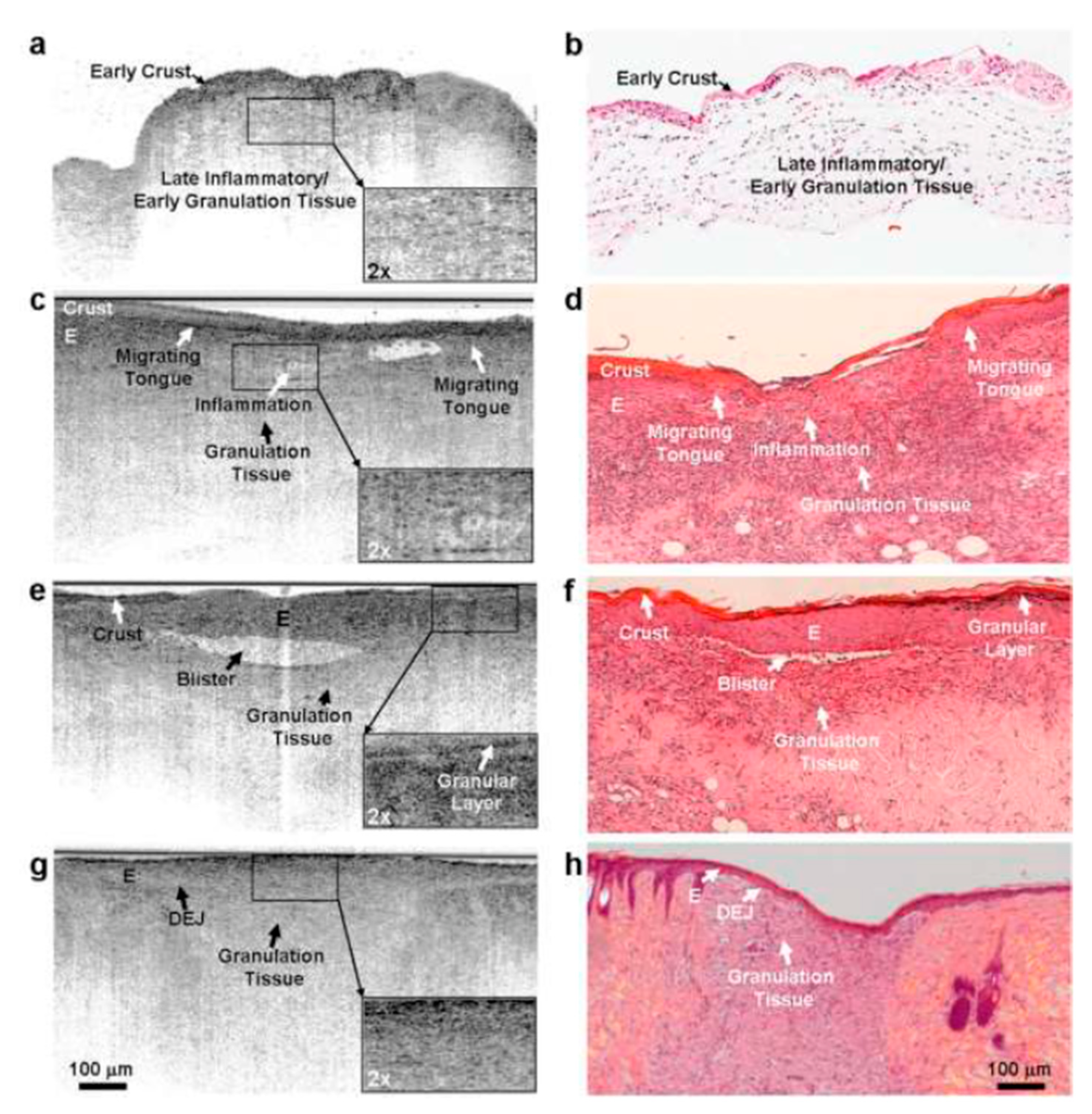
3.1.1. Optical Coherence Tomography
Summary
3.1.2. Confocal Microscopy
Summary
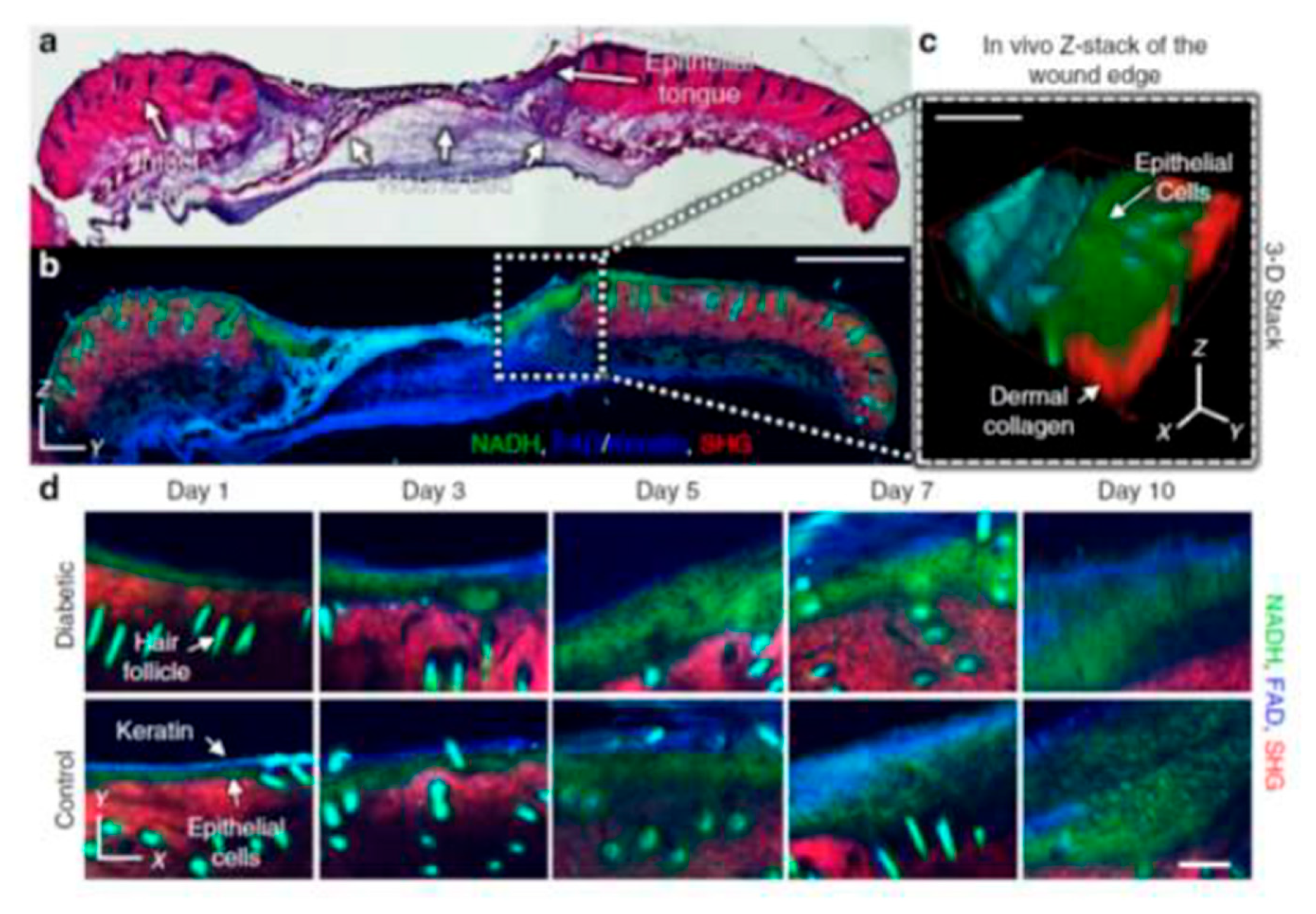
3.1.3. Multiphoton Tomography (MPT)
Summary
3.2. Physiology
3.2.1. Hyperspectral Imaging
Summary
3.3. Wound Size and Volume
3.3.1. 2D Imaging
Summary
3.3.2. 3D Imaging
Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.; Robinson, L.; Booth, H.; Knapp, M.; Jagger, C.; MODEM Project. Projections of multi-morbidity in the older population in England to 2035: Estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing 2018, 47, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ud-Din, S.; Bayat, A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Exp. Dermatol. 2016, 25, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Pillen, H.; Miller, M.; Thomas, J.; Puckridge, P.; Sandison, S.; Spark, J. Assessment of Wound Healing: Validity, Reliability and Sensitivity of Available Instruments. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2009, 17, 208–217. [Google Scholar]
- Santamaria, N.; Austin, D.; Clayton, L. A Multi-site Clinical Evaluation Trial of the Alfred/Medseed Wound Imaging System Prototype. Prim. Intent. Aust. J. Wound Manag. 2002, 10, 120–125. [Google Scholar]
- Khaodhiar, L.; Dinh, T.; Schomacker, K.T.; Panasyuk, S.V.; Freeman, J.E.; Lew, R.; Vo, T.; Panasyuk, A.A.; Lima, C.; Giurini, J.M.; et al. The Use of Medical Hyperspectral Technology to Evaluate Microcirculatory Changes in Diabetic Foot Ulcers and to Predict Clinical Outcomes. Diabetes Care 2007, 30, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Kuck, M.; Strese, H.; Alawi, M.S.A.; Meinke, M.C.; Fluhr, J.W.; Burbach, G.J.; Krah, M.; Sterry, W.; Lademann, J. Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing. Ski. Res. Technol. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- Cobb, M.J.; Chen, Y.; Underwood, R.A.; Usui, M.L.; Olerud, J.; Li, X. Noninvasive assessment of cutaneous wound healing using ultrahigh-resolution optical coherence tomography. J. Biomed. Opt. 2006, 11, 064002. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.; Brezinski, M.E. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Lange-Asschenfeldt, S.; Bob, A.; Terhorst, D.; Ulrich, M.; Fluhr, J.W.; Mendez, G.; Roewert-Huber, H.-J.; Stockfleth, E.; Lange-Asschenfeldt, B. Applicability of confocal laser scanning microscopy for evaluation and monitoring of cutaneous wound healing. J. Biomed. Opt. 2012, 17, 076016. [Google Scholar] [CrossRef]
- Deka, G.; Wu, W.-W.; Kao, F.-J. In vivowound healing diagnosis with second harmonic and fluorescence lifetime imaging. J. Biomed. Opt. 2012, 18, 061222. [Google Scholar] [CrossRef] [Green Version]
- Riemann, I.; Ehlers, A.; LeHarzic, R.; Martin, S.; Reif, A.; König, K. In vivo multiphoton tomography of skin during wound healing and scar formation. Multiphot. Microsc. Biomed. Sci. VII 2007, 6442, 644226. [Google Scholar] [CrossRef]
- König, K.; Weinigel, M.; Bückle, R.; Kaatz, M.; Hipler, C.; Zens, K.; Schneider, S.W.; Huck, V. Monitoring wound healing by multiphoton tomography/endoscopy. Photonic Ther. Diagn. XI 2015, 9303, 93030F. [Google Scholar] [CrossRef]
- Jones, J.; Ramser, H.E.; Woessner, A.E.; Quinn, K.P. In vivo multiphoton microscopy detects longitudinal metabolic changes associated with delayed skin wound healing. Commun. Biol. 2018, 1, 198. [Google Scholar] [CrossRef]
- Springer, S.; Zieger, M.; Böttcher, A.; Lademann, J.; Kaatz, M. Examination of wound healing after curettage by multiphoton tomography of human skin in vivo. Ski. Res. Technol. 2017, 23, 452–458. [Google Scholar] [CrossRef]
- Nouvong, A.; Hoogwerf, B.; Mohler, E.; Davis, B.; Tajaddini, A.; Medenilla, E. Evaluation of Diabetic Foot Ulcer Healing With Hyperspectral Imaging of Oxyhemoglobin and Deoxyhemoglobin. Diabetes Care 2009, 32, 2056–2061. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.H. A Survey of Optical Imaging Techniques for Assessing Wound Healing. Int. J. Intell. Control Syst. 2012, 17, 79–85. [Google Scholar]
- Plassmann, P.; Jones, T. MAVIS: A non-invasive instrument to measure area and volume of wounds. Med. Eng. Phys. 1998, 20, 332–338. [Google Scholar] [CrossRef]
- Sheehan, P.; Jones, P.; Caselli, A.; Giurini, J.M.; Veves, A. Percent Change in Wound Area of Diabetic Foot Ulcers Over a 4-Week Period Is a Robust Predictor of Complete Healing in a 12-Week Prospective Trial. Diabetes Care 2003, 26, 1879–1882. [Google Scholar] [CrossRef] [Green Version]
- Steed, D.L.; Attinger, C.; Colaizzi, T.; Rn, M.C.; Franz, M.; Harkless, L.; Bs, A.J.; Moosa, H.; Robson, M.; Serena, T.; et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006, 14, 680–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprigle, S.; Nemeth, M.; Gajjala, A. Iterative design and testing of a hand-held, non-contact wound measurement device. J. Tissue Viability 2012, 21, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.E.; Nixon, M.A. The Reliability of a Handheld Wound Measurement and Documentation Device in Clinical Practice. J. Wound Ostomy Cont. Nurs. 2011, 38, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Karimi, L.; Donohue, L.; Kapp, S. Interrater and Intrarater Reliability of Silhouette Wound Imaging Device. Adv. Ski. Wound Care 2012, 25, 513–518. [Google Scholar] [CrossRef]
- Foltynski, P.; Ladyzynski, P.; Sabalinska, S.; Wojcicki, J.M. Accuracy and Precision of Selected Wound Area Measurement Methods in Diabetic Foot Ulceration. Diabetes Technol. Ther. 2013, 15, 711–720. [Google Scholar] [CrossRef]
- Ladyzynski, P.; Foltynski, P.; Molik, M.; Tarwacka, J.; Migalska-Musial, K.; Mlynarczuk, M.; Wojcicki, J.M.; Krzymien, J.; Karnafel, W. Area of the Diabetic Ulcers Estimated Applying a Foot Scanner–Based Home Telecare System and Three Reference Methods. Diabetes Technol. Ther. 2011, 13, 1101–1107. [Google Scholar] [CrossRef]
- AThawer, H.; EHoughton, P.; Woodbury, M.G.; Keast, D.; Campbell, K. A comparison of computer-assisted and manual wound size measurement. Ostomy Wound Manag. 2002, 48, 46–53. [Google Scholar]
- Bhedi, A.; Saxena, A.K.; Gadani, R.; Patel, R. Digital Photography and Transparency-Based Methods for Measuring Wound Surface Area. Indian J. Surg. 2013, 75, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Rajbhandari, S.M.; Harris, N.D.; Sutton, M.; Lockett, C.; Eaton, S.; Gadour, M.; Tesfaye, S.; Ward, J.D. Digital imaging: An accurate and easy method of measuring foot ulcers. Diabet. Med. 1999, 16, 339–342. [Google Scholar] [CrossRef]
- Kecelj-Leskovec, N.; Jezeršek, M.; Možina, J.; Pavlović, M.D.; Lunder, T. Measurement of venous leg ulcers with a laser-based three-dimensional method: Comparison to computer planimetry with photography. Wound Repair Regen. 2007, 15, 767–771. [Google Scholar] [CrossRef]
- Gardner, S.E.; Frantz, R.A.; Hillis, S.; Blodgett, T.J.; Femino, L.M.; Lehman, S.M. Volume Measures Using a Digital Image Analysis System are Reliable in Diabetic Foot Ulcers. Wounds Compend. Clin. Res. Pract. 2012, 24, 146–151. [Google Scholar]
- Körber, A.; Rietkötter, J.; Grabbe, S.; Dissemond, J. Three-dimensional documentation of wound healing: First results of a new objective method for measurement. J. Der Dtsch. Dermatol. Ges. 2006, 4, 848–854. [Google Scholar] [CrossRef]
- Davis, A.; Nishimura, J.; Seton, J.; Goodman, B.; Ho, C.; Bogie, K. Repeatability and clinical utility in stereophotogrammetric measurements of wounds. J. Wound Care 2013, 22, 90–97. [Google Scholar] [CrossRef]
- Nemeth, M.; Sprigle, S.; Gajjala, A. Clinical usability of a wound measurement device. J. Spinal Cord Med. 2010. Available online: http://hdl.handle.net/1853/43276 (accessed on 19 November 2020).
- Chang, A.C.; Dearman, B.; Greenwood, J.E. A Comparison of Wound Area Measurement Techniques: Visitrak Versus Photography. Eplasty 2011, 11, e18. [Google Scholar]
- Haghpanah, S.; Bogie, K.; Wang, X.; Banks, P.G.; Ho, C.H. Reliability of Electronic Versus Manual Wound Measurement Techniques. Arch. Phys. Med. Rehabil. 2006, 87, 1396–1402. [Google Scholar] [CrossRef]
- Sirazitdinova, E.; Deserno, T.M. System design for 3D wound imaging using low-cost mobile devices. Burns 2017, 1, 761–769. [Google Scholar]
- Zvietcovich, F.; Castaneda, B.; Valencia, B.; Llanos-Cuentas, A. A 3D assessment tool for accurate volume measurement for monitoring the evolution of cutaneous Leishmaniasis wounds. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar]
- Pierce, M.C.; Strasswimmer, J.; Park, B.H.; Cense, B.; de Boer, J. Advances in Optical Coherence Tomography Imaging for Dermatology. J. Investig. Dermatol. 2004, 123, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Bowling, F.L.; King, L.; Fadavi, H.; Paterson, J.A.; Preece, K.; Daniel, R.W.; Matthews, D.J.; Boulton, A.J.M. An assessment of the accuracy and usability of a novel optical wound measurement system. Diabet. Med. 2009, 26, 93–96. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [Green Version]
- Galiano, R.D.; Michaels, V.J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; ASørensen, J.; Jemec, G.B.; Yderstraede, K.B. Methods to assess area and volume of wounds—A systematic review. Int. Wound J. 2015, 13, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef] [Green Version]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [Green Version]


| Anatomical | No. Study | Average | Range |
|---|---|---|---|
| Optical coherence tomography | 1 | 13 | 13 |
| Confocal microscopy | 1 | 13 | 13 |
| Multiphoton tomography | 2 | 12 | 12 |
| Physiological | |||
| Hyperspectral imaging | 2 | 14 | 14 |
| Wound size and volume | |||
| 3D imaging | 6 | 11 | 9–13 |
| 2D imaging | 9 | 14 | 10–14 |
| Paper | First Author | Year | Animal/Human | Patient NO | Wound Type | Statistical Method | Comparator | Outcome | Feasibility and Resolution | Companies |
|---|---|---|---|---|---|---|---|---|---|---|
| Optical coherence tomography | ||||||||||
| Evaluation of optical coherence tomography as a noninvasive diagnostic tool in Cutaneous wound healing. | Kuck, Monika | 2014 | human | 6 | Chronic wound | nil | histology | Visualisation of skin layers at each stage of healing | Lateral: 8 µm Axial: 5 µm Scan depth: 1–2 mm | Optovue, NinePoint Medical, and Thorlabs expensive |
| Multiphoton tomography | ||||||||||
| Monitoring Wound Healing by Multiphoton Tomography/Endoscopy | karsten konig | 2016 | human | 20 | Acutre and chronic | nil | nill | Detects tissue morphology and physiology changes during healing | Horizontal: 1 µm Vertical: 2 µm Scan depth: 200 µm 750 nm wavelength | Dermatinspectmost expensive |
| Invivo multiphoton micrsotoopy detects logitudinal metabolic changes associated with | jake D.jones | 2018 | animal | 37 | chronic and acute wound | ANOVA | normal wound | |||
| Confocal microscopy | ||||||||||
| Applicability of confocal laser scanning microscopy for evaluation and monitoring of cutaneous wound healing | susanne lange asschenfeldt | 2015 | human | 15 | acute woundchronic wounds | nil | nil | Detects tissue morphology and physiology changes during healing | Horizontal: 1.25 µm Vertical: 5.0 µm Scan depth: 200 µm | Vivascope less expensive |
| Hyperspectral imaging | ||||||||||
| Evaluation of Diabetic Foot Ulcer Healing With Hyperspectral Imaging of Oxyhemoglobin and Deoxyhemoglobin | Aksone Nouvong | 2009 | human | 72 | Foot ulcer | CHI square, T test | nil | H.T. index: sensitivity 80%, Specificity 74% positive predictive:90% | Lateral: 0.4–1.0 µm | Hyspex, Specim Cheapest |
| The Use of Medical Hyperspectral Technology to Evaluate Microcirculatory Changes in Diabetic Foot Ulcers and to Predict Clinical Outcomes. | Lalita Khaodhiar | 2007 | human | 21 | foot ulcer | ANOVA | nil | H.T. index: sensitivity 93%, Specificity 86% positive/negative predictive:93%/ 68% | ||
| 3D imaging | ||||||||||
| Three-dimensional documentation of wound healing: First results of a new objective method for measurement | Andreas Korber | 2006 | human | 3 | venous/arterial ulcer | non | non | Precise 3d rendition of the wound | 2–5 s | |
| MAVIS: a noninvasive instrument to measure area and volume of wounds | P.plassman | 1998 | human | 50 | variety | Pearson correlation coefficient standard deviation in % | manual planimetryalginate casts | STD deviation: Planetary: 4% (large wounds)-20% small wound mavis: 3–5% Wound volume Alginate: 5–40% Mavis: 5% smaller on average | non invasive fast and accurate | |
| Assessment of chronic wounds by three-dimensional optical imaging based on integrating geometrical, chromatic, and thermal data | Barone | 2011 | human | 7 | leg ulcers | Percentage difference to clinical assessment | Clinical assessment | Measurement of a known volume: 4–7% different on colour images, 5%–12% on thermal images | Lateral resolution: 0.2 mm Area 200 mm × 150 mm 3D scanner expensive | |
| Measurement of venous leg ulcers with a laser-based three-dimensional method comparison to computer planimetry with photography. | Kcelj leskovec | 2007 | human | 15 | venous ulcer | Bland altman analysis | computer planetary | Precision of 3d imaging Volume:7.5% Perimeter: 5.8% Area: 9.7% | area 100 × 100 × 100 mm | |
| Repeatability and clinical utility in stereophotogrammetric measurements of wounds | A.J.Davis | 2013 | human | 13 | chronic wound | Inter class correlation coefficient ANOVA | clinical assessment | Interclass correlation: 0.9867 | wound <6 cm | |
| Volume Measures Using a Digital Image Analysis System are Reliable in Diabetic Foot Ulcers | Gardner | 2012 | human | 34 | foot ulcer | inter rater and intrarater reliability | nil | inter rater reliability 0.970 intra rater reliability 0.981 | easy to use | |
| 2D Imaging | ||||||||||
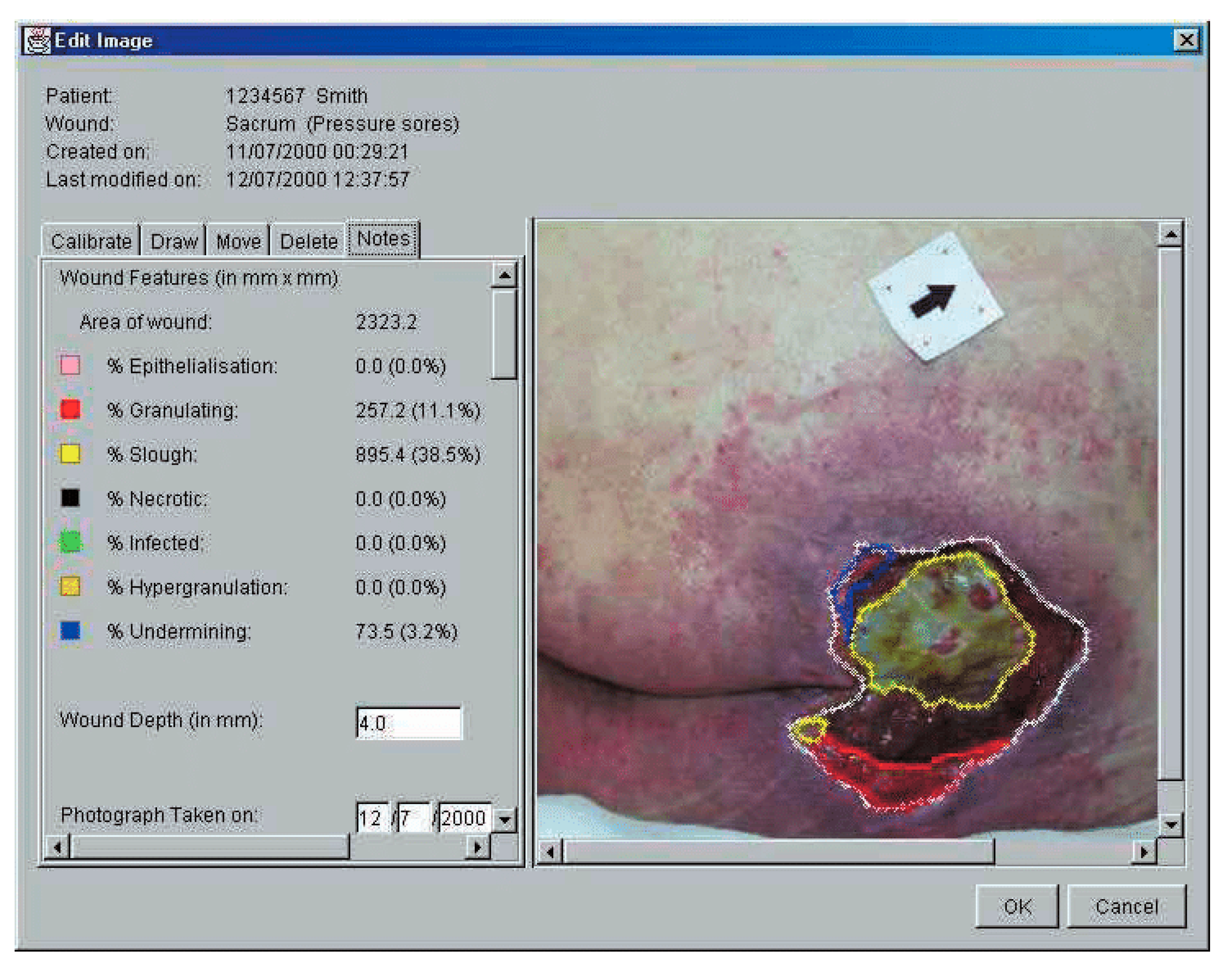
 | santamaria | 2002 | human | 100 | multiple | measurement error pearson correlation coefficient | nil | measurement error:5.1% ICC: 69.7 (p < 0.01) | easy to use, more effective costly | |
| A comparison of computer-assisted and manual wound size measure-ment. | thawar et al. | 2002 | human | 83 | chronic wound + acute | interclass cofficient: ANOVA Standard error of measurement | nil | Planetary: ICC 0.99, SEM 0.18 Digital imaging: ICC 0.94, SEM 0.94 | digital imaging was costly | |
| Digital photography and transparency-based methods for measuring wound surface area. | Bhedi | 2013 | human | 40 | various wounds | mean values and standard deviation | nil | no significant difference between manual planimerty and digital imaging | n/a | |
| Digital imaging: an accurate and easy method of measuring foot ulcers | S.M.Rajbhandari | 2001 | human | 30 | foot ulcer | Reliability coefficicnet of variation | nil | CV: 16% for digital imaging. CV:27% for graph method | digital imaging was faster and easier. | |
| Iterative design and testing of a handheld, non-contact wound measurement device. | springle | 2011 | human | 19 | preassure ulcer | Mean arror intrer and inter class coefficient | nil | Mean errors for WMD were 1.90% at 0 degrees, 1° 76% at 5° and 4⋅28% at 10 degrees Intra-rater ICC for WMD > 0.975. Inter-rater ICC was 0.966 | WMD, low cost | |
| The reliability of a handheld wound measurement and documentation device in clinical practice. | Hammond and Nixon | 2011 | human | 5 | chronic wound | inter and intra-rater variation | nil | intra-rater: 97.4% inter-rater: 96.8% ICC: 99⋅76%. | n/a | |
| interrater and Intrarater reliability of silhouette wound imaging device. | miller | 2012 | human | 14 | Mix wounds | inter and intra-rater variation | nil | Inter rater: 0.985 (large), 0.624 (small) Intra rater: no difference | n/a | |
| Accuracy and Precision of Selected Wound Area Measurement Methods in Diabetic Foot Ulceration | Foltynski | 2013 | human | 16 | diabetic wounds | Relative error cofficient of variation | nil | RE: Vistrak 6.3, TeleDiafos 2.1, Silhouette 2.3 CV: Vistrak 6.3, TeleDiafos 1.6, Silhouette 3.1 | System limited to foot wounds | |
| Area of the diabetic ulcers estimated applying a foot scanner-based home telecare system and three reference methods. | Ladyzynski | 2011 | human | 36 | diabetic wounds | linear regression, Bland althman analysis | nil | ICC: TeleDiafos vs. Vistrak:0.985 TeleDiafos vs. Silhoutte: 0.987 | System limited to foot wounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, P.; Lim, J.; Moiemen, N. Imaging Techniques Used for Wound Healing Assessment: A Systematic Review Part 1 Chronic Wounds. Eur. Burn J. 2021, 2, 194-214. https://doi.org/10.3390/ebj2040015
Tan P, Lim J, Moiemen N. Imaging Techniques Used for Wound Healing Assessment: A Systematic Review Part 1 Chronic Wounds. European Burn Journal. 2021; 2(4):194-214. https://doi.org/10.3390/ebj2040015
Chicago/Turabian StyleTan, Poh, Joanne Lim, and Naiem Moiemen. 2021. "Imaging Techniques Used for Wound Healing Assessment: A Systematic Review Part 1 Chronic Wounds" European Burn Journal 2, no. 4: 194-214. https://doi.org/10.3390/ebj2040015
APA StyleTan, P., Lim, J., & Moiemen, N. (2021). Imaging Techniques Used for Wound Healing Assessment: A Systematic Review Part 1 Chronic Wounds. European Burn Journal, 2(4), 194-214. https://doi.org/10.3390/ebj2040015







