Abstract
Bottlenose dolphins (Tursiops sp.) are opportunistic foragers with global distributions that utilize diverse feeding tactics based on environmental factors, habitat features, prey behavior, group dynamics, and genetics. We describe a unique foraging tactic regularly observed in the confluence of dredged shipping channels with high anthropogenic disturbance, and explore potential abiotic (temporal, tidal, habitat) drivers of the behavior. A shore-based digital theodolite was used from 2021 to 2022 to observe common bottlenose dolphins (T. truncatus) foraging within a current in a technique we term Orient-Against-Current (OAC). During OAC, dolphins position themselves facing into the flow of a current, swimming at a speed to maintain a stationary position within the current, and feed while prey move with the current towards them. Orient-Against-Current occurred in all seasons and throughout daylight hours, particularly during the winter and spring. Dolphins engaged in OAC during ebb tides and intermediate current speeds (1–2 knots), but not during slack tides. As OAC occurred closer to shoreline structures (i.e., seawalls, concrete blocks) than to mangroves and natural seagrass beds, it appears that hard human-engineered structures aid in prey capture during OAC. Knowledge of dolphin foraging techniques can aid in understanding behavioral plasticity shaped by anthropogenically altered environments in industrialized coastal areas.
1. Introduction
Marine mammals utilize a multitude of foraging tactics across a range of habitats that exploit prey availability and maximize feeding efficiency. Intra-individual and interindividual variations in marine mammal foraging tactics often arise due to differences in oceanographic features, habitat characteristics, group sizes and composition, genetics, prey types, and prey accessibility [1,2,3]. The variability of tactics used to find (e.g., migration, echolocation, diving), capture (e.g., herding, stalking, debilitation, ambushing, stunning, tool use), and consume prey (e.g., decapitation) likely developed from cost–benefit trade-offs to minimize predation risk and maximize energy intake [4,5]. The global distribution of bottlenose dolphins (Tursiops sp.), including long-term residency in diverse ecological habitats, has contributed to a high level of behavioral plasticity and development of diverse foraging techniques [6]. As dolphin distributions often overlap with anthropogenic operations in coastal areas, knowledge of specific dolphin foraging techniques is necessary to understand habitat use patterns and augment marine mammal management.
Dolphin foraging tactics can be influenced by temporal factors such as time of day and tidal characteristics. While dolphin foraging often occurs during daytime hours [2,7,8,9,10], some oceanic dolphins (common bottlenose dolphin, Tursiops truncatus; common dolphin, Delphinus delphis; dusky dolphin, Aethalodelphis obscurus; striped dolphin, Stenella coeruleoalba) forage nocturnally and opportunistically on prey that engage in diel vertical migrations to the surface of the water [9,11,12,13]. Tidal characteristics (e.g., current direction and strength, tidal state) can also prompt specific feeding behaviors. Foraging events have occurred among common bottlenose dolphins when ebb and flood currents were strongest, indicating potential increased chances of prey encounters during tidal flows [14,15]. In a few deep-water passes along the Texas coast, common bottlenose dolphins moved against tidal flows, mainly during ebb tide [7,16,17,18], positioning themselves against current flow and capturing fish carried with the tide [7]. Common bottlenose dolphins in the Moray Firth, Scotland, foraged near small-scale tidal fronts in areas of steep seabed gradients, suggesting that interaction between tidal and geographic features can improve foraging success [2].
Foraging tactics are also influenced by geographic and coastal habitat features. Dolphins can use natural barriers (e.g., seagrass, mangroves, sandy beaches, mud banks) and human-engineered structures (e.g., seawalls, concrete blocks, deep-dredged channel walls) to trap fish while foraging [1,9,10,19,20]. Common bottlenose dolphins in Sarasota Bay, Florida, and Indo-Pacific bottlenose dolphins (T. aduncus) in Western Australia use “kerplunking,” a tactic in which dolphins slap the surface of the water with their tails, generating a percussive sound to startle and capture prey that hide in shallow seagrass beds [9,21]. Common bottlenose dolphins in active shipping ports engage in “shipside feeding” year-round and pursue fish along the side of docked commercial ships [20]. Common bottlenose dolphins along the Texas coast preferentially use dredged ship channels and passes as foraging sites [10,16,17,22,23,24,25]. Additional foraging techniques include the use of unattached environmental objects as tools to directly and efficiently alter another object [26]. Indo-Pacific bottlenose dolphins in Western Australia engage in “sponging,” during which they position a marine sponge on their rostra for protection when digging for prey in the substrate [26,27,28,29], and “conching/shelling,” during which they lift conch shells from the substrate and feed on prey hidden in the shell’s aperture [30]. As dolphins continue to inhabit industrialized coastal areas and adapt to their environment, novel foraging techniques are likely to develop.
The Corpus Christi Ship Channel (CCSC) connects the Gulf of Mexico to the inshore Port of Corpus Christi and is a foraging hotspot in South Texas for common bottlenose dolphins [10]. The CCSC has experienced major infrastructure development over the last four decades including extensive dredging, pier construction, and energy resource exportation [31]. Despite the high levels of daily vessel traffic and anthropogenic operations in the area, common bottlenose dolphins occupy the CCSC year-round [16,17,25]. We observed dolphins engaged in a behavior we term Orient-Against-Current (OAC) in the CCSC; dolphins swim into the current while remaining relatively stationary in spatial positioning at the water’s surface while foraging. As fish are caught in the current and carried towards dolphins engaged in OAC, dolphins can increase prey encounters [7,15] and exhibit typical foraging behaviors (high arching dives, toss fish, chase fish) [17,25] (Video S1).
The objective of this study was to assess abiotic (temporal, tidal, habitat) drivers of OAC. We predict that Orient-Against-Current will occur throughout all seasons during morning and late afternoon [10,16], during strong ebb tidal current speeds [16,17,18], and near channel seawalls [19].
2. Materials and Methods
2.1. Study Area
The local confluence area where OAC was observed is the junction of the dredged Corpus Christi Ship Channel (CCSC), Lydia Ann Channel (LAC), and Aransas Channel (AC) between Mustang Island, San José Island, and Harbor Island along the South Texas coast (27°50′42.1” N 97°03′29.1” W; Figure 1). The three dredged channels support daily vessel transit through Aransas Pass between Corpus Christi Bay and the open Gulf of Mexico [32]. Coastal habitats (e.g., mangroves, seagrass beds) and natural shorelines (e.g., sand, rocks) border undeveloped flake and mud substrates along the LAC and AC, while human-engineered shorelines (e.g., granite rocks, concrete seawalls) border the CCSC and confluence area (Figure 1). The study area experiences current speeds averaging 2 kn and a mainly diurnal tide (0.42 m range, one low and one high tide daily) with mixed semi-diurnal (two low and two high tides daily) components [16,33,34]. Strong currents from each channel in the confluence area lead to potential nutrient mixing.
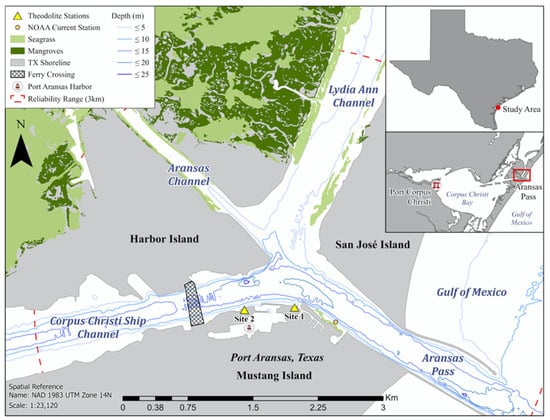
Figure 1.
Theodolite stations (site 1: 27°50.4867′ N, 97°3.4750′ W; site 2: 27°50.4767′ N, 97°3.8267′ W) in the Corpus Christi Ship Channel-Aransas Pass, Texas area.
2.2. Sampling Method
Data collection occurred in 6 h intervals using non-invasive techniques from one of two established elevated land-based theodolite stations along the CCSC between June 2021 and September 2022. Binoculars ((LAKWAR 10 × 50 mm binoculars, Shenzhen, China; Bushnell 12 × 50 mm binoculars, Overland Park, KS, USA)) were used to systematically scan the study area at the beginning and end of each field day and for approximately 20 min every hour. Dolphin geographic positions and behavior were recorded via theodolite. A digital theodolite (Sokkia Model DT5/DT5S, Atsugi, Kanagawa, Japan, 30× magnification) and two reference points were used to calculate the geographic positions (Global Positioning System (GPS) coordinates) of surfacing dolphins [35,36]. The theodolite was connected to a Dell laptop (Inspiron 3179, Round Rock, TX, USA) with Mysticetus software (version 2021.22, Preston, WA, USA) that converted x and y angle measurements into real-time GPS coordinates and recorded observed dolphin behaviors. The theodolite was positioned with a known eyepiece location and height (Site 1: 24.3–24.7 m; Site 2: 7–8 m) above sea level (Figure 1). Sampling was prioritized at Site 1 due to its higher elevation and proximity near the confluence area. To record the geographic positions of a single dolphin, the theodolite monocular crosshair was aligned on the focal dolphin at the waterline as it surfaced. To record the geographic positions of dolphins, the approximate middle of a dolphin group was recorded when the group was spread out (>10 m), while the middle individual’s position was recorded when the group was close together (≤10 m). A dolphin group consisted of adults with or without calves (<2/3 adult size and echelon swimming position [37]) within approximately 100 m of each other engaged in the same behavioral state [38]. To reduce observer bias, one computer operator and the same theodolite operator recorded data.
When behavioral states other than OAC occurred or were indiscernible, the behavioral state was excluded from analysis. Data were recorded until environmental conditions (e.g., fog, sunset, rain, Beaufort state > 3) restricted visibility or individuals were located beyond the theodolite’s reliable visibility range (>3 km; [39]; Figure 1).
2.3. Analyses
The frequency of occurrence of OAC behavior was assessed in Microsoft Excel (version 16.78.3, Redmond, WA, USA) across season, time of day, tidal state, current speed, and distances to habitats. Sampling occurred across summer (June–August), fall (September–November), winter (December–February), and spring (March–May) seasons during daylight hours. Times of day were categorized into morning (0800–1059 h), mid-day (1100–1359 h), early afternoon (1400–1659 h), and late afternoon (1700–2000 h). Current speed (knots) and direction (degrees true) were recorded automatically in 6 min intervals by a submerged (3.9 m) National Oceanic and Atmospheric Administration (NOAA) measurement station (cc0301) in the CCSC; the current data were obtained post hoc from the NOAA Center for Operational Oceanographic Products and Services [34]. Tidal states (ebb, slack, flood) were defined based on an azimuth circle to categorize approximate tidal ranges: ebb (83.25–172.75 degrees), slack (172.75–262.25 degrees and 351.75–83.25 degrees), and flood (262.25–351.75 degrees).
To account for unequal sampling effort across temporal variables, a relative abundance index (RAI) was calculated by dividing the number of observations of OAC by the sampling effort (h) and multiplying by a standardization factor (per 100 h). Count data of all geographic dolphin positions were used to standardize the tidal state and current speed sampling effort. The statistical software RStudio (version 2022.07.2, Vienna, Austria) was used to analyze the association between the number of OAC observations and temporal (season, time of day) and tidal (tidal state, current speed) variables. A Generalized Linear Model with a Poisson distribution was selected for analysis and fitted to the data, as it allows count response and explanatory variables with a non-normal distribution. The number of dolphin observations as counts was modeled with a log-transformed measure of effort, included as an offset. The model included season, time of day, tidal stage, and current speed with reference groups assigned for each categorical predictor variable based on the greatest effort during those periods: summer, mid-day, ebb, intermediate. The ratio of the residual deviance to the degrees of freedom was 0.7, meeting the key assumption of dispersion for the Poisson model, with a ratio value above one indicating overdispersion. No significant collinearity was detected among potential explanatory variables, based on variance inflation factor values with an upper threshold value of two indicating collinearity.
Kernel density analysis of OAC behavior was conducted in ArcGIS Pro (version 2.8.3, Redlands, CA, USA [40]) to determine spatial patterns in the confluence area. Kernel density calculates a magnitude per unit area from a feature (i.e., OAC point features) using a kernel function to fit a smoothly tapered surface to each point. The output cell value was set to densities, which represent the relative but not absolute concentration of OAC sightings in a spatial region. The number of sightings of OAC per square kilometer was calculated with a manually set search radius (150 m based on the size of the study area) and an output raster cell size of 15 m (derived by ArcGIS based on the search radius). The search radius defined the extent of the neighborhood around each output cell center where the density of each OAC point feature was calculated. The output raster was determined by the density of input point features within a defined search radius around the center of each output raster cell. The shortest distance between observations of OAC and habitat type (i.e., mangroves, seagrass, human-engineered shoreline) were calculated in ArcGIS Pro using the Generate Near Table tool and the Euclidean Distance tool. The shortest distance to a habitat type was used to assign a habitat type to a dolphin position. The Cost Path as Polyline tool was used to account for realistic paths a dolphin would take around land. Shapefile data of habitat types in the study area were obtained from NOAA, Texas Department of Transportation, and Texas Parks and Wildlife Department online archives. Several habitat types present in the area (i.e., emergent and coastal marsh, oyster beds) were inaccessible to dolphins and were not included in analysis.
3. Results
Sampling occurred for 63 days (287.8 h), and data were collected across all seasons (Summer: n = 124.6 h, average = 41.5 h/month; Fall: n = 61.7 h, average = 20.6 h/month; Winter: n = 40.7 h, average = 13.6 h/month; Spring: n = 60.8 h, average = 20.2 h/month) and times of day (Morning: n = 51.5 h, average = 1.4 h/day; Mid-Day: n = 112.3 h, average = 2.0 h/day; Early Afternoon: n = 81.9 h, average = 1.7 h/day; Late Afternoon: n = 24.9 h, average = 1.8 h/day). Dolphins were engaged in OAC behavior in a total of 36 positions out of 1187 geographic dolphin positions. The group sizes of dolphins engaged in OAC ranged from 1 to 14 dolphins (mean ± SD adults = 5.19 ± 2.87), with 0–2 calves present in 4 positions out of 36 (mean ± SD calves = 0.14 ± 0.42). Dolphins were observed engaging in feeding activity (i.e., chasing and tossing fish, high arching dives, trapping fish against structures [1,23]) during OAC behavior in the confluence area (Video S1). Fish species were indiscernible.
3.1. Temporal Patterns of Orient-Against-Current
Dolphin OAC behavior was observed across all seasons (nsummer = 5; nfall = 3; nwinter = 13; nspring = 15) and times of day (nmorning = 10; nmid-day = 12; nearly afternoon = 7; nlate afternoon = 7) (Figure 2). Season had a statistically significant effect on the probability of dolphins being observed engaging in OAC (winter: z = 2.17, p < 0.05, n = 33; spring: z = 3.02, p < 0.01, n = 3), explaining 36.8% of the deviance. There was no statistically significant effect of time of day on the probability of dolphins being observed engaging in OAC. Orient-Against-Current occurred throughout the day during the winter and spring seasons (Figure 2 and Figure 3A). Orient-Against-Current was not observed at mid-day in the summer nor in the morning and afternoon during the fall (Figure 2). When scaled by surveying effort, the number of observations of dolphins engaged in OAC was higher in the winter (RAI = 32 observations per 100 h) and spring (RAI = 25 observations per 100 h) compared to the summer (RAI = 4 observations per 100 h) and fall (RAI = 5 observations per 100 h) (Figure 3A; Table S1). The number of dolphins engaged in OAC, standardized by effort, was higher in the late afternoon (RAI = 28 observations per 100 h) and morning (RAI = 19 observations per 100 h) compared to mid-day (RAI = 11 observations per 100 h) and early afternoon (RAI = 9 observations per 100 h) (Figure 3B; Table S1).

Figure 2.
The number of Orient-Against-Current observations of common bottlenose dolphins (Tursiops truncatus) across seasons and times of day (n = 36 observations).

Figure 3.
The relative abundance (RAI, per 100 h) of Orient-Against-Current observations of common bottlenose dolphins (Tursiops truncatus) across (A) seasons and (B) times of day (n = 36 observations).
Dolphin OAC behavior was observed across all categories of current speeds (nweak = 5; nintermediate = 17; nstrong = 11) during ebb (n = 19) and flood (n = 14) tides (Figure 4). Three data points were not obtainable for tidal state. Tidal state and current speed did not have a statistically significant effect on the probability of dolphins being observed engaging in OAC. OAC behavior was observed more during ebb tides and strong and intermediate current speeds, and was not observed during slack tides (Figure 4 and Figure 5). When scaled by sampling effort, the number of common bottlenose dolphins engaged in OAC was higher during ebb tides (RAI = 3.78 observations per 100 counts) compared to flood tides (RAI = 3.36 observations per 100 counts) (Figure 5A, Table S2) and in strong (RAI = 6.08 observations per 100 counts) and intermediate (RAI = 3.74 observations per 100 counts) current speeds compared to weak current speeds (RAI = 1.58 observations per 100 counts) (Figure 5B; Table S2).

Figure 4.
The number of Orient-Against-Current observations across tidal states and current speeds (knots) (n = 33 observations). Three data points were not obtainable for tidal state.
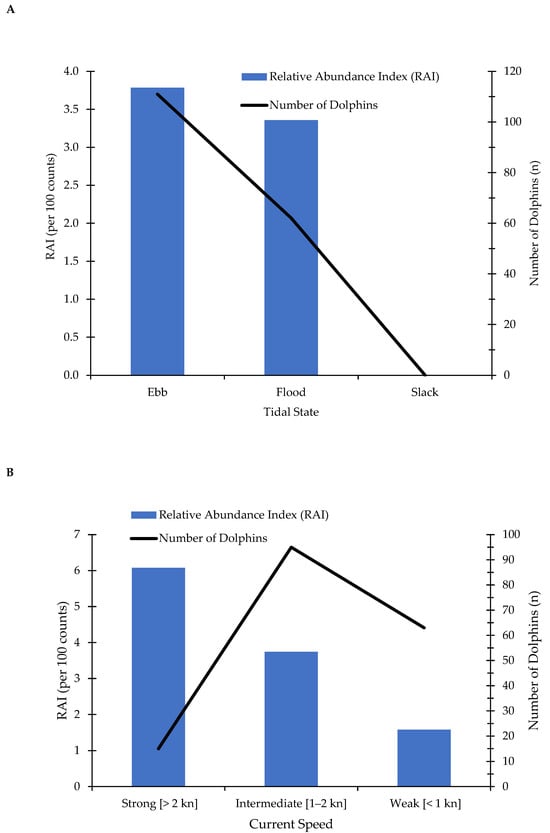
Figure 5.
The relative abundance (RAI, per 100 counts) of Orient-Against-Current observations of common bottlenose dolphins (Tursiops truncatus) across (A) tidal states and (B) current speeds (kn) (n = 33 observations).
3.2. Habitat Spatial Patterns of Orient-Against-Current
On average, dolphin OAC behavior appears to occur closer to human-engineered shorelines (mean ± SD = 60.3 m ± 23.6) than to seagrass beds (mean ± SD = 445 m ± 283) and mangroves (mean ± SD = 1830 m ± 316) (Figure 6). Three data points were not obtainable for habitat type. The densest occurrences of OAC per km occurred near the human-engineered rocky shoreline of Harbor Island and the seawall near Site 1 where the channels converge and the CCSC curves around a concrete outcropping (Figure 7).

Figure 6.
Mean distance (m) of common bottlenose dolphin (Tursiops truncatus) Orient-Against-Current behavioral observation to habitat types. Human-engineered habitat types included granite rocks and concrete seawalls (n = 33 observations). Three data points were not obtainable for habitat type.

Figure 7.
Kernel density map of common bottlenose dolphin (Tursiops truncatus) Orient-Against-Current behavior in Corpus Christi Ship Channel -Aransas Pass, Texas area (n = 36 observations). The kernel density analysis shows the relative sighting intensity (sightings km2) within the study area. The density surface was generated using a 150 m search radius and 15 m raster cell size.
4. Discussion
Several important trends emerged from this study. Orient-Against-Current behavior varied with abiotic (temporal, tidal, habitat) factors in the dredged Corpus Christi Ship Channel-Aransas Pass area. Systematic observations of OAC confirmed common bottlenose dolphins engaged in feeding activity (chasing fish, fish in dolphin’s mouth). While the CCSC-Aransas Pass is a known foraging area [10,16], the infrequent occurrence of OAC (3% of observations) in the region during daylight hours suggests that dolphins use additional foraging techniques to capture prey in the active ship channel, such as observed herding and trapping prey against hard-structures.
Common bottlenose dolphins in the CCSC-Aransas Pass area engaged in Orient-Against-Current behavior across all seasons during daylight hours. The occurrence of OAC in winter and spring seasons is congruent with prey migration patterns through Texas passes (red drum, Sciaenops ocellatus, [41]; spotted seatrout, Cynoscion nebulosus, [42]; Southern flounder, Paralichthys lethostigma, Gulf flounder, P. albigutta, [43]). Seasonal OAC patterns are also consistent with dolphin seasonal foraging patterns in the study area [10]. Among daylight hours, dolphins in the CCSC and other Texas passes forage most frequently in the morning and late afternoon [8,10,23] despite high vessel presence at these times [25]. As OAC was observed most during morning and late afternoon in the CCSC-Aransas Pass area, dolphins may alter foraging techniques during these times of day to avoid vessel traffic and improve feeding success. Updated data on dolphin prey abundance in the area may provide additional insights into the temporal patterns of OAC.
Orient-Against-Current behavior was observed across various hydrographic features (tidal state, current speed). Dolphins engaged in OAC during ebb and flood tides in the CCSC-Aransas Pass area, consistent with previous observations of dolphins moving primarily against ebb tides in dredged Texas passes [7,16,17,18]. In winter months when adult fish migrate and spawn near passes in the Gulf of Mexico, currents and tides carry larvae through passes to protected bays and estuaries [41,43]. The occurrence of OAC during ebb tides and during winter and spring months following spawning events suggests that dolphins may opportunistically feed on juvenile fish leaving the bay with a receding tide as water moves out to the Gulf of Mexico. The occurrence of OAC during flood tides aligns with the temporal patterns of mature flounder movement back into bays along the Texas coast [43]. Dolphins engaged in OAC appear to position themselves against the current in an attempt to capture fish carried with the tide, similar to dolphin foraging activity during strong tides or small-scale tidal fronts in other locations [2,14,15]. Dolphins in the CCSC-Aransas Pass area engaged in OAC when tidal currents were strong in strength (>2 knots). Additional research on hydrographic features in narrow ship channels is needed as the frequent operation of vessels can alter current velocities [44].
Common bottlenose dolphins Orient-Against-Current near human-engineered shorelines in two areas of the CCSC-Aransas Pass confluence area characterized by strong currents dependent on wind speed and tidal state. Deep channels and passes along the Texas coast are common foraging sites and experience mixing of waters and nutrients from protected bays and the Gulf of Mexico [10,16,17,22,23,24,25]. The interaction between topographic features like the steep seabed gradients in channels and variations in hydrographic features (tides, currents) could influence foraging success [2]. Dolphin feeding activity related to bathymetry (water depth) was not assessed in this study. Frequent vessel traffic and mixing of currents from the Lydia Ann Channel, Aransas Pass, and the Corpus Christi Ship Channel may promote deep water upwelling in the confluence area, which can bring nutrients to the surface, impacting prey availability and foraging activity. Visual observations of recreational fishers in both locations where OAC occurred along the channel periphery suggest high prey availability in the OAC locations. Hard structures along the sides of the active CCSC (e.g., seawalls, granite/concrete blocks) influence dolphin foraging activity in the area as they are used to trap prey [10]. Orient-Against-Current behavior occurred along the concrete seawall near Site 1 where a foraging hotspot was identified [10]. Dolphins likely transition between techniques of OAC and trapping prey against human-engineered shorelines along the channel periphery. The minimal amount of seagrass and mangroves located in the study area may contribute to the infrequent occurrence of OAC near natural habitats. While infrequently observed, OAC foraging appears to be influenced by human-engineered structures in dredged ship channels and may occur as an alternative to herding or trapping prey. Future studies with larger sample sizes may provide stronger insights into the factors influencing Orient-Against-Current behavior. Long-term monitoring of OAC behavior is recommended to better understand dolphin behavioral plasticity in industrialized seaports.
5. Conclusions
This study provides new insight into specialized common bottlenose dolphin foraging behavior observed in the CCSC -Aransas Pass area and the influences of abiotic factors on Orient-Against-Current. The temporal trends of OAC occurrence coincided with prey migration through Texas channels. Tidal state and current speed may provide hydrographic conditions for improved foraging success in dredged channels near human-engineered habitats. Consistent anthropogenic operations in active seaports could prompt dolphins to use varied foraging techniques. As anthropogenic activities (e.g., boating, commercial fishing and shipping, dredging, construction) continue to occupy and reshape marine coastal ecosystems, marine species and their habitats may be impacted. While dolphins Orient-Against-Current infrequently in the CCSC-Aransas Pass area, OAC appears to be localized and predictable. OAC may be an understudied foraging tactic that occurs in many industrialized shipping channels. Research on specialized dolphin foraging behavior in other deep-dredged passes and active seaports should be conducted to explore dolphin behavioral plasticity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oceans6040078/s1. Table S1: Relative abundance index (RAI) of the number of OAC observations across temporal variables (season, time of day) accounting for sampling effort (per 100 h). Table S2: Relative abundance index (RAI) of the number of OAC observations across tidal state and current speed (kn), accounting for sampling effort (per 100 counts). Video S1: Unoccupied aerial system (UAS) observations of common bottlenose dolphins (Tursiops truncatus) engaged in Orient-Against-Current in the CCSC-Aransas Pass, Texas confluence area. The UAS was operated by a licensed Federal Aviation Authority pilot and approved by the NOAA National Marine Fisheries Service (27973) and Texas A&M University–Corpus Christi (IACUC-2023-0061). Video credit: Lorenzo Fiori.
Author Contributions
Conceptualization, E.M.M.M. and D.N.O.; methodology, E.M.M.M. and S.P.; formal analysis, E.M.M.M. and S.P.; investigation, E.M.M.M.; writing—original draft preparation, E.M.M.M.; writing—review and editing, S.P. and D.N.O.; supervision, S.P. and D.N.O.; funding acquisition, E.M.M.M. and D.N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Texas Parks & Wildlife (State Wildlife Grant, CA-0000891), Texas A&M University—Corpus Christi (Center for Coastal Studies—Hans and Pat Suter Endowment Scholarship), the Southwestern Association of Naturalists (Howard McCarley Student Research Award), and the Texas Sea Grant College Program (Institutional Grant NA18OAR4170088) from the National Sea Grant Office, National Oceanic and Atmospheric Administration, U.S. Department of Commerce.
Institutional Review Board Statement
Ethical review and approval were waived for this study as the study was land-based and consisted exclusively of non-invasive observation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all the field data collection assistants: Allison Wilkins, Anya Ocampos, Audra Clute, Austin Willson, Brianna Hurst, Caitlynn Partin, Emily Cano, Jacquline Rich, Jennifer Gilmore, Kayla Torres, Madeleine Deel, Makayla Guinn, Peter Marra, Samantha Huron, Thaddeus Mills, and Tina Shield. We thank Dave Steckler for complementary use of Mysticetus software, Roy Roberts and Blair Sterba-Boatwright for statistical assistance, and Shawn McCracken for spatial analysis advice. We are grateful to the manager and staff at field station one.
Conflicts of Interest
Author Eliza M. M. Mills is employed by the company Environmental Systems Research Institute, Inc (Esri). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Torres, L.G.; Read, A.J. Where to catch a fish? The influence of foraging tactics on the ecology of bottlenose dolphins (Tursiops truncatus) in Florida Bay, Florida. Mar. Mamm. Sci. 2009, 25, 797–815. [Google Scholar] [CrossRef]
- Bailey, H.; Thompson, P. Effect of oceanographic features on fine-scale foraging movements of bottlenose dolphins. Mar. Ecol. Prog. Ser. 2010, 418, 223–233. [Google Scholar] [CrossRef]
- Hersh, T.A.; Marcondes, D.S.; Fonseca, G.F.; Valle-Pereira, J.V.S.; Kratofil, M.A.; Machado, A.M.S.; Atkins, S.; Bankhead, K.R.; McGarvey, K.; Rahman, M.M.; et al. Ecology and conservation of socially learned foraging tactics in odontocetes. Phil. Trans. R. Soc. B 2025, 380, 20240134. [Google Scholar] [CrossRef]
- Nowacek, D.P. Sequential foraging behaviour of bottlenose dolphins, Tursiops truncatus, in Sarasota Bay, FL. Behaviour 2002, 139, 1125–1145. [Google Scholar] [CrossRef]
- Ronje, E.I.; Barry, K.P.; Sinclair, C.; Grace, M.A.; Barros, N.; Allen, J.; Balmer, B.; Panike, A.; Toms, C.; Mullin, K.D.; et al. A common bottlenose dolphin (Tursiops truncatus) prey handling technique for marine catfish (Ariidae) in the northern Gulf of Mexico. PLoS ONE 2017, 12, e0181179. [Google Scholar] [CrossRef]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; American Press: San Diego, CA, USA, 2018; pp. 118–124. [Google Scholar]
- Shane, S.H.; Wells, R.S.; Würsig, B. Ecology, behavior, and social organization of the bottlenose dolphin: A review. Mar. Mamm. Sci. 1986, 2, 34–63. [Google Scholar] [CrossRef]
- Piwetz, S. Common bottlenose dolphin (Tursiops truncatus) behavior in an active narrow seaport. PLoS ONE 2019, 14, e0211971. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.S. Common bottlenose dolphin foraging: Behavioral solutions that incorporate habitat features and social associates. In Ethology and Behavioral Ecology of Odontocetes; Würsig, B., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 331–344. [Google Scholar]
- Mills, E.M.M.; Piwetz, S.; Orbach, D.N. Behavioral hotspots of bottlenose dolphins in industrialized ship channels. Front. Mar. Sci. 2024, 11, 1334252. [Google Scholar] [CrossRef]
- Caruso, F.; Alonge, G.; Bellia, G.; Domenico, E.D.; Grammauta, R.; Larosa, G.; Mazzola, S.; Riccobene, G.; Pavan, G.; Papale, E.; et al. Long-term monitoring of dolphin biosonar activity in deep pelagic waters of the Mediterranean Sea. Sci. Rep. 2017, 7, 4321. [Google Scholar] [CrossRef]
- Pearson, H.C. Dusky dolphins of continental shelves and deep canyons. In Ethology and Behavioral Ecology of Odontocetes; Würsig, B., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 387–411. [Google Scholar]
- Sol, M.; Ollier, C.; Boisseau, O.; Ridoux, V.; Virgili, A. Temporal patterns in dolphin foraging activity in the Mediterranean Sea: Insights from vocalisations recorded during the ACCOBAMS Survey Initiative. Front. Mar. Sci. 2024, 11, 1378524. [Google Scholar] [CrossRef]
- Gregory, P.R.; Rowden, A.A. Behaviour patterns of bottlenose dolphins (Tursiops truncatus) relative to tidal state, time-of-day, and boat traffic in Cardigan Bay, West Wales. Aquat. Mamm. 2001, 27, 105–113. [Google Scholar]
- Carmen, M.; Berrow, S.D.; O’Brien, J.M. Foraging behavior of bottlenose dolphins in the Shannon Estuary, Ireland as determined through static acoustic monitoring. J. Mar. Sci. Eng. 2021, 9, 275. [Google Scholar] [CrossRef]
- Shane, S.H. The Population Biology of the Atlantic Bottlenose Dolphin, Tursiops truncatus, in the Aransas Pass Area of Texas. Master’s Thesis, Texas A&M University-Galveston, Galveston, TX, USA, 1977. [Google Scholar]
- Shane, S.H. Occurrence, movements, and distribution of bottlenose dolphin, Tursiops truncatus, in southern Texas. Fish. Bull. 1980, 78, 593–601. [Google Scholar]
- Gruber, J.A. Ecology of the Atlantic bottlenosed dolphin (Tursiops truncatus) in the Pass Cavallo area of Matagorda Bay, Texas. Master’s Thesis, Texas A&M University, College Station, TX, USA, 1981. [Google Scholar]
- Weiss, J. Foraging habitats and associated preferential foraging specializations of bottlenose dolphin (Tursiops truncatus) mother-calf pairs. Aquat. Mamm. 2006, 32, 10–19. [Google Scholar] [CrossRef]
- Weinpress-Galipeau, M.; Baker, H.; Wolf, B.; Roumillat, B.; Fair, P.A. An adaptive bottlenose dolphin foraging tactic, “shipside feeding,” using container ships in an urban estuarine environment. Mar. Mamm. Sci. 2021, 37, 1159–1165. [Google Scholar] [CrossRef]
- Connor, R.C.; Heithaus, M.R.; Berggren, P.; Miksis, J.L. Kerplunking: Surface fluke-splashes during shallow-water bottom foraging by bottlenose dolphins. Mar. Mamm. Sci. 2000, 16, 646–653. [Google Scholar] [CrossRef]
- Leatherwood, S.; Reeves, R.R. Abundance of bottlenose dolphins in Corpus Christi Bay and coastal Southern Texas. Contrib. Mar. Sci. 1983, 26, 179–199. [Google Scholar]
- Henderson, E.E.; Würsig, B. Behavior patterns of bottlenose dolphins in San Luis Pass, Texas. Gulf Mex. Sci. 2007, 25, 153–161. [Google Scholar] [CrossRef]
- Ronje, E.I.; Whitehead, H.R.; Barry, K.; Piwetz, S.; Struve, J.; Lecours, V.; Garrison, L.; Wells, R.S.; Mullin, K.D. Abundance and occurrence of common bottlenose dolphins (Tursiops truncatus) in three estuaries of the northwestern Gulf of Mexico. Gulf Carib. Res. 2020, 31, 18–34. [Google Scholar] [CrossRef]
- Mills, E.M.M.; Piwetz, S.; Orbach, D.N. Vessels disturb bottlenose dolphin behavior and movement in an active ship channel. Animals 2023, 13, 3441. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Patterson, E.M. Tool use by aquatic animals. Phil. Trans. R. Soc. B 2013, 368, 20120424. [Google Scholar] [CrossRef]
- Smolker, R.; Richards, A.; Connor, R.; Mann, J.; Berggren, P. Sponge carving by dolphins (Delphinidae, Tursiops sp.): A foraging specialization involving tool use? Ethology 1997, 103, 454–465. [Google Scholar] [CrossRef]
- Krützen, M.; Kreicker, S.; MacLeod, C.D.; Learmonth, J.; Kopps, A.M.; Walsham, P.; Allen, S.J. Cultural transmission of tool use by Indo-Pacific bottlenose dolphins (Tursiops sp.) provides access to a novel foraging niche. Proc. Biol. Sci. 2014, 281, 20140374. [Google Scholar]
- Connor, R.C.; Sakai, M.; Morisaka, T.; Allen, S.J. The Indo-Pacific bottlenose dolphin (Tursiops aduncus). In Ethology and Behavioral Ecology of Odontocetes; Würsig, B., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 345–368. [Google Scholar]
- Allen, S.J.; Bejder, L.; Krützen, M. Why do Indo-Pacific bottlenose dolphins (Tursiops sp.) carry conch shells (Turbinella sp.) in Shark Bay, Western Australia? Mar. Mamm. Sci. 2011, 27, 449–454. [Google Scholar] [CrossRef]
- U.S. Army Corps of Engineers, Galveston District. USACE, Corpus Christi Celebrate Completion of Ship Channel Improvement Projects. Available online: https://www.swg.usace.army.mil/Media/News-Releases/Article/4200855/usace-corpus-christi-celebrate-completion-of-ship-channel-improvement-project/ (accessed on 2 June 2025).
- U.S. Coast Guard. Ports and Waterways Safety Assessment: Workshop Report Corpus Christi, Texas. United States Coast Guard Navigation Center, September 2019. Available online: https://navcen.uscg.gov/sites/default/files/pdf/pawsa/WorkshopReports/Corpus_Christi_Sep_2019.pdf (accessed on 1 August 2021).
- Tunnell, J.W., Jr.; Dokken, Q.; Smith, E.H.; Withers, K. Current status and historical trends of the estuarine living resources within the Corpus Christi Bay National Estuary Program study area. Tech. Rep. 1996, 1a. [Google Scholar] [CrossRef]
- NOAA National Ocean Service. Tides and Currents [Data Set]. Center for Operational Oceanographic Products and Services. Available online: https://tidesandcurrents.noaa.gov/map/index.html (accessed on 1 June 2022).
- Würsig, B.; Cipriano, F.; Würsig, M. Dolphin movement patterns: Information from radio and theodolite tracking studies. In Dolphin Societies: Discoveries and Puzzles; Pryor, K., Norris, K.S., Eds.; University of California Press: Berkeley, CA, USA, 1991; pp. 79–111. [Google Scholar]
- Harzen, S.E. Use of an electronic theodolite in the study of movements of the bottlenose dolphin (Tursiops truncatus) in the Sado Estuary, Portugal. Aquat. Mamm. 2002, 28, 251–260. [Google Scholar]
- Shane, S.H. Behavior and ecology of the bottlenose dolphin at Sanibel Island, Florida. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 245–265. [Google Scholar]
- Azzellino, A.; Gaspari, S.; Airoldi, S.; Nani, B. Habitat use and preferences of cetaceans along the continental slope and the adjacent pelagic waters in the western Ligurian Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 296–323. [Google Scholar] [CrossRef]
- Sagnol, O.; Reitsma, F.; Richter, C.; Field, L.H. Correcting positional error in shore-based theodolite measurements of animals at sea. J. Mar. Biol. 2014, 2014, 267917. [Google Scholar] [CrossRef]
- ESRI-Environmental Systems Research Institute, Inc. How Kernel Density Works (Spatial Statistics). Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-analyst/how-kernel-density-works.htm (accessed on 1 June 2022).
- Bushon, A. Recruitment, Spatial Distribution, and Fine-Scale Movement Patterns of Estuarine Dependent Species Through Tidal Inlets in Texas. Ph.D. Thesis, Texas A&M University-Corpus Christi, Corpus Christi, TX, USA, 2006. [Google Scholar]
- Payne, L.M. Evaluation of large-scale movement patterns of spotted seatrout (Cynoscion nebulosus) using acoustic telemetry. Master’s Thesis, Texas A&M University-Corpus Christi, Corpus Christi, TX, USA, 2011. [Google Scholar]
- Stokes, G.M. Life History Studies of Southern Flounder (Paralichthys lethostigma) and Gulf Flounder (P. albigutta) in the Aransas Bay Area of Texas; Texas Parks & Wildlife Department: Austin, TX, USA, 1977; Technical Series25; 37p, Available online: https://www.ccatexas.org/wp-content/uploads/2019/12/Technical-Series-No-25-Life-History-of-Southern-Flounder.pdf (accessed on 1 June 2022).
- Gabel, F.; Lorenz, S.; Stoll, S. Effects of ship-induced waves on aquatic ecosystems. Sci. Total Environ. 2017, 601–602, 926–939. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).