Role of Salinity on Phosphorous Removal by Chaetoceros muelleri
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation
2.2. Experimental Design and Microalgae Performance
2.3. Removal of Phosphorus from Culture Media by Microalgae
2.4. Biomass Recovery
2.5. Statistical Analysis
3. Results
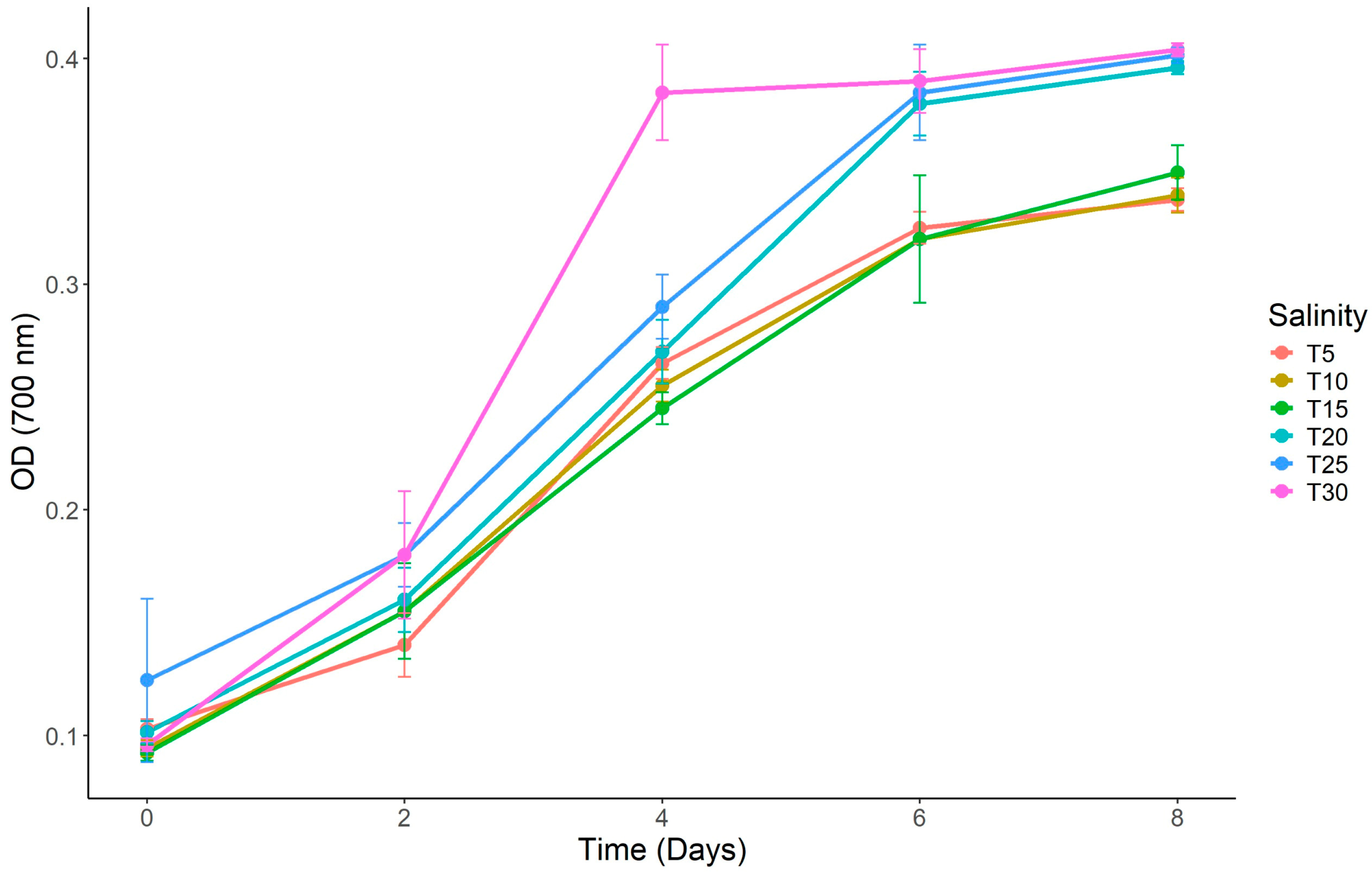
3.1. Culture Performance
3.2. Phosphorus Removal by C. muelleri
3.3. Biomass Yield and Harvesting Efficiency
4. Discussion
| Study | Microalgae Species | P Removal (%) | Biomass Yield (g L−1) | Culture Conditions |
|---|---|---|---|---|
| This study | Chaetoceros muelleri | 28–52% | 1.35–3.47 | Synthetic saline media, 6 salinity levels (T5–T30), 8-day culture |
| Huy et al. (2018) [54] | Chlorella sp. (dominant) | 100% | 0.4 | Textile effluent, open system, 13 days |
| Arias et al. (2018) [55] | Scenedesmus sp. | 100% | Not reported | Secondary effluent, closed 30 L PBR, 8-day HRT |
| Li et al. (2011) [56] | Chlorella sp. | 80.9% | Not reported | Domestic sewage, stationary culture |
| Abou-Shanab et al. (2013) [57] | Chlamydomonas sp. | 28% | Not reported | Synthetic wastewater, batch culture |
| Li et al. (2018) [58] | Coelastrella sp. | 12.6–84.9% | Not reported | Swine effluent, copper oxide stress, 16 days |
| Gao et al. (2013) [59] | C. muelleri | 64% | 0.24 | Nutrient-restricted F/2 medium, 12 days |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stead, S.M. Rethinking Marine Resource Governance for the United Nations Sustainable Development Goals. Curr. Opin. Environ. Sustain. 2018, 34, 54–61. [Google Scholar] [CrossRef]
- Techera, E.J. Supporting Blue Economy Agenda: Fisheries, Food Security and Climate Change in the Indian Ocean. J. Indian Ocean. Reg. 2018, 14, 7–27. [Google Scholar] [CrossRef]
- Ryan, M.H.; Tibbett, M.; Lambers, H.; Bicknell, D.; Brookes, P.; Barrett-Lennard, E.G.; Ocampo, C.; Nicol, D. Pronounced Surface Stratification of Soil Phosphorus, Potassium and Sulfur under Pastures Upstream of a Eutrophic Wetland and Estuarine System. Soil Res. 2017, 55, 657–669. [Google Scholar] [CrossRef]
- Sarelli, A.; Sykas, D.; Miltiadou, M.; Bliziotis, D.; Spastra, Y.; Ieronymaki, M. A Novel Automated Methodology That Estimates the United Nations (UN) Sustainable Development Goal (SDG) 14.1.1.: Index of Coastal Eutrophication Using the Copernicus Marine Environment Monitoring Service (CMEMS). In Proceedings of the Sixth International Conference on Remote Sensing and Geoinformation of the Environment (RSCy2018), Paphos, Cyprus, 6 August 2018; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; Volume 10773, p. 1077302. [Google Scholar]
- Shore, M.; Murphy, S.; Mellander, P.-E.; Shortle, G.; Melland, A.R.; Crockford, L.; O’Flaherty, V.; Williams, L.; Morgan, G.; Jordan, P. Influence of Stormflow and Baseflow Phosphorus Pressures on Stream Ecology in Agricultural Catchments. Sci. Total Environ. 2017, 590–591, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Jarvie, H.P.; Sharpley, A.N.; Withers, P.J.A.; Scott, J.T.; Haggard, B.E.; Neal, C. Phosphorus Mitigation to Control River Eutrophication: Murky Waters, Inconvenient Truths, and “Postnormal” Science. J. Environ. Qual. 2013, 42, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Rivers, M.R.; Weaver, D.M.; Smettem, K.R.J.; Davies, P.M. Estimating Farm to Catchment Nutrient Fluxes Using Dynamic Simulation Modelling—Can Agri-Environmental BMPs Really Do the Job? J. Environ. Manag. 2013, 130, 313–323. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Bergström, L.; Aronsson, H.; Bechmann, M.; Bolster, C.H.; Börling, K.; Djodjic, F.; Jarvie, H.P.; Schoumans, O.F.; Stamm, C.; et al. Future Agriculture with Minimized Phosphorus Losses to Waters: Research Needs and Direction. AMBIO 2015, 44, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and Perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Uddin, S.; Bebhehani, M.; Al-Musallam, L.; Kumar, V.V.; Sajid, S. Po Uptake in Microalgae at Different Seawater pH: An Experimental Study Simulating Ocean Acidification. Mar. Pollut. Bull. 2020, 151, 110844. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-Adapted Microalgae for Fucoxanthin Production: Effect of Incremental Increase in Salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Sigman, D.M.; Hain, M.P. The Biological Productivity of the Ocean. Nat. Educ. Knowl. 2012, 3, 21. [Google Scholar]
- de-Bashan, L.E.; Bashan, Y. Immobilized Microalgae for Removing Pollutants: Review of Practical Aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, L. The Microalgal Cell. In Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–19. ISBN 978-0-470-99528-0. [Google Scholar]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Kassim, M.A.; Yu, J.; Bhattacharya, S. Thermogravimetric Study of the Combustion of Tetraselmis suecica Microalgae and Its Blend with a Victorian Brown Coal in O2/N2 and O2/CO2 Atmospheres. Bioresour. Technol. 2013, 150, 15–27. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Falkowski, P.G. Ocean Science: The Power of Plankton. Nature 2012, 483, 17–20. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An Overview: Biomolecules from Microalgae for Animal Feed and Aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Lourenço, S.O. Cultivo de Microalgas Marinhas: Princípios e Aplicações; RiMa: Miami, FL, USA, 2006; ISBN 978-85-7656-113-2. [Google Scholar]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Reijnders, M.J.M.F.; van Heck, R.G.A.; Lam, C.M.C.; Scaife, M.A.; dos Santos, V.A.P.M.; Smith, A.G.; Schaap, P.J. Green Genes: Bioinformatics and Systems-Biology Innovations Drive Algal Biotechnology. Trends Biotechnol. 2014, 32, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, A.; Bilal Khan, M. Progress in Energy from Microalgae: A Review. Renew. Sustain. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Sanhueza, C.J.; Valenzuela, B.R. Las microalgas: Una fuente renovable para la obtención de ácidos grasos omega-3 de cadena larga para la nutrición humana y animal. Rev. Chil. Nutr. 2015, 42, 306–310. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Benning, C. Triacylglycerol Accumulation in Photosynthetic Cells in Plants and Algae. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2016; pp. 179–205. ISBN 978-3-319-25979-6. [Google Scholar]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Gangl, D.; Zedler, J.A.Z.; Rajakumar, P.D.; Martinez, E.M.R.; Riseley, A.; Włodarczyk, A.; Purton, S.; Sakuragi, Y.; Howe, C.J.; Jensen, P.E.; et al. Biotechnological Exploitation of Microalgae. J. Exp. Bot. 2015, 66, 6975–6990. [Google Scholar] [CrossRef]
- Leu, S.; Boussiba, S. Advances in the Production of High-Value Products by Microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional Evaluation of Microalgae Oils Rich in Omega-3 Long Chain Polyunsaturated Fatty Acids as an Alternative for Fish Oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of Biodiesel from Microalgae through Biological Carbon Capture: A Review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef]
- Kenny, P.; Flynn, K.J. In Silico Optimization for Production of Biomass and Biofuel Feedstocks from Microalgae. J. Appl. Phycol. 2015, 27, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.-L.; Montero, Z.; Cuaresma, M.; Ruiz-Domínguez, M.-C.; Mogedas, B.; Nores, I.G.; González del Valle, M.; Vílchez, C. Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa Onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes 2020, 8, 324. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds-a Brief Review of Recent Work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of Growth Rate and Nutrient Content of Five Microalgae Species Cultivated in Greenhouses. Plants 2019, 8, 279. [Google Scholar] [CrossRef]
- Jaramillo-Madrid, A.C.; Ashworth, J.; Ralph, P.J. Levels of Diatom Minor Sterols Respond to Changes in Temperature and Salinity. J. Mar. Sci. Eng. 2020, 8, 85. [Google Scholar] [CrossRef]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Sinninghe Damste, J.S. A Comprehensive Study of Sterols in Marine Diatoms (Bacillariophyta): Implications for Their Use as Tracers for Diatom Productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and Dissolution of Biogenic Silica in the Ocean: Revised Global Estimates, Comparison with Regional Data and Relationship to Biogenic Sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Wang, L.; Shi, L.; Xu, D.; Zhang, X.; Ye, N. Features of Metabolic Regulation Revealed by Transcriptomic Adaptions Driven by Long-Term Elevated p CO2 in Chaetoceros muelleri: Metabolic Regulation Driven by Long-Term Elevated p CO2. Phycol. Res. 2020, 68, 236–248. [Google Scholar] [CrossRef]
- de Jesús-Campos, D.; López-Elías, J.A.; Medina-Juarez, L.Á.; Carvallo-Ruiz, G.; Fimbres-Olivarria, D.; Hayano-Kanashiro, C. Chemical Composition, Fatty Acid Profile and Molecular Changes Derived from Nitrogen Stress in the Diatom Chaetoceros muelleri. Aquac. Rep. 2020, 16, 100281. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid Productivity as a Key Characteristic for Choosing Algal Species for Biodiesel Production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Indrayani, I. Isolation and Characterization of Microalgae with Commercial Potential. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2017; p. 225.
- Ishika, T.; Laird, D.W.; Bahri, P.A.; Moheimani, N.R. Co-Cultivation and Stepwise Cultivation of Chaetoceros muelleri and Amphora sp. for Fucoxanthin Production under Gradual Salinity Increase. J. Appl. Phycol. 2019, 31, 1535–1544. [Google Scholar] [CrossRef]
- Minggat, E.; Roseli, W.; Tanaka, Y. Nutrient Absorption and Biomass Production by the Marine Diatom Chaetoceros muelleri: Effects of Temperature, Salinity, Photoperiod, and Light Intensity. J. Ecol. Eng. 2021, 22, 231–240. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8716-3. [Google Scholar]
- Henry-Silva, G.G.; Camargo, A.F.M. Tratamento de efluentes de carcinicultura por macrófitas aquáticas flutuantes. Rev. Bras. Zootec. 2008, 37, 181–188. [Google Scholar] [CrossRef]
- Cotas, J.; Figueirinha, A.; Pereira, L.; Batista, T. The effect of salinity on Fucus ceranoides (Ochrophyta, Phaeophyceae) in the Mondego River (Portugal). J. Oceanol. Limnol. 2019, 37, 881–891. [Google Scholar] [CrossRef]
- Duarte, A.A.; Vieira, J.M.; Neto, J.M.; Pardal, M.A. Monitorização da Hidrodinâmica e da Qualidade da Água no Estuário do Rio Mondego. Eng. Civ. 2008, 33, 65–74. [Google Scholar]
- Lovio-Fragoso, J.P.; Hayano-Kanashiro, C.; López-Elías, J.A. Effect of Different Phosphorus Concentrations on Growth and Biochemical Composition of Chaetoceros muelleri. Lat. Am. J. Aquat. Res. 2019, 47, 361–366. [Google Scholar] [CrossRef]
- Huy, M.; Kumar, G.; Kim, H.-W.; Kim, S.-H. Photoautotrophic Cultivation of Mixed Microalgae Consortia Using Various Organic Waste Streams towards Remediation and Resource Recovery. Bioresour. Technol. 2018, 247, 576–581. [Google Scholar] [CrossRef]
- Arias, D.M.; Solé-Bundó, M.; Garfí, M.; Ferrer, I.; García, J.; Uggetti, E. Integrating Microalgae Tertiary Treatment into Activated Sludge Systems for Energy and Nutrients Recovery from Wastewater. Bioresour. Technol. 2018, 247, 513–519. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a Microalga chlorella sp. Well Adapted to Highly Concentrated Municipal Wastewater for Nutrient Removal and Biodiesel Production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shanab, R.A.I.; Ji, M.-K.; Kim, H.-C.; Paeng, K.-J.; Jeon, B.-H. Microalgal Species Growing on Piggery Wastewater as a Valuable Candidate for Nutrient Removal and Biodiesel Production. J. Environ. Manag. 2013, 115, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W.L.; He, H.; Wu, S.; Zhou, Q.; Yang, C.; Zeng, G.; Luo, L.; Lou, W. Responses of Microalgae coelastrella sp. to Stress of Cupric Ions in Treatment of Anaerobically Digested Swine Wastewater. Bioresour. Technol. 2018, 251, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, M.; Wang, C. Nutrient Deprivation Enhances Lipid Content in Marine Microalgae. Bioresour. Technol. 2013, 147, 484–491. [Google Scholar] [CrossRef] [PubMed]

| Chaetoceros muelleri | Treatment | |||||
|---|---|---|---|---|---|---|
| T30 | T25 | T20 | T15 | T10 | T5 | |
| Salinity | 30 | 25 | 20 | 15 | 10 | 5 |
| Optical Densities (OD700 nm) | ||||||
|---|---|---|---|---|---|---|
| T30 | T25 | T20 | T15 | T10 | T5 | |
| Initial | 0.096 ± 0.007 | 0.110 ± 0.012 | 0.103 ± 0.01 | 0.092 ± 0.001 | 0.094 ± 0.002 | 0.103 ± 0.005 |
| Final | 0.404 ± 0.004 a | 0.401 ± 0.003 b | 0.396 ± 0.004 c | 0.347 ± 0.002 d | 0.339 ± 0.002 e | 0.338 ± 0.003 e |
| Percentage of Change | 320.83% | 264.55% | 284.47% | 277.17% | 260.64% | 228.16% |
| Time Period | Quantity (mg L−1) | |||||
|---|---|---|---|---|---|---|
| T30 | T25 | T20 | T15 | T10 | T5 | |
| Initial | 0.562 ± 0.0010 | 0.573 ± 0.0010 | 0.529 ± 0.0010 | 0.571 ± 0.0006 | 0.554 ± 0.0010 | 0.589 ± 0.0010 |
| Final P % removal | 0.303 ± 0.0036 46.08 ± 0.67% a | 0.320 ± 0.0029 44.15 ± 0.77% b | 0.305 ± 0.0018 42.34 ± 0.30% c | 0.350 ± 0.0021 38.80 ± 0.30% d | 0.346 ± 0.0017 37.54 ± 0.41% e | 0.375 ± 0.0026 36.33 ± 0.55% f |
| Microalgae | Biomass Yields (g L−1) | |||||
|---|---|---|---|---|---|---|
| T30 | T25 | T20 | T15 | T10 | T5 | |
| Chaetoceros muelleri | 3.38 ± 0.07 a | 3.47 ± 0.04 a | 2.88 ± 0.04 b | 2.08 ± 0.02 c | 1.53 ± 0.03 d | 1.35 ± 0.08 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, G.S.; Santigado, C.S.; Silva, L.C.B.; Cotas, J.; Pereira, L. Role of Salinity on Phosphorous Removal by Chaetoceros muelleri. Oceans 2025, 6, 79. https://doi.org/10.3390/oceans6040079
Araújo GS, Santigado CS, Silva LCB, Cotas J, Pereira L. Role of Salinity on Phosphorous Removal by Chaetoceros muelleri. Oceans. 2025; 6(4):79. https://doi.org/10.3390/oceans6040079
Chicago/Turabian StyleAraújo, Glacio S., Clarice S. Santigado, Lucas C. B. Silva, João Cotas, and Leonel Pereira. 2025. "Role of Salinity on Phosphorous Removal by Chaetoceros muelleri" Oceans 6, no. 4: 79. https://doi.org/10.3390/oceans6040079
APA StyleAraújo, G. S., Santigado, C. S., Silva, L. C. B., Cotas, J., & Pereira, L. (2025). Role of Salinity on Phosphorous Removal by Chaetoceros muelleri. Oceans, 6(4), 79. https://doi.org/10.3390/oceans6040079








