Systematic Analysis of Threats to Sea Turtles in Mexico: Trends, Knowledge Gaps, and Implications for Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Selection Criteria
2.2. Data Collection and Analysis
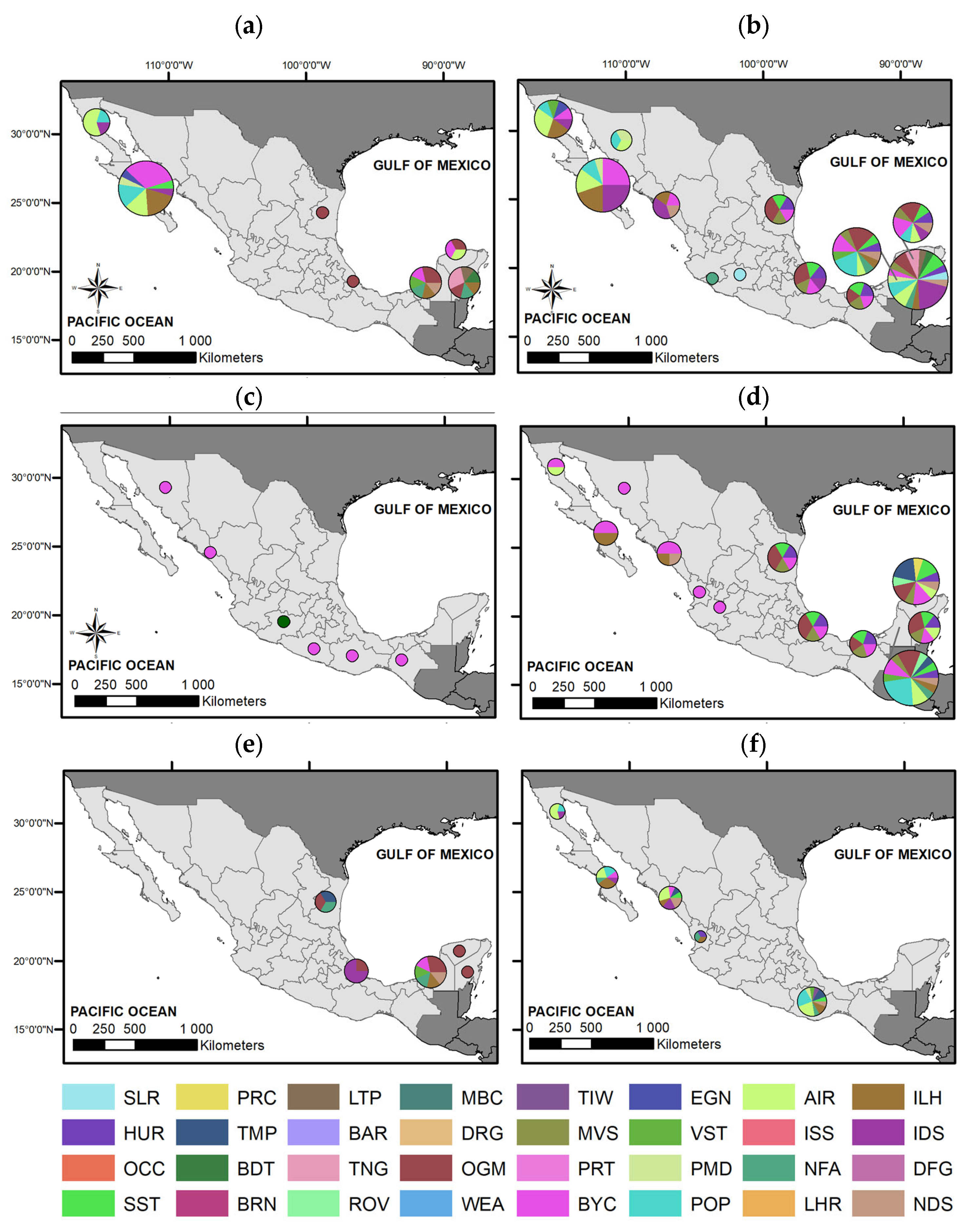
2.2.1. Geographic Analysis and Gap Mapping
2.2.2. The Mann–Kendall Test
2.3. Comparative Threat Assessment
3. Results
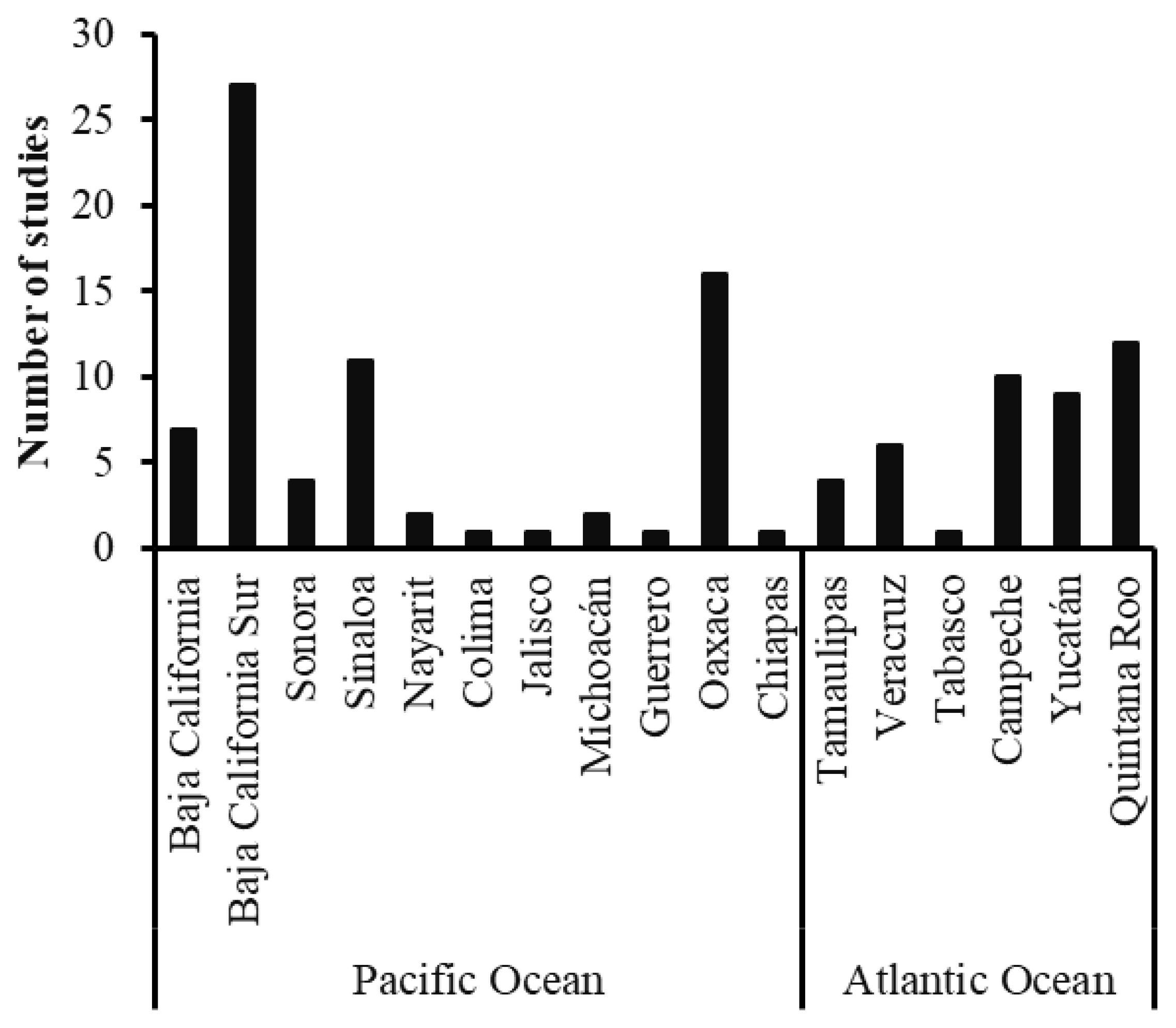
3.1. Temporal and Spatial Research Trends
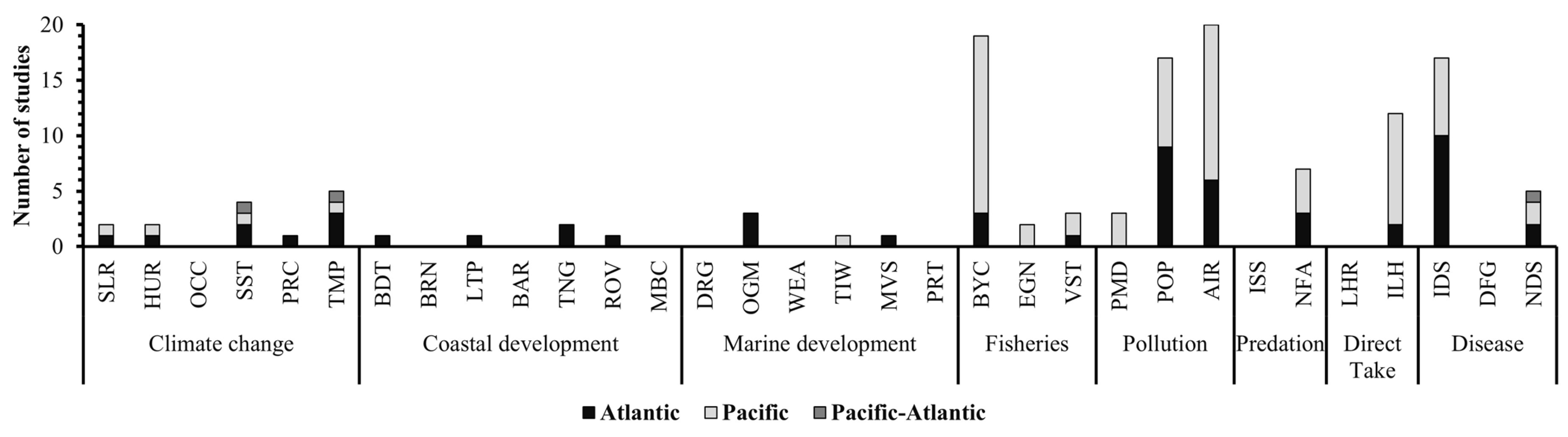
3.2. Threats to Sea Turtles: Frequency and Species Impacted
3.3. Species-Specific Threats and Gaps in Public Policy and Research
- D. coriacea and L. kempii have multiple threats listed in PACE without scientific support, highlighting the need for specific studies.
- C. mydas and E. imbricata have threats that are better documented in recent literature, suggesting more advanced research and conservation efforts.
3.3.1. Caretta caretta
3.3.2. Chelonia mydas
3.3.3. Eretmochelys imbricata
3.3.4. Dermochelys coriacea
3.3.5. Lepidochelys kempii
3.3.6. Lepidochelys olivacea
4. Discussion
4.1. Geographic Disparities and Knowledge Gaps
4.2. Policy Implications and Research Alignment
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heithaus, M.R. Predators, Prey, and the Ecological Roles of Sea Turtles. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 249–285. [Google Scholar]
- Bouchard, S.S.; Bjorndal, K.A. Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 2000, 81, 2305–2313. [Google Scholar] [CrossRef]
- Liceaga-Correa, M.D.L.A.; Uribe-Martínez, A.; Cuevas, E. Ecological vulnerability of adult female marine turtles as indicators of opportunities for regional socioecosystem management in the southern Gulf of Mexico. Sustainability 2022, 14, 184. [Google Scholar] [CrossRef]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Hannan, L.B.; Roth, J.D.; Ehrhart, L.; Weishampel, J.F. Dune vegetation fertilization by nesting sea turtles. Ecology 2007, 88, 1053–1058. [Google Scholar] [CrossRef]
- CONANP (Comisión Nacional de Áreas Naturales Protegidas). Programa Nacional de Conservación de Tortugas Marinas; Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2022; 80p. [Google Scholar]
- Lamont, M.M.; Putman, N.F.; Fujisaki, I.; Hart, K.M. Spatial requirements of different life-stages of the loggerhead turtle (Caretta caretta) from a distinct population segment in the northern Gulf of Mexico. Herpetol. Conserv. Biol. 2015, 10, 26–43. [Google Scholar]
- Rguez-Baron, J.M.; Kelez, S.; Liles, M.; Zavala-Norzagaray, A.; Torres-Suárez, O.L.; Amorocho, D.F.; Gaos, A.R. Sea Turtles in the East Pacific Ocean Region; MTSG Annual Regional Report; IUCN-SSC Marine Turtle Specialist Group: Gland, Switzerland, 2019. [Google Scholar]
- Gallegos-Fernández, S.A.; Trujillo-Córdova, J.A.; Guzmán-Hernández, V.; Abreu-Grobois, F.A.; Huerta-Rodríguez, P.; Gómez-Ruiz, P.A.; Uribe-Martínez, A.; Cuevas, E. Marine turtles, umbrella species undergoing recovery. Front. Amphib. Reptile Sci. 2023, 1, 1303373. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Schofield, G.; Gkazinou, C.; Almpanidou, V.; Hays, G.C. Global sea turtle conservation successes. Sci. Adv. 2017, 3, e1600730. [Google Scholar] [CrossRef] [PubMed]
- Cohello, D.; Herrera, M. Línea base de conocimiento sobre el estado actual de las tortugas marinas en el Ecuador. Bol. Esp. Inst. Nac. Pesca 2011, 2, 85. [Google Scholar]
- Levy, Y.; Frid, O.; Weinberger, A.; Sade, R.; Adam, Y.; Kandanyan, U.; Berkun, V.; Perry, N.; Edelist, D.; Goren, M.; et al. A small fishery with a high impact on sea turtle populations in the eastern Mediterranean. Zool. Middle East 2015, 61, 300–317. [Google Scholar] [CrossRef]
- DOF (Diario Oficial de la Federación). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para Su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Secretaría del Medio Ambiente y Recursos Naturales: Ciudad de México, México, 2010. [Google Scholar]
- IUCN (International Union for Conservation of Nature). The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 12 February 2025).
- Calderon-Aguilera, L.E.; Rivera-Monroy, V.H.; Porter-Bolland, L.; Martínez-Yrízar, A.; Ladah, L.B.; Martínez-Ramos, M.; Búrquez, A. An assessment of natural and human disturbance effects on Mexican ecosystems: Current trends and research gaps. Biodivers. Conserv. 2012, 21, 589–617. [Google Scholar] [CrossRef]
- Strongin, K.; Polidoro, B.; Linardich, C.; Ralph, G.; Saul, S.; Carpenter, K. Translating globally threatened marine species information into regional guidance for the Gulf of Mexico. Glob. Ecol. Conserv. 2020, 23, e01010. [Google Scholar] [CrossRef]
- Shaver, D.J.; Gredzens, C.; Walker, J.S.; Godard-Codding, C.A.; Yacabucci, J.E.; Frey, A.; Dutton, P.H.; Schmitt, C.J. Embryo deformities and nesting trends in Kemp’s ridley sea turtles Lepidochelys kempii before and after the Deepwater Horizon oil spill. Endanger. Species Res. 2021, 44, 277–289. [Google Scholar] [CrossRef]
- Seminoff, J.A. Sea turtles, red listing, and the need for regional assessments. Mar. Turtle Newsl. 2004, 106, 4–6. [Google Scholar]
- Aguilar-González, M.E.; Luna-González, A.; Aguirre, A.; Zavala-Norzagaray, A.A.; Mundo-Ocampo, M.; González-Ocampo, H.A. Perceptions of fishers to sea turtle bycatch, illegal capture and consumption in the San Ignacio-Navachiste-Macapule lagoon complex, Gulf of California, Mexico. Integr. Zool. 2014, 9, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Merrick, R.; Silber, G.K.; Demaster, D.P.; Reynolds, J.E., III. Endangered species and populations. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 368–375. [Google Scholar]
- Morzaria-Luna, H.N.; Turk-Boyer, P.; Moreno-Baez, M. Social indicators of vulnerability for fishing communities in the Northern Gulf of California, Mexico: Implications for climate change. Mar. Policy 2014, 45, 182–193. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Limpus, C.J.; Hays, G.C. Vulnerability of marine turtles to climate change. In Advances in Marine Biology; Sims, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 56, pp. 151–211. [Google Scholar]
- Hazel, J.; Gyuris, E. Vessel-related mortality of sea turtles in Queensland, Australia. Wildl. Res. 2006, 33, 149–154. [Google Scholar] [CrossRef]
- Márquez, R. FAO Species Catalogue, Volume 11: Sea Turtles of the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990. [Google Scholar]
- Frazier, J. Prehistoric and ancient historic interactions between humans and marine turtles. In The Biology of Sea Turtles, Volume II; Lutz, P.L., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–38. [Google Scholar]
- Seminoff, J.A.; Resendiz, A.; Resendiz, B.; Nichols, W.J. Occurrence of loggerhead sea turtles (Caretta caretta) in the Gulf of California, Mexico: Evidence of life-history variation in the Pacific Ocean. Herpetol. Rev. 2004, 35, 24–27. [Google Scholar]
- Mast, R.B.; Hutchinson, B.J.; Howgate, E.; Pilcher, N.J. MTSG update: IUCN/SSC marine turtle specialist group hosts the second burning issues assessment workshop. Mar. Turtle Newsl. 2005, 110, 13–15. [Google Scholar]
- Fuentes, M.; McMichael, E.; Kot, C.Y.; Silver-Gorges, I.; Wallace, B.P.; Godley, B.J.; Brooks, A.M.L.; Ceriani, S.A.; Cortés-Gómez, A.A.; Dawson, T.M.; et al. Key issues in assessing threats to sea turtles: Knowledge gaps and future directions. Endanger. Species Res. 2023, 52, 303–341. [Google Scholar] [CrossRef]
- Pohlert, T. Non-parametric trend tests and change-point detection. CC BY-ND 2016, 4, 1–18. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Caguama (Caretta caretta); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP), Programa de las Naciones Unidad para el Desarrollo (PNUD): Ciudad de México, México, 2018. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Golfina (Lepidochelys olivacea); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2018. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Laúd (Dermochelys coriacea); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2018. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Verde/Negra (Chelonia mydas); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2018. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Lora (Lepidochelys kempii); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2018. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Programa de Acción para la Conservación de la Especie Tortuga Carey (Eretmochelys imbricata); Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Comisión Nacional de Áreas Naturales Protegidas (CONANP): Ciudad de México, México, 2020. [Google Scholar]
- Garduño-Andrade, M.; Guzman, V.; Miranda, E.; Briseño-Dueñas, R.; Abreu-Grobois, F.A. Increases in hawksbill turtle (Eretmochelys imbricata) nestings in the Yucatan Peninsula, Mexico, 1977–1996: Data in support of successful conservation? Chelonian Conserv. Biol. 1999, 3, 286–295. [Google Scholar]
- Tiburcio-Pintos, G.; Cariño-Olvera, M.M. Collective efforts for the conservation of sea turtles in the Gulf of California. Let. Verdes Rev. Latinoam. Estud. Socioambientales 2017, 22, 7–26. [Google Scholar] [CrossRef]
- Cordon, C.; Carmena, B.; Giménez, M.C.; García, J.L.; Calderon-Guerrero, C. Evolution of ecotourism in coastal indigenous communities: Comparison of the case studies of La Ventanilla and La Escobilla in Oaxaca, Mexico. Sustainability 2023, 15, 2207. [Google Scholar] [CrossRef]
- Craig, R.K. Marine biodiversity, climate change, and governance of the oceans. Diversity 2012, 4, 224–238. [Google Scholar] [CrossRef]
- Vallarino-Castillo, R.; Negro-Valdecantos, V.; del Campo, J.M. Understanding the impact of hydrodynamics on coastal erosion in Latin America: A systematic review. Front. Environ. Sci. 2023, 11, 1267402. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q. Evaluating the mechanisms of coastal circulation and their responses to climate change. Int. J. Mar. Sci. 2024, 14, 182–192. [Google Scholar] [CrossRef]
- Grain, D.A.; Bolten, A.B.; Bjorndal, K.A. Effects of beach nourishment on sea turtles: Review and research initiatives. Restor. Ecol. 1995, 3, 95–104. [Google Scholar] [CrossRef]
- Dellert, L.J.; O’Neil, D.; Cassill, D.L. Effects of beach renourishment and clutch relocation on the success of the loggerhead sea turtle (Caretta caretta) eggs and hatchlings. J. Herpetol. 2014, 48, 186–187. [Google Scholar] [CrossRef]
- Hill, M.K.; Monroe, M.C.; Ankersen, T.T.; Carthy, R.R.; Kay, T.A. Coastal armoring and sea turtles: Beachfront homeowners’ opinions and intent. Coast. Manag. 2019, 47, 594–610. [Google Scholar] [CrossRef]
- Nelson-Sella, K.A.; Fuentes, M. Exposure of marine turtle nesting grounds to coastal modifications: Implications for management. Ocean Coast. Manag. 2019, 169, 182–190. [Google Scholar] [CrossRef]
- Hirsch, S.E.; Toonder, M.; Reilly, J.D.; Hoover, S.R.; Perrault, J.R. Responses of three nesting sea turtle species to hard-armoring structures. Front. Mar. Sci. 2022, 9, 980715. [Google Scholar] [CrossRef]
- Reine, K.J. A Literature Review of Beach Nourishment Impacts on Marine Turtles; US Army Corps of Engineers: Washington, DC, USA, 2022. [Google Scholar]
- Fuentes, M.; Limpus, C.J.; Hamann, M. Vulnerability of sea turtle nesting grounds to climate change. Glob. Change Biol. 2011, 17, 140–153. [Google Scholar] [CrossRef]
- Whittock, P.A.; Pendoley, K.L.; Larsen, R.; Hamann, M. Effects of a dredging operation on the movement and dive behaviour of marine turtles during breeding. Biol. Conserv. 2017, 206, 190–200. [Google Scholar] [CrossRef]
- Gleason, F.H.; Allerstorfer, M.; Lilje, O. Newly emerging diseases of marine turtles, especially sea turtle egg fusariosis (SEFT), caused by species in the Fusarium solani complex (FSSC). Mycology 2020, 11, 184–194. [Google Scholar] [CrossRef]
- Komyakova, V.; Jaffrés, J.B.; Strain, E.M.; Cullen-Knox, C.; Fudge, M.; Langhamer, O.; Bender, A.; Yaakub, S.M.; Wilson, E.; Allan, B.J.M.; et al. Conceptualisation of multiple impacts interacting in the marine environment using marine infrastructure as an example. Sci. Total Environ. 2022, 830, 154748. [Google Scholar] [CrossRef]
- Pat-Fernández, L.A.; Calderón-Gómez, G. Caracterización del perfil turístico en un destino emergente, caso de estudio de ciudad del Carmen, Campeche. México. Gestión Turística 2012, 18, 47–70. [Google Scholar] [CrossRef]
- Frisk, G.V. Noiseonomics: The relationship between ambient noise levels in the sea and global economic trends. Sci. Rep. 2012, 2, 437. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, X.; Liu, G. Noise in the sea and its impacts on marine organisms. Int. J. Environ. Res. Public Health 2015, 12, 12304–12323. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Viada, S.T.; Hammer, R.M.; Racca, R.; Hannay, D.; Thompson, M.J.; Balcom, B.J.; Phillips, N.W. Review of potential impacts to sea turtles from underwater explosive removal of offshore structures. Environ. Impact Assess. Rev. 2008, 28, 267–285. [Google Scholar] [CrossRef]
- Díaz, M.P.; Kunc, H.P.; Houghton, J.D. Anthropogenic noise predicts sea turtle behavioural responses. Mar. Pollut. Bull. 2024, 198, 115907. [Google Scholar] [CrossRef]
- Eckert, K.L.; Bjorndal, K.A.; Abreu-Grobois, F.A.; Donnelly, M. Técnicas de Investigación y Manejo para la Conservación de las Tortugas Marinas; Marine Turtle Specialist Group UICN/CSE: Washington, DC, USA, 1999. [Google Scholar]
- Antworth, R.L.; Pike, D.A.; Stiner, J.C. Nesting ecology, current status, and conservation of sea turtles on an uninhabited beach in Florida, USA. Biol. Conserv. 2006, 130, 10–15. [Google Scholar] [CrossRef]
- Hendrix, H.; Pérez-Espona, S. A Systematic Review of Population Monitoring Studies of Sea Turtles and Its Application to Conservation. Diversity 2024, 16, 177. [Google Scholar] [CrossRef]
- Vazquez, G.F.; Reyes, M.C.; Fernandez, G.; Aguayo, J.E.C.; Sharma, V.K. Contamination in marine turtle (Dermochelys coriaca) egg shells of Playon de Mexiquillo, Michoacan, Mexico. Bull. Environ. Contam. Toxicol. 1997, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.C.; Pier, M.D.; Wesselman, R.; Juárez, J.A. Organochlorine contaminants in sea turtles from the Eastern Pacific. Mar. Pollut. Bull. 2003, 46, 1082–1089. [Google Scholar] [CrossRef]
- Seminoff, J.A.; Jones, T.T.; Resendiz, A.; Nichols, W.J.; Chaloupka, M.Y. Monitoring green turtles (Chelonia mydas) at a coastal foraging area in Baja California, Mexico: Multiple indices to describe population status. J. Mar. Biol. Assoc. U. K. 2003, 83, 1355–1362. [Google Scholar] [CrossRef]
- Cordero-Tapia, A.; Gardner, S.C.; Arellano-Blanco, J.; Inohuye-Rivera, R.B. Learedius learedi infection in black turtles (Chelonia mydas agassizii), Baja California Sur, Mexico. J. Parasitol. 2004, 90, 645–647. [Google Scholar] [CrossRef]
- Inohuye-Rivera, R.B.; Cordero-Tapia, A.; Arellano-Blanco, J.; Gardner, S.C. Learedius learedi (Trematoda: Spirorchiidae), in Black Turtle (Chelonia mydas agassizii) Hearts from Magdalena Bay, Baja California Sur, Mexico. Comp. Parasitol. 2004, 71, 37–41. [Google Scholar] [CrossRef]
- Gardner, S.C.; Fitzgerald, S.L.; Vargas, B.A.; Rodríguez, L.M. Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. Biometals 2006, 19, 91–99. [Google Scholar] [CrossRef]
- Koch, V.; Nichols, W.J.; Peckham, H.; De La Toba, V. Estimates of sea turtle mortality from poaching and bycatch in Bahía Magdalena, Baja California Sur, Mexico. Biol. Conserv. 2006, 128, 327–334. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Osuna-López, J.I.; Ruiz-Telles, A.; Quintero-Alvarez, J.M.; López-López, G.; Izaguirre-Fierro, G.; Voltolina, D. Heavy metals in the tissues of the sea turtle Lepidochelys olivacea from a nesting site of the northwest coast of Mexico. Bull. Environ. Contam. Toxicol. 2006, 77, 179–185. [Google Scholar] [CrossRef]
- Kampalath, R.; Gardner, S.C.; Méndez-Rodríguez, L.; Jay, J.A. Total and methylmercury in three species of sea turtles of Baja California Sur. Mar. Pollut. Bull. 2006, 52, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Talavera-Saenz, A.; Gardner, S.C.; Rodriquez, R.R.; Acosta-Vargas, B. Metal profiles used as environmental markers of Green Turtle (Chelonia mydas) foraging resources. Sci. Total Environ. 2007, 373, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pechham, S.H.; Maldonado Diaz, D.; Walli, A.; Ruiz, G.; Crowder, L.B.; Nichols, W.J. Small-scale fisheries bycatch jeopardizez endangered pacific loggerhead turtles. PLoS ONE 2007, 2, e1041. [Google Scholar] [CrossRef]
- Peckham, S.H.; Maldonado-Diaz, D.; Koch, V.; Mancini, A.; Gaos, A.; Tinker, T.M.; Nicholas, W.J. High mortality of loggerhead turtles due to bycatch, human consumption and strandings at Baja California Sur, Mexico, 2003 to 2007. Endanger. Species Res. 2008, 5, 171–183. [Google Scholar] [CrossRef]
- Mancini, A.; Koch, V. Sea turtle consumption and black market trade in Baja California Sur, Mexico. Endanger. Species Res. 2009, 7, 1–10. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Calderón-Campuzano, M.F.; Soto-Jiménez, M.F.; Ruelas-Inzunza, J.R. Lead in blood and eggs of the sea turtle, Lepidochelys olivacea, from the Eastern Pacific: Concentration, isotopic composition and maternal transfer. Mar. Pollut. Bull. 2010, 60, 433–439. [Google Scholar] [CrossRef]
- Richardson, K.L.; Lopez Castro, M.; Gardner, S.C.; Schlenk, D. Polychlorinated biphenyls and biotransformation enzymes in three species of sea turtles from the Baja California peninsula of Mexico. Arch. Environ. Contam. Toxicol. 2010, 58, 183–193. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Calderón-Campuzano, M.F.; Soto-Jiménez, M.F.; Ruelas-Inzunza, J.R. Trace Metals (Cd, Cu, Ni, and Zn) in Blood and Eggs of the Sea Turtle Lepidochelys olivacea from a Nesting Colony of Oaxaca, Mexico. Arch. Environ. Contam. Toxicol. 2010, 59, 632–641. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Calderón-Campuzano, M.F.; Soto-Jiménez, M.F.; Ruelas-Inzunza, J.R. Mercury in blood and eggs of the sea turtle Lepidochelys olivacea from a nesting colony in Oaxaca, Mexico. Mar. Pollut. Bull. 2011, 62, 1320–1323. [Google Scholar] [CrossRef]
- Ley-Quiñónez, C.; Zavala-Norzagaray, A.A.; Espinosa-Carreón, T.L.; Peckham, H.; Marquez-Herrera, C.; Campos-Villegas, L.; Aguirre, A.A. Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur, Mexico. Mar. Pollut. Bull. 2011, 62, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Senko, J.; Borquez-Reyes, R.; Póo, J.G.; Seminoff, J.A.; Koch, V. To Poach or Not to Poach an Endangered Species: Elucidating the Economic and Social Drivers Behind Illegal Sea Turtle Hunting in Baja California Sur, Mexico. Hum. Ecol. 2011, 39, 743–756. [Google Scholar] [CrossRef]
- Benítez, J.A.; Vidal, J.; Brichieri-Colombi, T.; Delgado-Estrella, A. Monitoring ecosystem health of the Terminos Lagoon region using heavy metals as environmental indicators. Environ. Impact 2012, 162, 349–358. [Google Scholar] [CrossRef]
- Mancini, A.; Koch, V.; Seminoff, J.A.; Madon, B. Small-scale gill-net fisheries cause massive green turtle Chelonia mydas mortality in Baja California Sur, Mexico. Oryx 2012, 46, 69–77. [Google Scholar] [CrossRef]
- Serrano, A.; Vázquez-Castán, L.; Silva, C.G.S.; De Jesús Basañez-Muñoz, A.; Naval-Avila, C. Identification of the bacterial flora in Kemp’s Ridley sea turtle (Lepidochelys kempii) in Barra Galindo, Tuxpan, Veracruz, Mexico [Identificación de la flora bacteriana en la tortuga lora (Lepidochelys kempii) en el ejido Barra Galindo, Tuxpan, Veracruz, México]. Hidrobiologia 2012, 22, 142–146. [Google Scholar]
- Ley-Quiñónez, C.P.; Zavala-Norzagaray, A.A.; Réndon-Maldonado, J.G.; Espinosa-Carreón, T.L.; Canizales-Román, A.; Escobedo-Urías, D.C.; Leal-Acosta, M.L.; Hart, C.E.; Aguirre, A.A. Selected heavy metals and selenium in the blood of black sea turtle (Chelonia mydas agasiizzi) from Sonora, Mexico. Bull. Environ. Contam. Toxicol. 2013, 91, 645–651. [Google Scholar] [CrossRef]
- Senko, J.; Mancini, A.; Seminoff, J.A.; Koch, V. Bycatch and directed harvest drive high green turtle mortality at Baja California Sur, Mexico. Biol. Conserv. 2014, 169, 24–30. [Google Scholar] [CrossRef]
- Zavala-Norzagaray, A.A.; Ley-Quiñónez, C.P.; Espinosa-Carreón, T.L.; Canizalez-Román, A.; Hart, C.E.; Aguirre, A.A. Trace elements in blood of sea turtles Lepidochelys olivacea in the gulf of California, Mexico. Bull. Environ. Contam. Toxicol. 2014, 93, 536–541. [Google Scholar] [CrossRef]
- Bevan, E.; Wibbels, T.; Najera, B.M.; Martinez, M.A.; Martinez, L.A.; Reyes, D.J.; Hernandez, M.H.; Gamez, D.G.; Pena, L.J.; Burchfield, P.M. In situ nest and hatchling survival at Rancho Nuevo, the primary nesting beach of the Kemp’s ridley sea turtle, Lepidochelys Kempii. Herpetol. Conserv. Biol. 2014, 9, 563–577. [Google Scholar]
- Cortés-Gómez, A.A.; Fuentes-Mascorro, G.; Romero, D. Metals and metalloids in whole blood and tissues of Olive Ridley turtles (Lepidochelys olivacea) from La Escobilla Beach (Oaxaca, Mexico). Mar. Pollut. Bull. 2014, 89, 367–375. [Google Scholar] [CrossRef]
- Zavala-Norzagaray, A.A.; Aguirre, A.A.; Velazquez-Roman, J.; Flores-Villaseñor, H.; León-Sicairos, N.; Ley-Quiñonez, C.P.; Hernández-Díaz, L.D.; Canizalez-Roman, A. Isolation, characterization, and antibiotic resistance of Vibrio spp. in sea turtles from northwestern Mexico. Front. Microbiol. 2015, 6, 635. [Google Scholar] [CrossRef]
- Calvillo-García, Y.; Ramírez-Herrera, M.T.; Delgado-Trejo, C.; Legorreta-Paulin, G.; Corona, N. Modeling sea-level change, inundation scenarios, and their effect on the colola beach reserve—A nesting-habitat of the black sea turtle, Michoacán, Mexico. Geofísica Int. 2015, 54, 179–190. [Google Scholar] [CrossRef]
- Bárcenas-Ibarra, A.; de la Cueva, H.; Rojas-Lleonart, I.; Abreu-Grobois, F.A.; Lozano-Guzmán, R.I.; Cuevas, E.; García-Gasca, A. First approximation to congenital malformation rates in embryos and hatchlings of sea turtles. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Baena, M.L.; Escobar, F.; Halffter, G.; García-Chávez, J.H.; Rummer, J.L. Distribution and feeding behavior of Omorgus suberosus (Coleoptera: Trogidae) in Lepidochelys olivacea Turtle Nests. PLoS ONE 2015, 10, e0139538. [Google Scholar] [CrossRef] [PubMed]
- Reséndiz, E.; Flores-Ramírez, S.; Koch, V.; Cordero-Tapia, A. First record of fibropapillomatosis in a green turtle Chelonia mydas from the Baja California Peninsula. J. Aquat. Anim. Health 2016, 28, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Rodríguez, L.; Álvarez-Castañeda, S.T. Predation on turtle nests in the southwestern coast of the Baja California Peninsula. Rev. Mex. Biodivers. 2016, 87, 483–488. [Google Scholar]
- Pasanisi, E.; Cortés-Gómez, A.A.; Pérez-López, M.; Soler, F.; Hernández-Moreno, D.; Guerranti, C.; Martellini, T.; Fuentes-Mascorro, G.; Romero, D.; Cincinelli, A. Levels of perfluorinated acids (PFCAs) in different tissues of Lepidochelys olivacea sea turtles from the Escobilla beach (Oaxaca, Mexico). Sci. Total Environ. 2016, 572, 1059–1065. [Google Scholar] [CrossRef]
- Santos, K.C.; Livesey, M.; Fish, M.; Lorences, A.C. Climate change implications for the nest site selection process and subsequent hatching success of a green turtle population. Mitig. Adapt. Strateg. Glob. Change 2017, 22, 121–135. [Google Scholar] [CrossRef]
- Oliver de la Esperanza, A.; Arenas Martínez, A.; Tzeek-Tuz, M.; Pérez-Collazos, E. Are anthropogenic factors affecting nesting habitat of sea turtles? The case of Kanzul beach, Riviera Maya-Tulum (Mexico). J. Coast. Conserv. 2017, 21, 85–93. [Google Scholar] [CrossRef]
- Tremblay, N.; Ortíz-Arana, A.; González-Jáuregui, M.; Rendón-von Osten, J. Relationship between organochlorine pesticides and stress indicators in hawksbill sea turtle (Eretmochelys imbricata) nesting at Punta Xen (Campeche), Southern Gulf of Mexico. Ecotoxicology 2017, 26, 173–183. [Google Scholar] [CrossRef]
- Ley-Quiñónez, C.P.; Rossi-Lafferriere, N.A.; Espinoza-Carreon, T.L.; Hart, C.E.; Peckham, S.H.; Aguirre, A.A.; Zavala-Norzagaray, A.A. Associations between trace elements and clinical health parameters in the North Pacific loggerhead sea turtle (Caretta caretta) from Baja California Sur, Mexico. Environ. Sci. Pollut. Res. 2017, 24, 9530–9537. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez-Tapia, L.A.; Pedroza, D.; Ponce-Díaz, G.; Díaz de León, A.J.; Lluch-Belda, D. A continual engagement framework to tackle wicked problems: Curtailing loggerhead sea turtle fishing bycatch in Gulf of Ulloa, Mexico. Sustain. Sci. 2017, 12, 535–548. [Google Scholar] [CrossRef]
- Early-Capistrán, M.-M.; Sáenz-Arroyo, A.; Cardoso-Mohedano, J.-G.; Garibay-Melo, G.; Peckham, S.H.; Koch, V. Reconstructing 290 years of a data-poor fishery through ethnographic and archival research: The East Pacific green turtle (Chelonia mydas) in Baja California, Mexico. Fish Fish. 2018, 19, 57–77. [Google Scholar] [CrossRef]
- Cuevas, E.; Guzmán-Hernández, V.; Uribe-Martínez, A.; Raymundo-Sánchez, A.; Herrera-Pavon, R. Identification of Potential Sea Turtle Bycatch Hotspots Using a Spatially Explicit Approach in the Yucatan Peninsula, Mexico. Chelonian Conserv. Biol. 2018, 17, 78–93. [Google Scholar] [CrossRef]
- Cortés-Gómez, A.A.; Tvarijonaviciute, A.; Teles, M.; Cuenca, R.; Fuentes-Mascorro, G.; Romero, D. p-Nitrophenyl Acetate Esterase Activity and Cortisol as Biomarkers of Metal Pollution in Blood of Olive Ridley Turtles (Lepidochelys olivacea). Arch. Environ. Contam. Toxicol. 2018, 75, 25–36. [Google Scholar] [CrossRef]
- Salvarani, P.I.; Vieira, L.R.; Ku-Peralta, W.; Morgado, F.; Osten, J.R.V. Oxidative stress biomarkers and organochlorine pesticides in nesting female hawksbill turtles Eretmochelys imbricata from Mexican coast (Punta Xen, Mexico). Environ. Sci. Pollut. Res. 2018, 25, 23809–23816. [Google Scholar] [CrossRef]
- Vega-Manriquez, D.X.; Dávila-Arrellano, R.P.; Eslava-Campos, C.A.; Salazar Jiménez, E.; Negrete-Philippe, A.C.; Raigoza-Figueras, R.; Muñoz-Tenería, F.A. Identification of bacteria present in ulcerative stomatitis lesions of captive sea turtles Chelonia mydas. Vet. Res. Commun. 2018, 42, 251–254. [Google Scholar] [CrossRef]
- Cuevas, E.; Liceaga-Correa, M.; Uribe-Martínez, A. Ecological vulnerability of two sea turtle species in the Gulf of Mexico: An integrated spatial approach. Endanger. Species Res. 2019, 40, 337–356. [Google Scholar] [CrossRef]
- Mejía-Radillo, R.Y.; Zavala-Norzagaray, A.A.; Chávez-Medina, J.; Aguirre, A.; Escobedo-Bonilla, C.M. Presence of chelonid herpesvirus 5 (ChHV5) in sea turtles in northern Sinaloa, Mexico. Dis. Aquat. Org. 2019, 132, 99–108. [Google Scholar] [CrossRef]
- Salvarani, P.I.; Morgado, F.; Vieira, L.R.; Osten, J.R.V. Organochlorines Contaminants in Eggs of Hawksbill (Eretmochelys imbricata) and Green Sea Turtles (Chelonia mydas) from Mexico coast. Arch. Environ. Contam. Toxicol. 2019, 76, 425–434. [Google Scholar] [CrossRef]
- Bevan, E.M.; Wibbels, T.; Shaver, D.; Walker, J.S.; Illescas, F.; Montano, J.; Ortiz, J.; Peña, J.J.; Sarti, L.; Najera, B.M.; et al. Comparison of beach temperatures in the nesting range of Kemp’s ridley sea turtles in the Gulf of Mexico, Mexico and USA. Endanger. Species Res. 2019, 40, 31–40. [Google Scholar] [CrossRef]
- Flores-Aguirre, C.D.; Díaz-Hernández, V.; Salgado-Ugarte, I.H.; Caballero, L.E.S.; de la Cruz, F.R.M. Feminization tendency of Hawksbill Turtles (Eretmochelys imbricata) in the western Yucatan Peninsula, Mexico. Amphib. Reptile Conserv. 2020, 14, 190–202. [Google Scholar]
- Suárez-Domínguez, E.A.; Martínez-Serrano, I.; Righini, N.; Chamlaty-Fayad, Y.E.; Bello-Sánchez, E.A.; Ramos-Díaz, A.H. Fibropapillomatosis in free-ranging green sea turtles (Chelonia mydas) off the Central Coast of Veracruz, Mexico [Fibropapilomatosis en tortuga verde (Chelonia mydas) de vida libre en la Costa Central de Veracruz, México]. Cienc. Mar. 2020, 46, 133–143. [Google Scholar] [CrossRef]
- Salinas-Zavala, C.A.; Morales-Zárate, M.V.; Martínez-Rincón, R.O. An empirical relationship between sea surface temperature and massive stranding of the loggerhead turtle (Caretta caretta) in the Gulf of Ulloa, Mexico. Lat. Am. J. Aquat. Res. 2020, 48, 214–225. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, C.; Pajuelo, M.; Grados, D.; Abrego, M.E.; Rebeca Barragán-Rocha, A.; Barrantes, M.; Cotto Sánchez, A.; Fonseca, L.G.; Gadea Espinal, V.; Mangel, J.C.; et al. Rapid Assessments of Leatherback Small-Scale Fishery Bycatch in Internesting Areas in the Eastern Pacific Ocean. Front. Mar. Sci. 2020, 6, 813. [Google Scholar] [CrossRef]
- Espinoza, J.; Hernández, E.; Lara-Uc, M.M.; Reséndiz, E.; Alfaro-Núñez, A.; Hori-Oshima, S.; Medina-Basulto, G. Genetic Analysis of Chelonid Herpesvirus 5 in Marine Turtles from Baja California Peninsula. EcoHealth 2020, 17, 258–263. [Google Scholar] [CrossRef]
- McLoughlin, M.; Comer-Santos, K. Twenty-eight Years of Buggy Adventures on a Mexican Turtle Beach. Sea Turt. Res. Conserv. 2021, 15–20. [Google Scholar] [CrossRef]
- Reséndiz, E.; Fernández-Sanz, H.; Domínguez-Contreras, J.F.; Ramos-Díaz, A.H.; Mancini, A.; Zavala-Norzagaray, A.A.; Alonso-Aguirre, A. Molecular characterization of Chelonid Alphaherpesvirus 5 in a black turtle (Chelonia mydas) Fibropapilloma from Baja California Sur, Mexico. Animals 2021, 11, 105. [Google Scholar] [CrossRef]
- Mondragón, M.E.; Luzardo, O.P.; Zumbado, M.; Rodríguez-Hernández, Á.; Berriel, C.R.; Ramírez-Gomez, H.V.; Islas, C.G.; Fisher, R.F.; Martínez, J.R. Incidence of 49 elements in the blood and scute tissues of nesting hawksbill turtles (Eretmochelys imbricata) in Holbox Island. Reg. Stud. Mar. Sci. 2021, 41, 101566. [Google Scholar] [CrossRef]
- Masés-García, C.A.; Briones-Salas, M.; Sosa-Escalante, J.E. Assessment of wildlife crime in a high-biodiversity region of Mexico. J. Nat. Conserv. 2021, 59, 125932. [Google Scholar] [CrossRef]
- Cortés-Gómez, A.A.; Romero, D.; Santos, J.; Rivera-Hernández, J.R.; Girondot, M. Inorganic elements in live vs dead nesting olive ridley marine turtles in the Mexican Pacific: Introducing a new statistical methodology in ecotoxicology. Sci. Total Environ. 2021, 761, 143249. [Google Scholar] [CrossRef]
- Bojórquez-Tapia, L.A.; Ponce-Díaz, G.; Pedroza-Páez, D.; Díaz de León, A.J.; Arreguín-Sánchez, F. Application of Exploratory Modeling in Support of Transdisciplinary Inquiry: Regulation of Fishing Bycatch of Loggerhead Sea Turtles in Gulf of Ulloa, Mexico. Front. Mar. Sci. 2021, 8, 643347. [Google Scholar] [CrossRef]
- Olimón-Andalón, V.; Valdés-Flores, J.; Ley-Quiñonez, C.P.; Zavala-Norzagaray, A.A.; Aguirre, A.A.; León-Sicairos, N.; Velázquez-Román, J.; Flores-Villaseñor, H.; Acosta-Smith, E.; Sosa-Cornejo, I.; et al. Essential and trace metals in a post-nesting olive ridley turtles (Lepidochelys olivacea) in Ceuta beach, Sinaloa, Mexico. Environ. Sci. Pollut. Res. 2021, 28, 29998–30006. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tenería, F.A.; Labrada-Martagón, V.; Herrera-Pavón, R.L.; Work, T.M.; González-Ballesteros, E.; Negrete-Philippe, A.C.; Maldonado-Saldaña, G. Fibropapillomatosis dynamics in green sea turtles Chelonia mydas over 15 years of monitoring in Akumal Bay, Quintana Roo, Mexico. Dis. Aquat. Org. 2022, 149, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Cornejo, I.; Martín-Del-Campo, R.; González-Flores, J.A.; González-Camacho, Z.B.; Cabrera-Cuellar, B.A.; Bielli, A.; Valdes-Flores, J.E.; Olimón-Andalón, V. Leucism: The prevalent congenital malformation in the Olive ridley sea turtle of northwestern Mexico. Dis. Aquat. Org. 2022, 152, 61–71. [Google Scholar] [CrossRef]
- Senko, J.F.; Peckham, S.H.; Aguilar-Ramirez, D.; Wang, J.H. Net illumination reduces fisheries bycatch, maintains catch value, and increases operational efficiency. Curr. Biol. 2022, 32, 911–918. [Google Scholar] [CrossRef]
- Muñoz-Tenería, F.A.; Calderón-Amador, J.; Negrete-Philippe, A.C.; Flores-Romo, L.A. A subpopulation of green turtle suprabasal epidermal cells are Langerin+ and migrate under in vitro stimulation of the chemokine CCL21. Vet. Res. Commun. 2022, 46, 939–945. [Google Scholar] [CrossRef]
- Buenrostro-Silva, A.; García-Grajales, J.; Sánchez-Nava, P.; Ruíz-Gómez, M. Paresis as a limiting factor in the reproductive efficiency of a nesting colony of Lepidochelys olivacea (Eschscholtz, 1829) in La Escobilla beach, Oaxaca, Mexico. J. Threat. Taxa 2022, 14, 22133–22138. [Google Scholar] [CrossRef]
- Flores-Aguirre, C.D.; Díaz-Hernández, V.; Moreno, D.A.; de la Cruz, F.R.M. Effect of moisture, temperature, and maternal influence on the hatching, phenotype, and performance of hawksbill turtles Eretmochelys imbricata. Endanger. Species Res. 2023, 50, 217–234. [Google Scholar] [CrossRef]
- Martínez-Estévez, L.; Angulo-Angulo, A.; Astorga, M.E.; Becerra, C.D.; Leyva, N.C.; Amador, F.C.; Amador, J.P.C. Exploring the demography and conservation needs of hawksbill sea turtles Eretmochelys imbricata in north-west Mexico. Oryx 2023, 57, 392–400. [Google Scholar] [CrossRef]
- Escobedo-Mondragón, M.; Pérez-Luzardo, O.; Henríquez-Hernández, L.A.; Rodríguez-Hernández, Á.; Zumbado, M.; Rosiles-Martínez, J.R. Trophic behavior of inorganic elements in nesting sea turtles (Chelonia mydas, Eretmochelys imbricata, and Caretta caretta) in Quintana Roo: Biomagnification and biodilution effect in blood and scute tissues. Mar. Pollut. Bull. 2023, 187, 114582. [Google Scholar] [CrossRef]
- Pavón, R.G.S. Tourist use limits with turtles in Cozumel. Environ. Chall. 2023, 10, 100669. [Google Scholar] [CrossRef]
- Salvarani, P.I.; Vieira, L.R.; Rendón-von Osten, J.; Morgado, F. Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico). Toxics 2023, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Villanueva, R.I.; Gumeta-Gómez, F.; Lara-Uc, M.; López-Vivas, J.M.; Hinojosa-Arango, G. The use of the territorial sea by Lepidochelys olivacea (Eschscholtz, 1829) in front of nesting beaches in Oaxaca, Mexico. Reg. Stud. Mar. Sci. 2023, 65, 103065. [Google Scholar] [CrossRef]
- Guevara-Meléndez, A.M.; Comas-Garcia, M.; Labrada-Martagón, V. Description and quantification of micronucleus and nuclear abnormalities in erythrocytes of the sentinel green turtle (Chelonia mydas) with fluorescence microscopy. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 887, 503596. [Google Scholar] [CrossRef]
- Rousso, S.J.; Perezrul, M.D.H.; Mancini, A.; Zavala-Norzagaray, A.A.; Senko, J.F. Citizen science enhances understanding of sea turtle distribution in the Gulf of California. PeerJ 2024, 12, e18203. [Google Scholar] [CrossRef]
- Fuentes, M.M.; Santos, A.; Abreu-Grobois, A.; Briseño-Dueñas, R.; Al-Khayat, J.; Hamza, S.; Saliba, S.; Anderson, D.; Rusenko, K.W.; Mitchell, N.; et al. Adaptation of sea turtles to climate warming: Will phenological responses be sufficient to counteract changes in reproductive output? Glob. Change Biol. 2024, 30, e16991. [Google Scholar] [CrossRef]
- Labrada-Martagón, V.; Islas, N.L.; Yáñez-Estrada, L.; Muñoz-Tenería, F.A.; Solé, M.; Zenteno-Savín, T. Evidence of oxidative stress responses of green turtles (Chelonia mydas) to differential habitat conditions in the Mexican Caribbean. Sci. Total Environ. 2024, 946, 174151. [Google Scholar] [CrossRef]
- Labastida-Estrada, E.; González-Cortés, L.; Karam-Martínez, S.G.; Montoya-Márquez, J.A.; Zúñiga-Marroquín, T.; Becerril-Morales, F.; Islas-Villanueva, V. Influence of incubation temperature, maternal effects, and paternity on quality of olive ridley hatchlings (Lepidochelys olivacea) from a mass-nesting beach in the Mexican Pacific. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2024, 341, 563–577. [Google Scholar] [CrossRef]
- Martínez-Pacheco, M.; Diaz-Barba, K.; Perez-Molina, R.; Marmolejo-Valencia, A.; Collazo-Saldana, P.; Escobar-Rodriguez, M.; Sanchez-Perez, M.; Meneses-Acosta, A.; Martínez-Rizo, A.B.; Sanchez-Pacheco, A.U.; et al. Gene expression dynamics during temperature-dependent sex determination in a sea turtle. Dev. Biol. 2024, 514, 99–108. [Google Scholar] [CrossRef]
- Guzmán-Hernández, V.; Rivas-Hernández, G.A.; García-Alvarado, P.A.; Huerta-Rodríguez, P.; Peralta-Jiménez, X.; Cuevas, E. Long-term (29-year) spatiotemporal patterns of sea turtle deaths in the southern Gulf of Mexico. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4201. [Google Scholar] [CrossRef]
- Lopez, J.; Griffiths, S.; Wallace, B.P.; Caceres, V.; Rodríguez, L.H.; Abrego, M.; Alfaro-Shigueto, J.; Andraka, S.; Brito, M.J.; Bustos, L.C.; et al. Vulnerability of the Critically Endangered leatherback turtle to fisheries bycatch in the eastern Pacific Ocean. I. A machine-learning species distribution model. Endanger. Species Res. 2024, 53, 271–293. [Google Scholar] [CrossRef]
- Ramos-Díaz, A.H.; Reséndiz, E.; Fernández-Sanz, H.; Collinot-Martínez, R.; Suárez-Domínguez, E.A. Proliferative lesions related to fibropapillomatosis in the Kemp’s Ridley Sea Turtle, Lepidochelys kempii Garman, 1880, at nesting sites in Veracruz State, Mexico. Herpetol. Notes 2024, 17, 87–92. [Google Scholar]
- Flores-Ramírez, R.; Mendoza-Rivera, S.P.; García-Grajales, J.; Buenrostro-Silva, A.; Sanjuan-Meza, E.U.; Berumen-Rodríguez, A.A.; Espinosa-Reyes, G. Persistent organic pollutants in the olive ridley turtle (Lepidochelys olivacea) during the nesting stage in the “La Escobilla” Sanctuary, Oaxaca, Mexico. Environ. Sci. Pollut. Res. 2024, 31, 10911–10919. [Google Scholar] [CrossRef]
- Flores-Gasca, L.A.; Fernández-Sanz, H.; Hernández-Burgos, F.I.; Reséndiz, E. Predation of Black Turtle Hatchlings (Chelonia mydas) on Clarion Island in Revillagigedo National Park, Mexico; [Depredación de Crías de Tortuga Negra (Chelonia mydas) en Isla Clarión del Parque Nacional Revillagigedo, México]. Rev. Latinoam. Herpetol. 2024, 7, e1098-35. [Google Scholar] [CrossRef]
- Rivas-Hernández, G.; Rodríguez-Fuentes, G.; Noreña-Barroso, E.; Cobos-Gasca, V. Biomarkers of Oxidative Stress and Persistent Organic Pollutants in Plasma and Eggs of Chelonia mydas Nesting in the Southern Gulf of Mexico. Estuaries Coasts 2024, 47, 2616–2630. [Google Scholar] [CrossRef]
- Aranda, D.A.; Sindou, P.; Rodriguez, J.V.; Saldaña, G.M.; Coronado, R.F.; González, W.D.; Díaz, M.E.; Escalante, V.C. A non-invasive method of microplastics pollution quantification in green sea turtle Chelonia mydas of the Mexican Caribbean. Mar. Pollut. Bull. 2024, 200, 116092. [Google Scholar] [CrossRef]
- Espinoza, J.; Alfaro-Núñez, A.; Cedillo-Peláez, C.; Fernández-Sanz, H.; Mancini, A.; Zavala-Norzagaray, A.A. Epidemiology of marine turtle fibropapillomatosis and tumour-associated chelonid alphaherpesvirus 5 (ChHV5; Scutavirus chelonidalpha5) in North-Western Mexico: A scoping review implementing the one health approach. Vet. Res. Commun. 2024, 48, 2943–2961. [Google Scholar] [CrossRef]
- Rodriguez-Salazar, C.L.; Comas-Garcia, M.; Muñoz-Teneria, F.A.; Zenteno-Savin, T.; Labrada-Martagon, V. Genotoxic damage in green turtles (Chelonia mydas) exhibits regional and annual fluctuations. Mar. Environ. Res. 2025, 204, 106877. [Google Scholar] [CrossRef]

| Threats | Associated Stressors |
|---|---|
| Climate change | SLR: Sea level rise |
| HUR: Hurricanes/storms | |
| OCC: Ocean circulation | |
| SST: Changes in sea surface temperature | |
| PRC: Changes in precipitation | |
| TMP: Changes in temperature | |
| Coastal development | BDT: Beach driving/beach traffic |
| BRN: Beach renourishment | |
| LTP: Light pollution | |
| BAR: Beach armoring | |
| TNG: Tourism (nesting ground) | |
| ROV: Removal of vegetation | |
| MBC: Mechanical beach cleaning | |
| Marine development | DRG: Dredging |
| OGM: Oil and gas mining | |
| WEA: Wind energy/aquaculture | |
| TIW: Tourism (in water) | |
| MVS: Marine traffic/vessel strike 1 | |
| PRT: Ports | |
| Fisheries | BYC: Bycatch |
| EGN: Entanglement/ghost nets | |
| VST: Vessel strike 2 | |
| Pollution | PMD: Plastics/marine debris |
| POP: Persistent organic pollutants | |
| AIR: Agricultural and industrial runoff | |
| Predation | ISS: Invasive species |
| NFA: Native/feral animals | |
| Direct take | LHR: Legal harvest |
| ILH: Illegal harvest | |
| Disease | IDS: Infectious disease |
| DFG: Disease fungus | |
| NDS: Non-infectious disease |
| Threats | Associated Stressors | Number of Studies | |
|---|---|---|---|
| Pollution | Plastics/marine debris | 3 | 40 |
| Persistent organic pollutants | 17 | ||
| Agricultural and industrial runoff | 20 | ||
| Fisheries | Bycatch | 19 | 24 |
| Entanglement/ghost nets | 2 | ||
| Vessel strike | 3 | ||
| Disease | Infectious disease | 17 | 22 |
| Disease fungus | 0 | ||
| Non-infectious disease | 5 | ||
| Climate change | Sea level rise | 2 | 14 |
| Hurricanes/storms | 2 | ||
| Ocean circulation | 0 | ||
| Changes in sea surface temperature | 4 | ||
| Changes in precipitation | 1 | ||
| Changes in temperature | 5 | ||
| Direct take | Legal harvest | 0 | 12 |
| Illegal harvest | 12 | ||
| Predation | Invasive species | 0 | 7 |
| Native/feral animals | 7 | ||
| Coastal development | Beach driving/beach traffic | 1 | 5 |
| Beach renourishment | 0 | ||
| Light pollution | 1 | ||
| Beach armoring | 0 | ||
| Tourism (nesting ground) | 2 | ||
| Removal of vegetation | 1 | ||
| Mechanical beach cleaning | 0 | ||
| Marine development | Dredging | 0 | 5 |
| Oil and gas mining | 3 | ||
| Wind energy/aquaculture | 0 | ||
| Tourism (in water) | 1 | ||
| Marine traffic/vessel strike | 1 | ||
| Ports | 0 | ||
| Single Species | Number of Studies | % |
|---|---|---|
| Chelonia mydas | 22 | 25.00 |
| Lepidochelys olivacea | 20 | 22.73 |
| Caretta caretta | 9 | 10.23 |
| Eretmochelys imbricata | 7 | 7.95 |
| Lepidochelys kempii | 5 | 5.68 |
| Dermochelys coriacea | 3 | 3.41 |
| Multiple Species | Number of Studies | % |
| C. caretta, C. mydas, L. olivacea | 5 | 5.68 |
| C. caretta, C. mydas, E. imbricata, L. olivacea | 3 | 3.41 |
| C. mydas, E. imbricata | 3 | 3.41 |
| C. caretta, C. mydas | 2 | 2.27 |
| C. caretta, C. mydas, E. imbricata | 2 | 2.27 |
| C. caretta, C. mydas, E. imbricata, L. kempii | 2 | 2.27 |
| C. mydas, E. imbricata, L. olivacea | 2 | 2.27 |
| C. mydas, L. olivacea | 2 | 2.27 |
| E. imbricata, L. olivacea | 1 | 1.14 |
| Total | 88 | 100 |
| Threats |
Associated
Stressors | C. caretta | C. mydas | E. imbricata | D. coriacea | L. kempii | L. olivacea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PACE | PR | PACE | PR | PACE | PR | PACE | PR | PACE | PR | PACE | PR | ||
| Climate change | SLR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| HUR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| OCC | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| SST | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| PRC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| TMP | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Coastal development | BDT | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| BRN | 1 | 1 | 1 | 1 | 1 | ||||||||
| LTP | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| BAR | 1 | 1 | 1 | 1 | 1 | ||||||||
| TNG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| ROV | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| MBC | 1 | 1 | |||||||||||
| Marine development | DRG | 1 | |||||||||||
| OGM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| WEA | |||||||||||||
| TIW | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| MVS | 1 | 1 | 1 | 1 | |||||||||
| PRT | 1 | 1 | 1 | 1 | |||||||||
| Fisheries | BYC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| EGN | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| VST | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Pollution | PMD | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| POP | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| AIR | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Predation | ISS | 1 | 1 | 1 | |||||||||
| NFA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Direct take | LHR | ||||||||||||
| ILH | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Disease | IDS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| DFG | 1 | ||||||||||||
| NDS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total | 21 | 15 | 22 | 18 | 27 | 14 | 17 | 2 | 30 | 8 | 23 | 13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Villanueva, R.I.; Gumeta-Gómez, F.; Hinojosa-Arango, G. Systematic Analysis of Threats to Sea Turtles in Mexico: Trends, Knowledge Gaps, and Implications for Conservation. Oceans 2025, 6, 71. https://doi.org/10.3390/oceans6040071
Ramírez-Villanueva RI, Gumeta-Gómez F, Hinojosa-Arango G. Systematic Analysis of Threats to Sea Turtles in Mexico: Trends, Knowledge Gaps, and Implications for Conservation. Oceans. 2025; 6(4):71. https://doi.org/10.3390/oceans6040071
Chicago/Turabian StyleRamírez-Villanueva, Ruth I., Fernando Gumeta-Gómez, and Gustavo Hinojosa-Arango. 2025. "Systematic Analysis of Threats to Sea Turtles in Mexico: Trends, Knowledge Gaps, and Implications for Conservation" Oceans 6, no. 4: 71. https://doi.org/10.3390/oceans6040071
APA StyleRamírez-Villanueva, R. I., Gumeta-Gómez, F., & Hinojosa-Arango, G. (2025). Systematic Analysis of Threats to Sea Turtles in Mexico: Trends, Knowledge Gaps, and Implications for Conservation. Oceans, 6(4), 71. https://doi.org/10.3390/oceans6040071






