Low Genetic Diversity and Decreased Effective Population Sizes of Acropora hyacinthus Populations Inhabiting Inshore and Offshore Reefs in the South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. Sequencing Analysis
2.2.1. ddRAD Sequencing Library Construction and Data Generation
2.2.2. PCR Amplification and Sequencing of mtCR Fragment
2.3. Data Analysis
2.3.1. Data Processing, SNP Calling, and Population Genetic Analysis of A. hyacinthus
2.3.2. Genetic and Phylogenetic Analysis of A. hyacinthus Using mtCR Sequences
3. Results
3.1. ddRAD Sequencing Data
3.1.1. Sequencing Data and Genetic Diversity
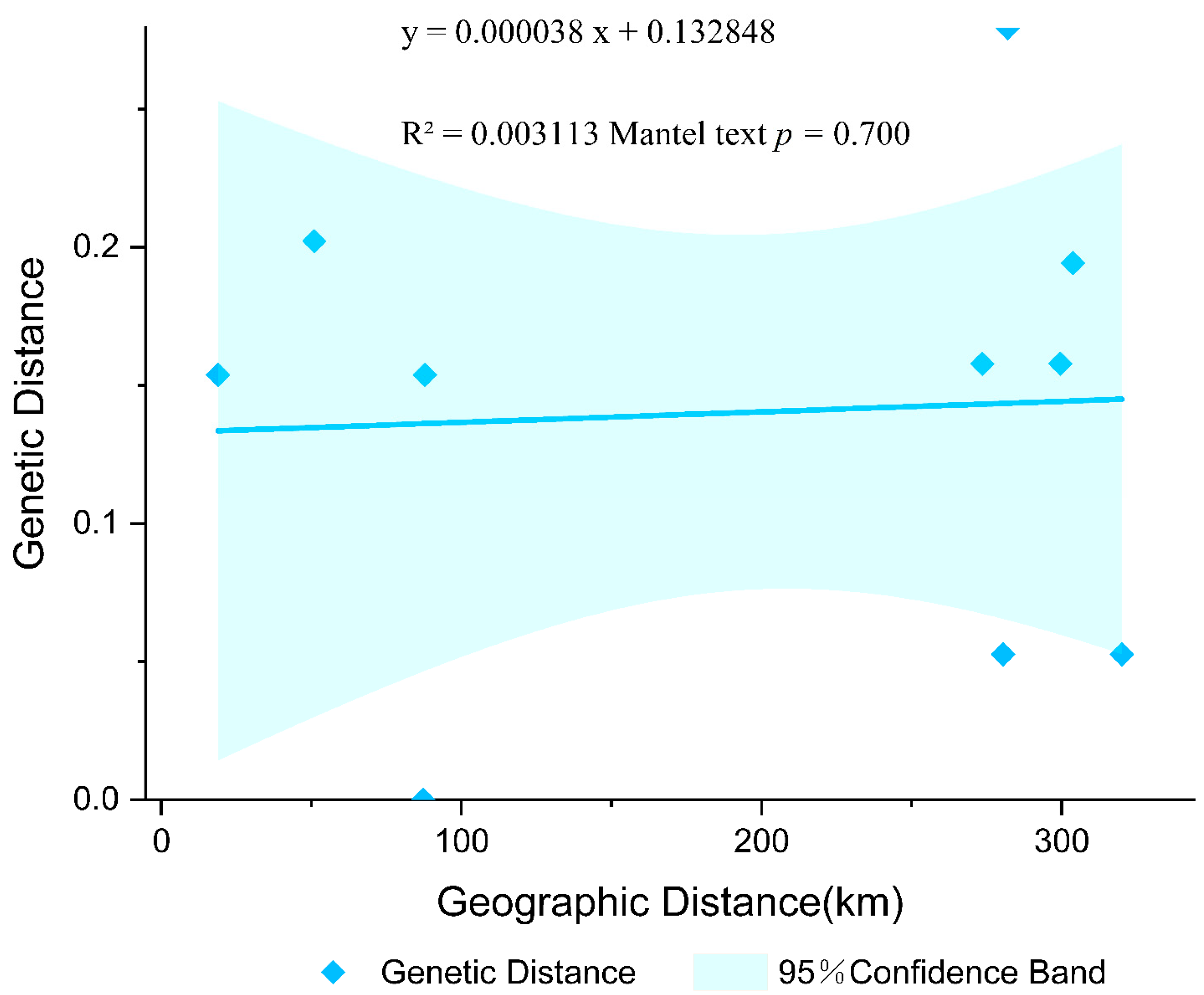
3.1.2. Population Connectivity
3.1.3. Demographic History
3.2. Mitochondrial Putative Control Region Sequences
3.2.1. Sequence Data
3.2.2. Phylogenetic Analysis
3.2.3. Genetic Diversity
3.2.4. Population Structure
4. Discussion
4.1. The Lineages of A. hyacinthus Used in This Study
4.2. Genetic Structure and Connectivity
4.3. Low Genetic Diversity and Limited Adaptive Potential of A. hyacinthus in the SCS
4.4. Implications for Conserving A. hyacinthus Populations in the SCS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakajima, Y.; Nishikawa, A.; Iguchi, A.; Sakai, K. Gene Flow and Genetic Diversity of a Broadcast-Spawning Coral in Northern Peripheral Populations. PLoS ONE 2010, 5, e11149. [Google Scholar] [CrossRef]
- Elliff, C.I.; Silva, I.R. Coral Reefs as the First Line of Defense: Shoreline Protection in Face of Climate Change. Mar. Environ. Res. 2017, 127, 148–154. [Google Scholar] [CrossRef]
- Reaka, M.L.; Lombardi, S.A. Hotspots on Global Coral Reefs. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 471–501. ISBN 978-3-642-20992-5. [Google Scholar]
- Huang, D.; Licuanan, W.Y.; Hoeksema, B.W.; Chen, C.A.; Ang, P.O.; Huang, H.; Lane, D.J.W.; Vo, S.T.; Waheed, Z.; Affendi, Y.A.; et al. Extraordinary Diversity of Reef Corals in the South China Sea. Mar. Biodivers. 2015, 45, 157–168. [Google Scholar] [CrossRef]
- Taguchi, T.; Tagami, E.; Mezaki, T.; Vacarizas, J.M.; Canson, K.L.; Avila, T.N.; Bataan, D.A.U.; Tominaga, A.; Kubota, S. Karyotypic Mosaicism and Molecular Cytogenetic Markers in the Scleractinian Coral Acropora pruinosa Brook, 1982 (Hexacorallia, Anthozoa, Cnidaria). Coral Reefs 2020, 39, 1415–1425. [Google Scholar] [CrossRef]
- Tuwo, A.; Tresnati, J. Coral Reef Ecosystem. In Advances in Biological Sciences and Biotechnology; Integrated Publications: Delhi, India, 2021; pp. 75–104. ISBN 978-81-945787-5-8. [Google Scholar]
- Xiao, J.; Wang, W.; Wang, X.; Tian, P.; Niu, W. Recent Deterioration of Coral Reefs in the South China Sea Due to Multiple Disturbances. PeerJ 2022, 10, e13634. [Google Scholar] [CrossRef]
- McCook, L.; Almany, G.; Berumen, M.; Day, J.; Green, A.; Jones, G.; Leis, J.; Planes, S.; Russ, G.; Sale, P.; et al. Management under Uncertainty: Guide-Lines for Incorporating Connectivity into the Protection of Coral Reefs. Coral Reefs 2009, 28, 353–366. [Google Scholar] [CrossRef]
- Hughes, T.P.; Huang, H.; Young, M.A.L. The Wicked Problem of China’s Disappearing Coral Reefs. Conserv. Biol. 2013, 27, 261–269. [Google Scholar] [CrossRef]
- Zhao, M.; Yu, K.; Zhang, Q.; Shi, Q.; Price, G.J. Long-Term Decline of a Fringing Coral Reef in the Northern South China Sea. J. Coast. Res. 2012, 28, 1088–1099. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, G.H.; Yan, H.Q.; Zhang, H.L. Black Disease (Terpios Hoshinota): A Probable Cause for the Rapid Coral Mortality at the Northern Reef of Yongxing Island in the South China Sea. Ambio 2012, 41, 446–455. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, W.; Chen, R.; Rinkevich, B.; Shafir, S.; Xia, J.; Zhu, M.; Chen, R.; Wang, A.; Li, X. Framed Reef Modules: A New and Cost-Effective Tool for Coral Restoration. Restor. Ecol. 2024, 32, e13997. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral Restoration—A Systematic Review of Current Methods, Successes, Failures and Future Directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Kenyon, J.C. Models of Reticulate Evolution in the Coral Genus Acropora Based on Chromosome Numbers: Parallels with Plants. Evolution 1997, 51, 756–767. [Google Scholar] [CrossRef]
- Jamodiong, E.A.; Maboloc, E.A.; Villanueva, R.D.; Cabaitan, P.C. Gametogenesis and Inter-Annual Variability in the Spawning Pattern of Acropora hyacinthus in Northwestern Philippines. Zool. Stud. 2018, 57, e46. [Google Scholar] [CrossRef]
- Leinbach, S.E.; Speare, K.E.; Rossin, A.M.; Holstein, D.M.; Strader, M.E. Energetic and Reproductive Costs of Coral Recovery in Divergent Bleaching Responses. Sci. Rep. 2021, 11, 23546. [Google Scholar] [CrossRef]
- Randall, C.J.; Giuliano, C.; Stephenson, B.; Whitman, T.N.; Page, C.A.; Treml, E.A.; Logan, M.; Negri, A.P. Larval Precompetency and Settlement Behaviour in 25 Indo-Pacific Coral Species. Commun. Biol. 2024, 7, 142. [Google Scholar] [CrossRef]
- Ladner, J.T.; Palumbi, S.R. Extensive Sympatry, Cryptic Diversity and Introgression throughout the Geographic Distribution of Two Coral Species Complexes. Mol. Ecol. 2012, 21, 2224–2238. [Google Scholar] [CrossRef]
- Suzuki, G.; Keshavmurthy, S.; Hayashibara, T.; Wallace, C.C.; Shirayama, Y.; Chen, C.A.; Fukami, H. Genetic Evidence of Peripheral Isolation and Low Diversity in Marginal Populations of the Acropora hyacinthus Complex. Coral Reefs 2016, 35, 1419–1432. [Google Scholar] [CrossRef]
- Ssg, I.N.L. “Acropora Hyacinthus” IUCN Red List of Threatened Species, 2023. Available online: https://www.iucnredlist.org (accessed on 31 July 2025).
- Henry, J.A.; O’Neil, K.L.; Pilnick, A.R.; Patterson, J.T. Strategies for Integrating Sexually Propagated Corals into Caribbean Reef Restoration: Experimental Results and Considerations. Coral Reefs 2021, 40, 1667–1677. [Google Scholar] [CrossRef]
- López-Nandam, E.H.; Payne, C.Y.; Delbeek, J.C.; Dunker, F.; Krol, L.; Larkin, L.; Lev, K.; Ross, R.; Schaeffer, R.; Yong, S.; et al. Kinship and Genetic Variation in Aquarium-Spawned Acropora hyacinthus Corals. Front. Mar. Sci. 2022, 9, 961106. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Marshall, D.J. Genetic Diversity Increases Population Productivity in a Sessile Marine Invertebrate. Ecology 2012, 93, 1134–1142. [Google Scholar] [CrossRef]
- Schweinsberg, M.; González-Pech, R.; Tollrian, R.; Lampert, K. Transfer of Intracolonial Genetic Variability through Gametes in Acropora hyacinthus Corals. Coral Reefs 2014, 33, 77–87. [Google Scholar] [CrossRef]
- Shinzato, C.; Khalturin, K.; Inoue, J.; Zayasu, Y.; Kanda, M.; Kawamitsu, M.; Yoshioka, Y.; Yamashita, H.; Suzuki, G.; Satoh, N. Eighteen Coral Genomes Reveal the Evolutionary Origin of Acropora Strategies to Accommodate Environmental Changes. Mol. Biol. Evol. 2021, 38, 16–30. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Wu, Q.; Feng, Y.; Wang, Y.; Lu, Z.; Chen, J.; Chen, B.; Xiao, Z.; Meng, L.; et al. Reduced Genetic Diversity and Restricted Gene Flow of Broadcast-Spawning Coral Galaxea fascicularis in the South China Sea Reveals Potential Degradation under Environmental Change. Mar. Pollut. Bull. 2023, 193, 115147. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, W.; Chen, B.; Yang, E.; Meng, L.; Chen, Y.; Li, J.; Huang, X.; Liang, J.; Yap, T.-K.; et al. Genetic Structure of Turbinaria Peltata in the Northern South China Sea Suggest Insufficient Genetic Adaptability of Relatively High-Latitude Scleractinian Corals to Environment Stress. Sci. Total. Environ. 2021, 775, 145775. [Google Scholar] [CrossRef]
- Li, M.; Huang, W.; Wu, Q.; Feng, Y.; Chen, Y.; Yu, K.; Chen, B.; Yang, E.; Meng, L.; Huang, X.; et al. High Genetic Differentiation and Moderate Genetic Diversity of the Degenerative Branching Coral Pocillopora verrucosa in the Tropical South China Sea. Sci. Total. Environ. 2022, 819, 153076. [Google Scholar] [CrossRef]
- Lord, K.S.; Lesneski, K.C.; Buston, P.M.; Davies, S.W.; D’Aloia, C.C.; Finnerty, J.R. Rampant Asexual Reproduction and Limited Dispersal in a Mangrove Population of the Coral Porites divaricata. Proc. R. Soc. B 2023, 290, 20231070. [Google Scholar] [CrossRef]
- Pääbo, S.; Wilson, A.C. Polymerase Chain Reaction Reveals Cloning Artefacts. Nature 1988, 334, 387–388. [Google Scholar] [CrossRef]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double Digest RADseq: An Inexpensive Method for de Novo SNP Discovery and Genotyping in Model and Non-Model Species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An Analysis Tool Set for Population Genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Zn, K.; Jf, T.; Nj, G. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Slatkin, M.; Barton, N.H. A Comparison of Three Indirect Methods for Estimating Average Levels of Gene Flow. Evolution 1989, 43, 1349–1368. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.-X. Stairway Plot 2: Demographic History Inference with Folded SNP Frequency Spectra. Genome Biol. 2020, 21, 280. [Google Scholar] [CrossRef]
- Fuller, Z.; Mocellin, V.; Morris, L.; Cantin, N.; Shepherd, J.; Sarre, L.; Peng, J.; Liao, Y.; Pickrell, J.; Andolfatto, P.; et al. Population Genetics of the Coral Acropora millepora: Toward Genomic Prediction of Bleaching. Science 2020, 369, eaba4674. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Swindell, S.R.; Plasterer, T.N. SEQMAN. In Sequence Data Analysis Guidebook; Swindell, S.R., Ed.; Springer: Totowa, NJ, USA, 1997. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (Version 3.0): An Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. Online 2007, 1, 47–50. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. Partitionfinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Puechmaille, S.J. The Program Structure Does Not Reliably Recover the Correct Population Structure When Sampling Is Uneven: Subsampling and New Estimators Alleviate the Problem. Mol. Ecol. Resour. 2016, 16, 608–627. [Google Scholar] [CrossRef]
- Huang, W.; Li, M.; Yu, K.; Wang, Y.; Li, J.; Liang, J.; Luo, Y.; Huang, X.; Qin, Z.; Wang, G.; et al. Genetic Diversity and Large-Scale Connectivity of the Scleractinian Coral Porites lutea in the South China Sea. Coral Reefs 2018, 37, 1259–1271. [Google Scholar] [CrossRef]
- van der Ven, R.M.; Triest, L.; De Ryck, D.J.R.; Mwaura, J.M.; Mohammed, M.S.; Kochzius, M. Population Genetic Structure of the Stony Coral Acropora tenuis Shows High but Variable Connectivity in East Africa. J. Biogeogr. 2016, 43, 510–519. [Google Scholar] [CrossRef]
- van der Ven, R.M.; Heynderickx, H.; Kochzius, M. Differences in Genetic Diversity and Divergence between Brooding and Broadcast Spawning Corals across Two Spatial Scales in the Coral Triangle Region. Mar. Biol. 2021, 168, 17. [Google Scholar] [CrossRef]
- Li, J.-J.; Hu, Z.-M.; Gao, X.; Sun, Z.-M.; Choi, H.-G.; Duan, D.-L.; Endo, H. Oceanic Currents Drove Population Genetic Connectivity of the Brown Alga Sargassum Thunbergii in the North-West Pacific. J. Biogeogr. 2017, 44, 230–242. [Google Scholar] [CrossRef]
- Tisthammer, K.H.; Forsman, Z.H.; Toonen, R.J.; Richmond, R.H. Genetic Structure Is Stronger across Human-Impacted Habitats than among Islands in the Coral Porites lobata. PeerJ 2020, 8, e8550. [Google Scholar] [CrossRef] [PubMed]
- Ward, S. Evidence for Broadcast Spawning as Well as Brooding in the Scleractinian Coral Pocillopora damicornis. Mar. Biol. 1992, 112, 641–646. [Google Scholar] [CrossRef]
- Knittweis, L.; Kraemer, W.E.; Timm, J.; Kochzius, M. Genetic Structure of Heliofungia Actiniformis (Scleractinia: Fungiidae) Populations in the Indo-Malay Archipelago: Implications for Live Coral Trade Management Efforts. Conserv. Genet. 2009, 10, 241–249. [Google Scholar] [CrossRef]
- Hu, J.; Kawamura, H.; Hong, H.; Qi, Y. A Review on the Currents in the South China Sea: Seasonal Circulation, South China Sea Warm Current and Kuroshio Intrusion. J. Oceanogr. 2000, 56, 607–624. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Jiang, L.; Yu, X.; Huang, L.; Yuan, T.; Yang, J.; Lian, J.; Liu, C.; Ang, P.; et al. Coral Spawning Patterns on the Luhuitou Fringing Reef in Hainan Island of the Northern South China Sea. Front. Mar. Sci. 2024, 11, 1418942. [Google Scholar] [CrossRef]
- Kuriiwa, K.; Chiba, S.N.; Motomura, H.; Matsuura, K. Phylogeography of Blacktip Grouper, Epinephelus Fasciatus (Perciformes: Serranidae), and Influence of the Kuroshio Current on Cryptic Lineages and Genetic Population Structure. Ichthyol. Res. 2014, 61, 361–374. [Google Scholar] [CrossRef]
- Neo, M.L.; Liu, L.-L.; Huang, D.; Soong, K. Thriving Populations with Low Genetic Diversity in Giant Clam Species, Tridacna Maxima and Tridacna Noae, at Dongsha Atoll, South China Sea. Reg. Stud. Mar. Sci. 2018, 24, 278–287. [Google Scholar] [CrossRef]
- Kennett, J.P.; Ingram, B.L. A 20,000-Year Record of Ocean Circulation and Climate Change from the Santa Barbara Basin. Nature 1995, 377, 510–514. [Google Scholar] [CrossRef]
- Bond, G.; Showers, W.; Cheseby, M.; Lotti, R.; Almasi, P.; deMenocal, P.; Priore, P.; Cullen, H.; Hajdas, I.; Bonani, G. A Pervasive Millennial-Scale Cycle in North Atlantic Holocene and Glacial Climates. Science 1997, 278, 1257–1266. [Google Scholar] [CrossRef]
- Wang, P. Response of Western Pacific Marginal Seas to Glacial Cycles: Paleoceanographic and Sedimentological Features1. Mar. Geol. 1999, 156, 5–39. [Google Scholar] [CrossRef]
- Yan, S.; Catanese, G.; Brown, C.; Wang, M.; Yang, C.; Yang, T. Phylogeographic Study on the Chub Mackerel (Scomber Japonicus) in the Northwestern Pacific Indicates the Late Pleistocene Population Isolation. Mar. Ecol. 2015, 36, 753–765. [Google Scholar] [CrossRef]
- Fifer, J.E.; Yasuda, N.; Yamakita, T.; Bove, C.B.; Davies, S.W. Genetic Divergence and Range Expansion in a Western North Pacific Coral. Sci. Total Environ. 2022, 813, 152423. [Google Scholar] [CrossRef]
- Robledo, D.; Palaiokostas, C.; Bargelloni, L.; Martínez, P.; Houston, R. Applications of Genotyping by Sequencing in Aquaculture Breeding and Genetics. Rev. Aquacult. 2018, 10, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, F.; Tranchina, D.; Gresham, D. Fluctuating Environments Maintain Genetic Diversity through Neutral Fitness Effects and Balancing Selection. Mol. Biol. Evol. 2021, 38, 4362–4375. [Google Scholar] [CrossRef]
- Yu, K. Coral Reefs in the South China Sea: Their Response to and Records on Past Environmental Changes. Sci. China Earth Sci. 2012, 55, 1217–1229. [Google Scholar] [CrossRef]
- McManus, J. The Spratly Islands: A Marine Park Alternative. Naga–ICLARM Quart. 1992, 15, 4–8. [Google Scholar]
- Li, S.; Yu, K.; Chen, T.; Shi, Q.; Zhang, H. Assessment of Coral Bleaching Using Symbiotic Zooxanthellae Density and Satellite Remote Sensing Data in the Nansha Islands, South China Sea. Chin. Sci. Bull. 2011, 56, 1031–1037. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Huang, H.; Huang, L.; Jing, Z.; Zhang, C. Coral Bleaching Caused by an Abnormal Water Temperature Rise at Luhuitou Fringing Reef, Sanya Bay, China. Aquat. Ecosyst. Health Manage. 2012, 15, 227–233. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, J.; Ren, Y.; Xie, M.; Yin, H.; Liu, X.; Huang, J.; Zhu, M.; Li, X. Coastal Corals during Heat Stress and Eutrophication: A Case Study in Northwest Hainan Coastal Areas. Mar. Pollut. Bull. 2021, 173, 113048. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhou, Z.; Zhang, Y.; Chen, Z.; Deng, W.; Shi, R. The Mass Coral Bleaching Event of Inshore Corals Form South China Sea Witnessed in 2020: Insight into the Causes, Process and Consequence. Coral Reefs 2022, 41, 1351–1364. [Google Scholar] [CrossRef]
- Lacy, R.C. Importance of Genetic Variation to the Viability of Mammalian Populations. J. Mammal. 1997, 78, 320–335. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and Extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Peters, R.L. Conservation Biology: The Science of Scarcity and Diversity. Ecol. Restor. 1987, 5, 23–24. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological Consequences of Genetic Diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Toh, E.-C.; Liu, K.-L.; Tsai, S.; Lin, C. Cryopreservation and Cryobanking of Cells from 100 Coral Species. Cells 2022, 11, 2668. [Google Scholar] [CrossRef] [PubMed]
- Vardi, T.; Hoot, W.; Levy, J.; Shaver, E.; Winters, R.S.; Banaszak, A.; Baums, I.; Chamberland, V.; Cook, N.; Gulko, D.; et al. Six Priorities to Advance the Science and Practice of Coral Reef Restoration Worldwide. Restor. Ecol. 2021, 29, e13498. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Marhaver, K.L.; Miller, M.W.; Hartmann, A.C.; Albright, R.; Hagedorn, M.; Harrison, P.L.; Latijnhouwers, K.R.W.; Mendoza Quiroz, S.; Pizarro, V.; et al. Applying Coral Breeding to Reef Restoration: Best Practices, Knowledge Gaps, and Priority Actions in a Rapidly-Evolving Field. Restor. Ecol. 2023, 31, e13913. [Google Scholar] [CrossRef]
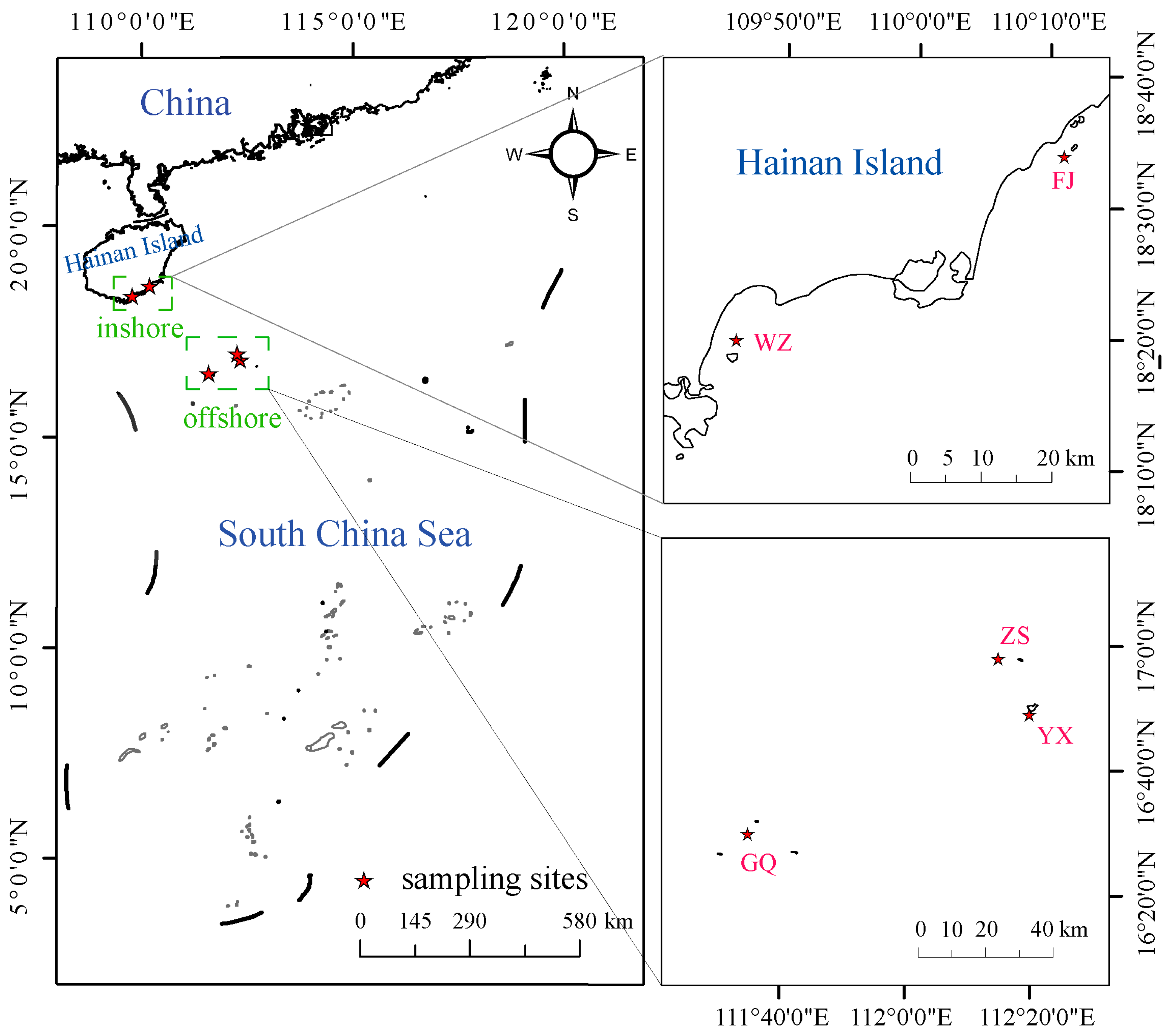
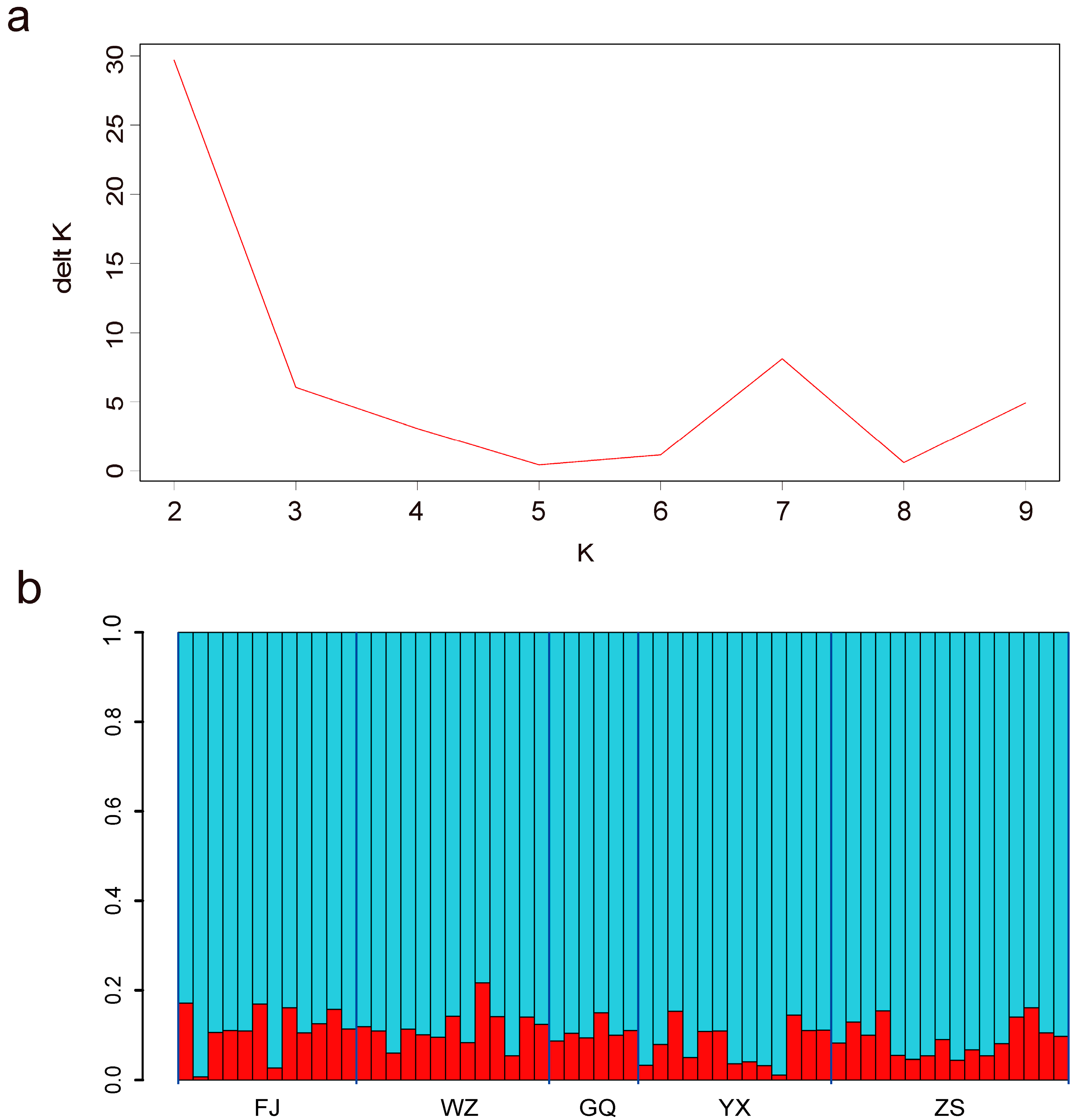
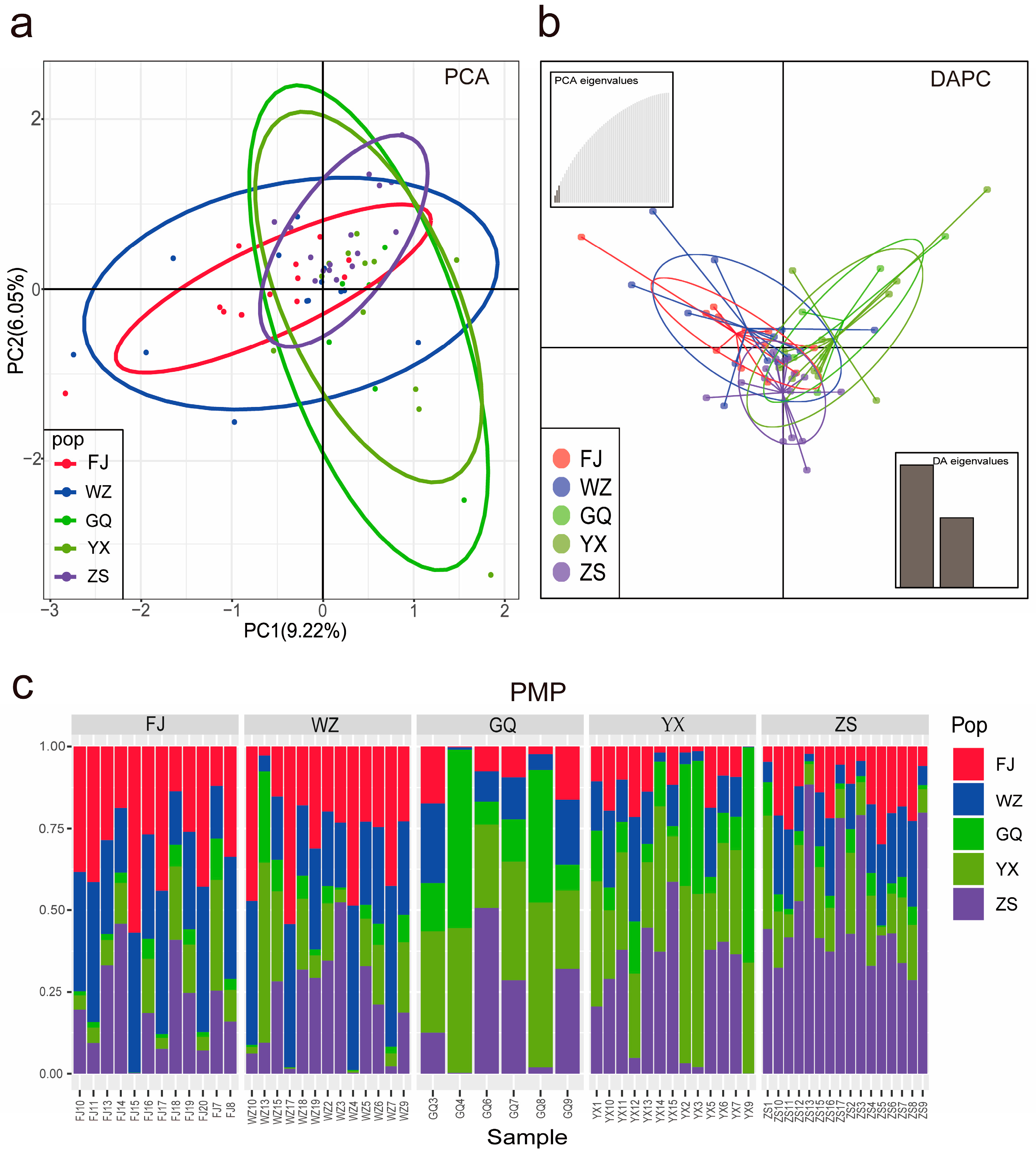
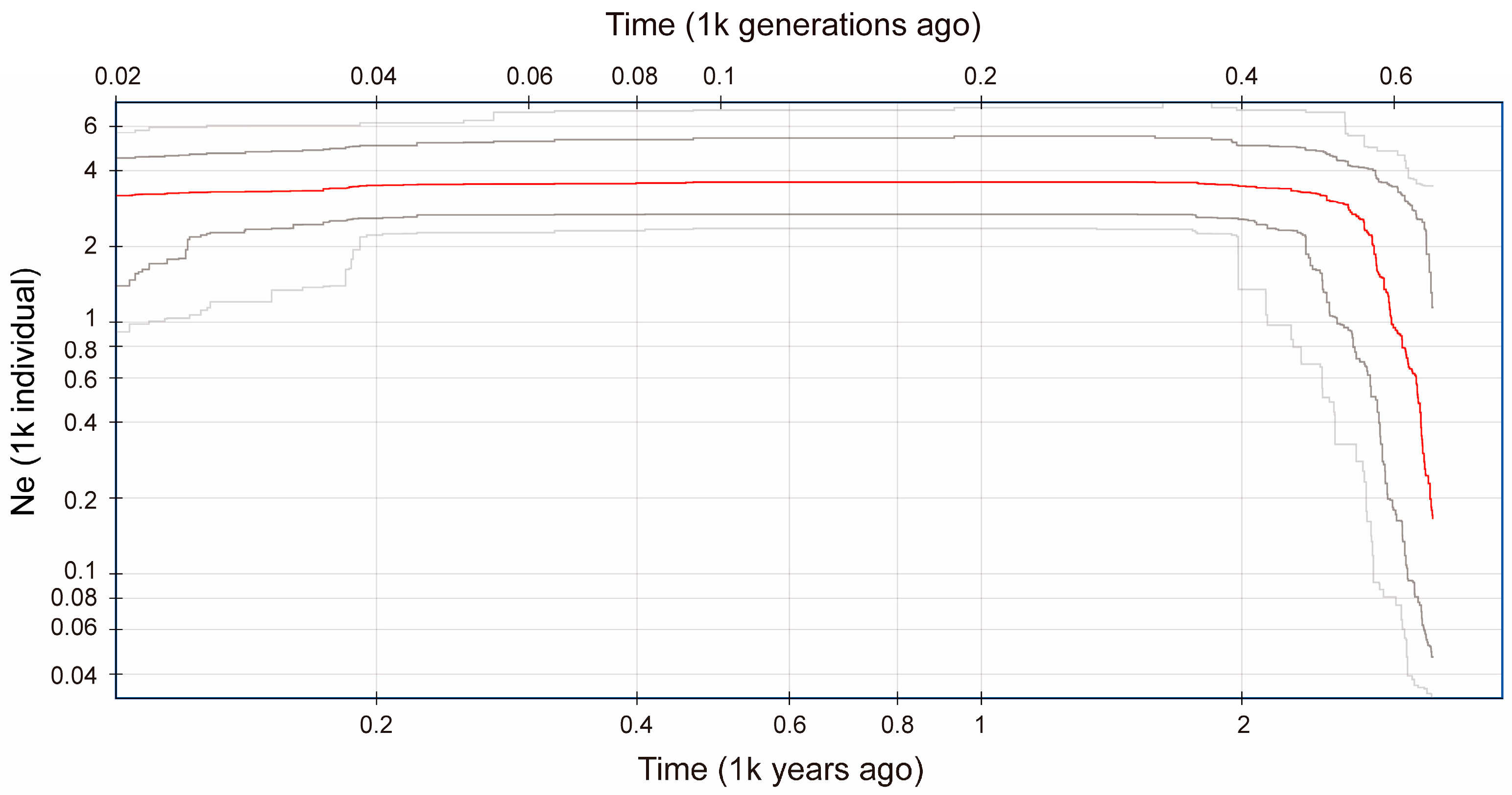

| Location | POP | N | PA | Ho | He | π | Fis |
|---|---|---|---|---|---|---|---|
| Inshore | WZ | 13 | 0.0013 | 0.03951 | 0.08080 | 0.08619 | 0.1728 |
| FJ | 12 | 0.0011 | 0.03843 | 0.07269 | 0.07779 | 0.1368 | |
| Offshore | ZS | 16 | 0.0014 | 0.03011 | 0.06077 | 0.06366 | 0.1600 |
| YX | 13 | 0.0012 | 0.03211 | 0.05573 | 0.05869 | 0.1251 | |
| GQ | 6 | 0.0004 | 0.04962 | 0.06537 | 0.07609 | 0.0513 |
| FST/Nm | FJ | WZ | GQ | YX | ZS |
|---|---|---|---|---|---|
| FJ | 5.3604 | 2.9737 | 4.4038 | 4.8909 | |
| WZ | 0.04456 | 3.6417 | 4.4956 | 5.2385 | |
| GQ | 0.07755 | 0.06424 | 3.9404 | 3.5761 | |
| YX | 0.05372 | 0.05268 | 0.05966 | 5.7081 | |
| ZS | 0.04863 | 0.04555 | 0.06534 | 0.04196 |
| Location | Pop | N | S | Hd ± SD | Pi ± SD | H |
|---|---|---|---|---|---|---|
| SCS | 74 | 14 | 0.130 ± 0.053 | 0.00134 ± 0.00079 | 4 | |
| WZ | 19 | 0 | 0.000 ± 0.000 | 0.00000 ± 0.00000 | Hap_1 (19) | |
| FJ | 19 | 11 | 0.292 ± 0.127 | 0.00293 ± 0.00217 | Hap_1 (16) Hap_2 (1) Hap_3 (2) | |
| ZS | 13 | 9 | 0.295 ± 0.156 | 0.00332 ± 0.00244 | Hap_1 (11) Hap_3 (1) Hap_4 (1) | |
| YX | 16 | 0 | 0.000 ± 0.000 | 0.00000 ± 0.00000 | Hap_1 (16) | |
| GQ | 7 | 0 | 0.000 ± 0.000 | 0.00000 ± 0.00000 | Hap_1 (7) | |
| Taiwan | 54 | 19 | 0.848 ± 0.027 | 0.01650 ± 0.00078 | 10 | |
| Ken | 19 | 18 | 0.743 ± 0.072 | 0.01338 ± 0.00155 | Hap_1 (8) Hap_3 (1) Hap_4 (6) Hap_5 (1) Hap_7 (2) Hap_8 (1) | |
| Don | 25 | 19 | 0.837 ± 0.042 | 0.01642 ± 0.00146 | Hap_1 (3) Hap_4 (1) Hap_5 (4) Hap_6 (8) Hap_7 (1) Hap_9 (4) Hap_10 (4) | |
| Lon | 5 | 12 | 0.800 ± 0.164 | 0.01542 ± 0.00421 | Hap_1 (2) Hap_2 (2) Hap_5 (1) | |
| San | 5 | 11 | 0.600 ± 0.175 | 0.01451 ± 0.00424 | Hap_1 (3) Hap_6 (2) | |
| Japan | 51 | 17 | 0.701 ± 0.032 | 0.01336 ± 0.00115 | 4 | |
| Shi | 20 | 17 | 0.658 ± 0.065 | 0.01383 ± 0.00154 | Hap_1 (8) Hap_4 (2) Hap_5 (1) Hap_6 (9) | |
| Yok | 13 | 17 | 0.782 ± 0.069 | 0.01514 ± 0.00270 | Hap_1 (2) Hap_4 (3) Hap_5 (3) Hap_6 (5) | |
| AMA | 18 | 11 | 0.699 ± 0.043 | 0.01152 ± 0.00156 | Hap_1 (6) Hap_4 (5) Hap_6 (7) | |
| Mean | 179 | 19 | 0.640 ± 0.035 | 0.01327 ± 0.00070 | 10 |
| Group | Source of Variation | df | Sum of Squares | Variance Components | Percentage of Variation | Φ-Statistics |
|---|---|---|---|---|---|---|
| Inshore vs. Offshore | Among groups | 1 | 0.131 | −0.00652 Va | −2.15 | ΦCT = −0.02152 |
| Among populations | 3 | 1.090 | 0.00414 Vb | 1.37 | ΦSC = 0.01338 | |
| Within populations | 69 | 21.077 | 0.30546 Vc | 100.78 | ΦST = −0.00785 | |
| SCS vs. Taiwan | Among groups | 1 | 5.662 | 0.03173 Va | 3.85 | ΦCT = 0.03847 |
| Among populations | 7 | 18.582 | 0.11893 Vb | 14.42 | ΦSC = 0.14995 * | |
| Within populations | 123 | 107.874 | 0.67421 Vc | 81.73 | ΦST = 0.18265 * | |
| SCS vs. Japan | Among groups | 1 | 6.490 | 0.06006 Va | 9.47 | ΦCT = −0.09473 |
| Among populations | 6 | 11.956 | 0.08258 Vb | 13.02 | ΦSC = 0.14387 * | |
| Within populations | 147 | 72.238 | 0.49141 Vc | 77.50 | ΦST = 0.22497 * |
| SCS | Taiwan | Japan | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WZ | FJ | YX | ZS | GQ | Lon | San | Ken | Don | Shi | Yok | AMA | |
| WZ | ||||||||||||
| FJ | 0.02564 | |||||||||||
| YX | 0.00000 | 0.01459 | ||||||||||
| ZS | 0.04994 | −0.03874 | 0.03465 | |||||||||
| GQ | 0.00000 | −0.04724 | 0.00000 | −0.04111 | ||||||||
| Lon | 0.75103 * | 0.55375 * | 0.72091 * | 0.51570 * | 0.55696 * | |||||||
| San | 0.57271 * | 0.34515 * | 0.53171 * | 0.22964 | 0.33121 | 0.20561 | ||||||
| Ken | 0.42739 * | 0.32991 * | 0.40294 * | 0.25139 * | 0.30311 * | 0.21738 * | −0.07688 | |||||
| Don | 0.57824 * | 0.50893 * | 0.55847 * | 0.45853 * | 0.47943 * | 0.15012 | 0.12429 | 0.11652 * | ||||
| Shi | 0.50473 * | 0.42003 * | 0.48116 * | 0.34618 * | 0.38530 * | 0.26786 * | −0.08060 | −0.00161 | 0.07601 | |||
| Yok | 0.67571 * | 0.58128 * | 0.65082 * | 0.51638 * | 0.54110 * | 0.20217 | 0.08864 | 0.08237 | −0.04500 | 0.02330 | ||
| AMA | 0.62061 * | 0.53066 * | 0.59775 * | 0.45619 * | 0.50280 * | 0.36387 * | −0.01151 | 0.02128 | 0.08765 * | −0.03762 | 0.02323 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Y.; Zheng, L.; Ke, J.; Zhou, Y.; Mo, S.; Liu, X.; Lin, J.; Ren, Y.; Huang, D.; Chen, R.; et al. Low Genetic Diversity and Decreased Effective Population Sizes of Acropora hyacinthus Populations Inhabiting Inshore and Offshore Reefs in the South China Sea. Oceans 2025, 6, 72. https://doi.org/10.3390/oceans6040072
Di Y, Zheng L, Ke J, Zhou Y, Mo S, Liu X, Lin J, Ren Y, Huang D, Chen R, et al. Low Genetic Diversity and Decreased Effective Population Sizes of Acropora hyacinthus Populations Inhabiting Inshore and Offshore Reefs in the South China Sea. Oceans. 2025; 6(4):72. https://doi.org/10.3390/oceans6040072
Chicago/Turabian StyleDi, Yijin, Lingyu Zheng, Jingzhao Ke, Yinyin Zhou, Shaoyang Mo, Xiangbo Liu, Jiquan Lin, Yuxiao Ren, Duanjie Huang, Rouwen Chen, and et al. 2025. "Low Genetic Diversity and Decreased Effective Population Sizes of Acropora hyacinthus Populations Inhabiting Inshore and Offshore Reefs in the South China Sea" Oceans 6, no. 4: 72. https://doi.org/10.3390/oceans6040072
APA StyleDi, Y., Zheng, L., Ke, J., Zhou, Y., Mo, S., Liu, X., Lin, J., Ren, Y., Huang, D., Chen, R., & Li, X. (2025). Low Genetic Diversity and Decreased Effective Population Sizes of Acropora hyacinthus Populations Inhabiting Inshore and Offshore Reefs in the South China Sea. Oceans, 6(4), 72. https://doi.org/10.3390/oceans6040072






