Combining Adequate BRUV Deployment Times with Individual Photo-Identification Improves Monitoring of Shark Populations in the Caribbean
Abstract
1. Introduction
2. Materials and Methods
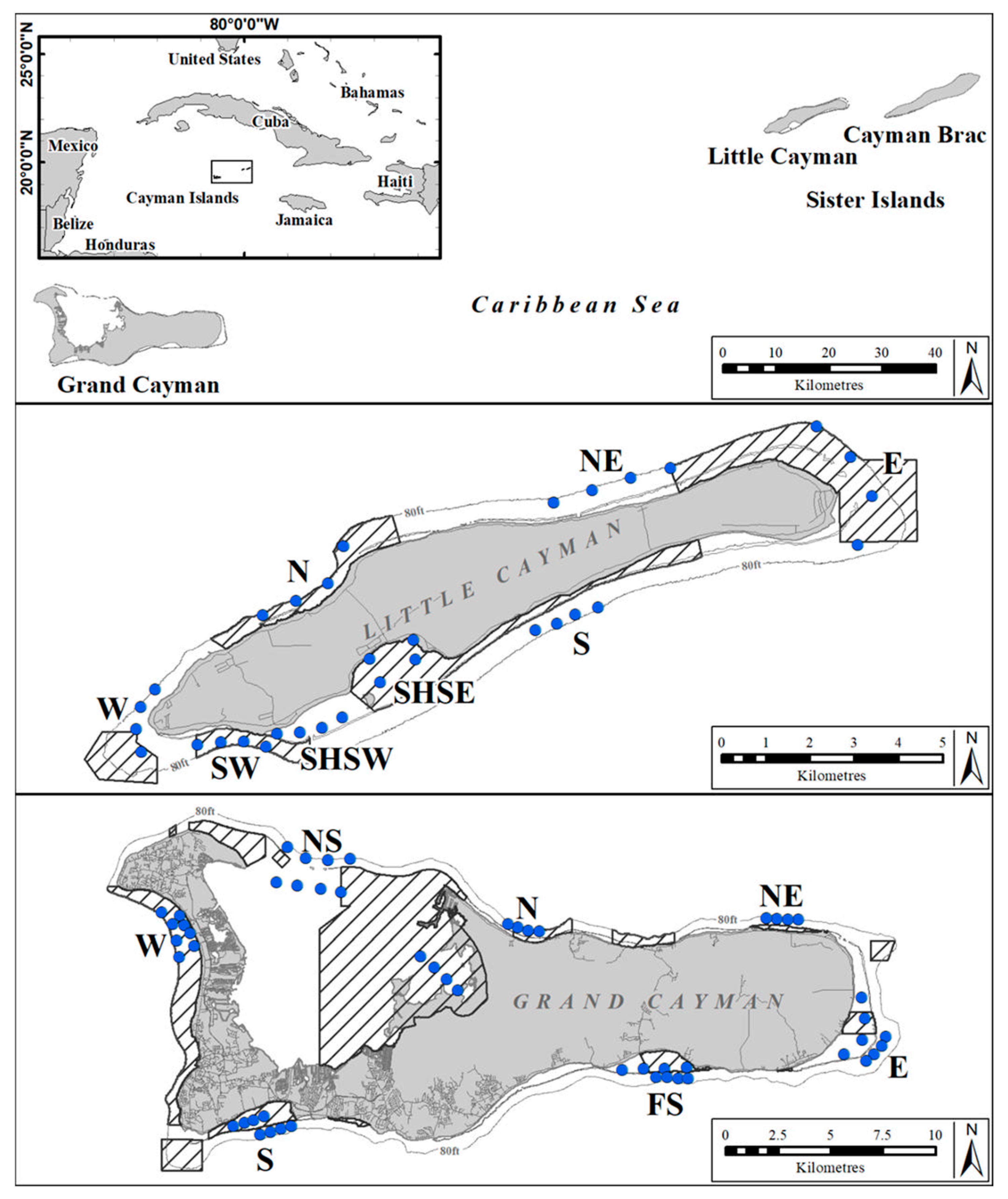
2.1. Study Area
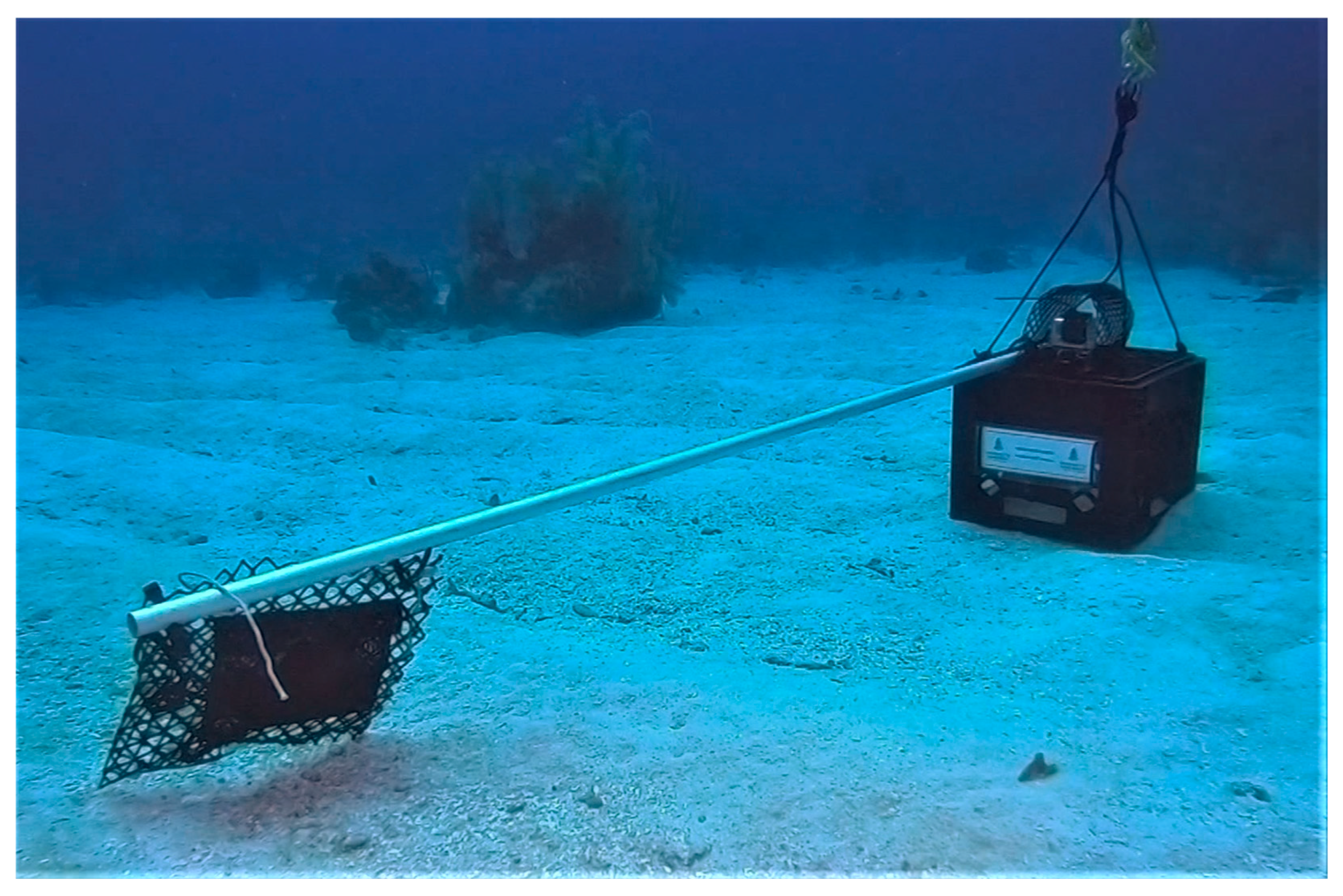
2.2. Data Collection
2.3. Video Analysis
2.3.1. Recording Times
2.3.2. Abundance Metrics: MaxN and MaxIND
2.3.3. Biological Estimates
2.3.4. Behaviour
2.4. Statistical Analysis
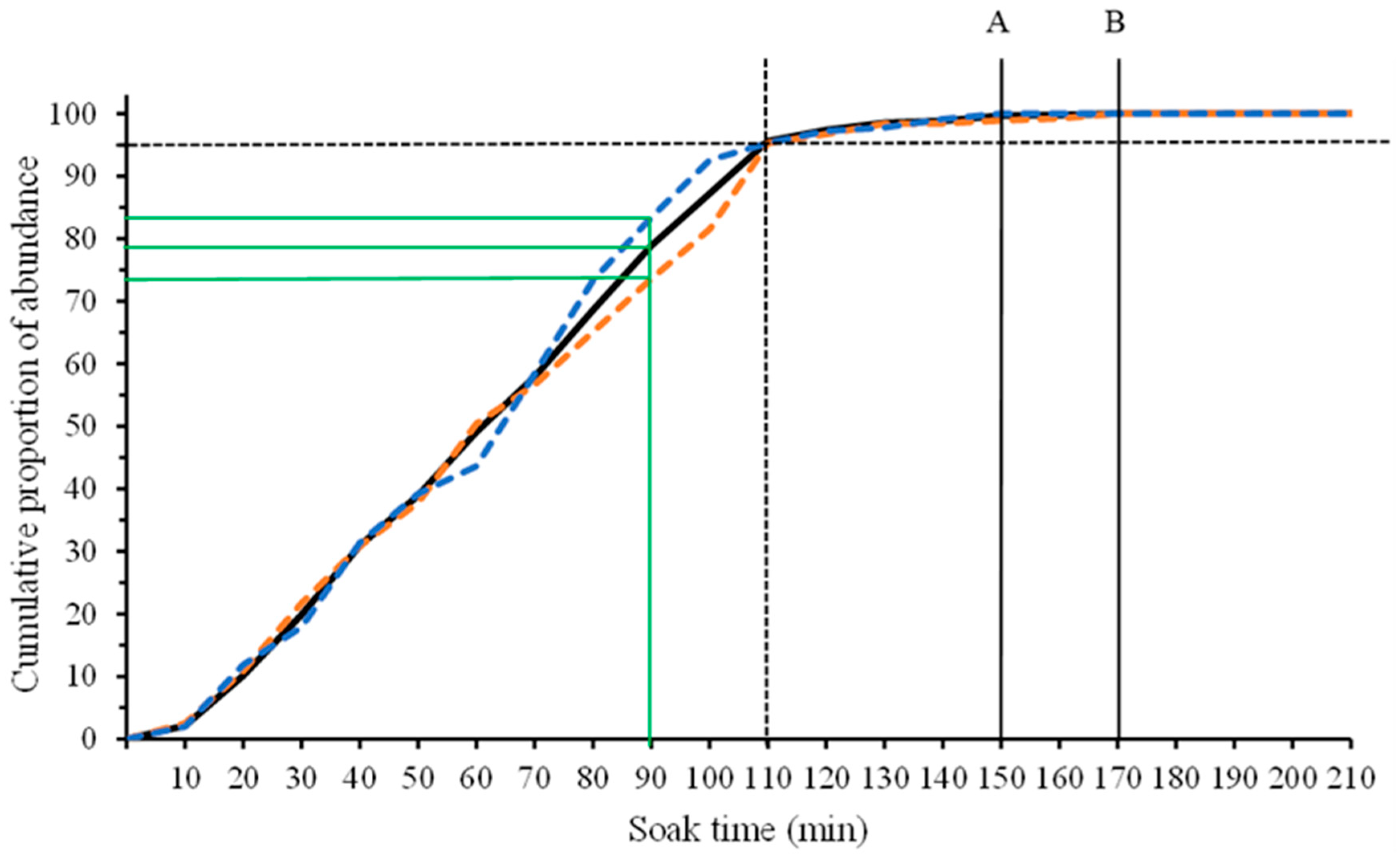
2.4.1. Adequate Recording Time
2.4.2. MaxN and MaxIND
2.4.3. Shark Behaviour
3. Results
3.1. Assessment for Adequate Recording Time
3.2. Comparison of MaxN and MaxIND
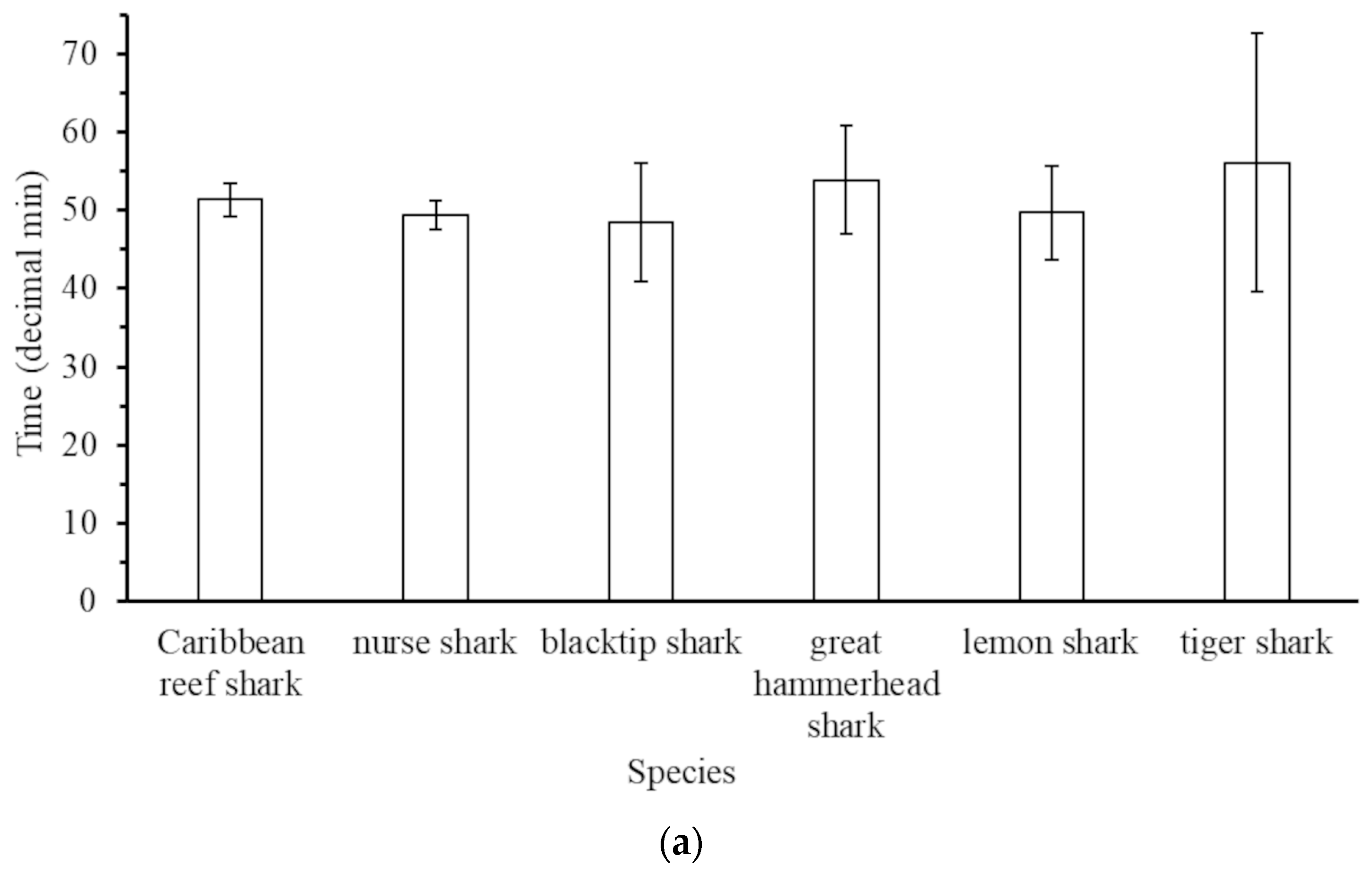
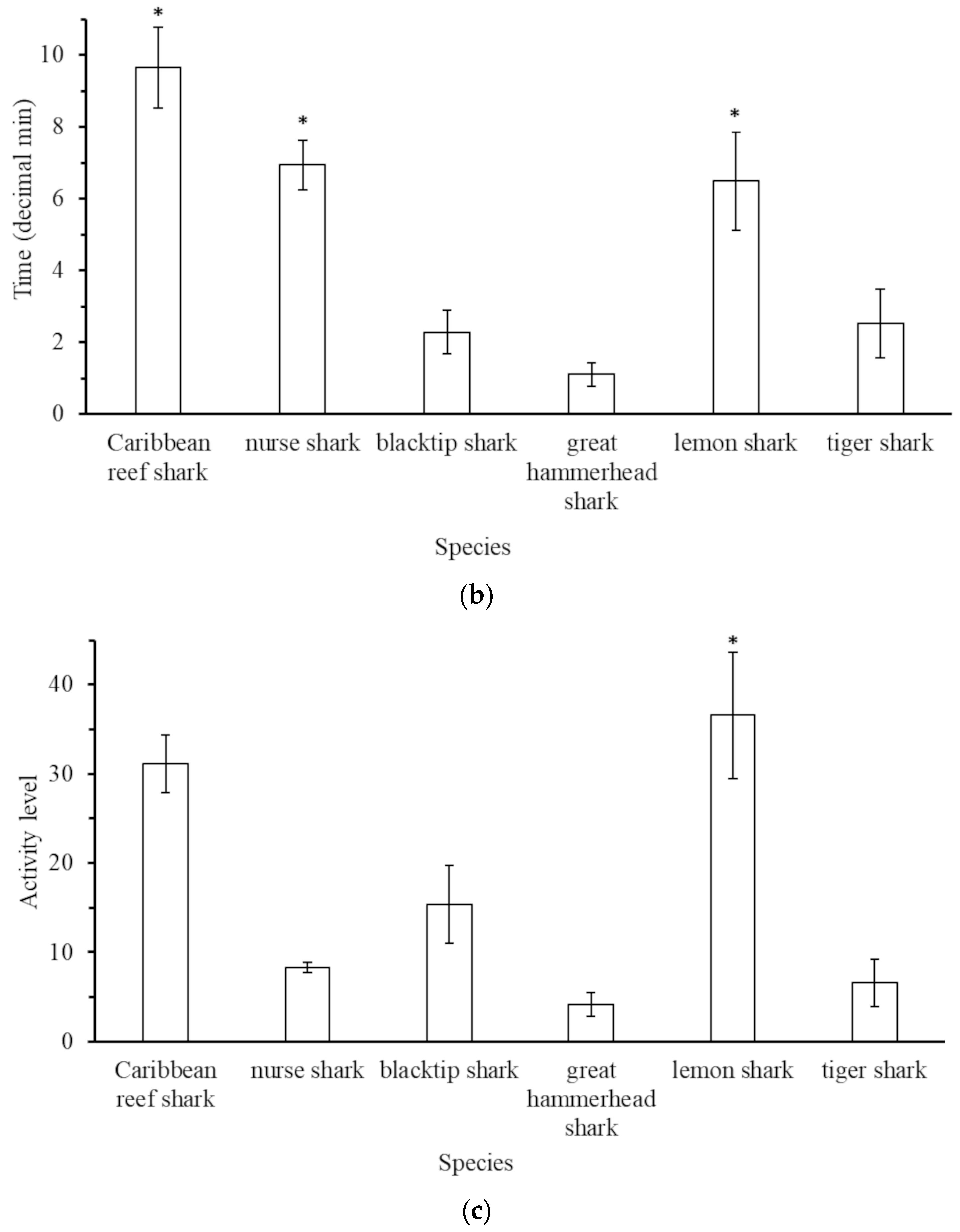
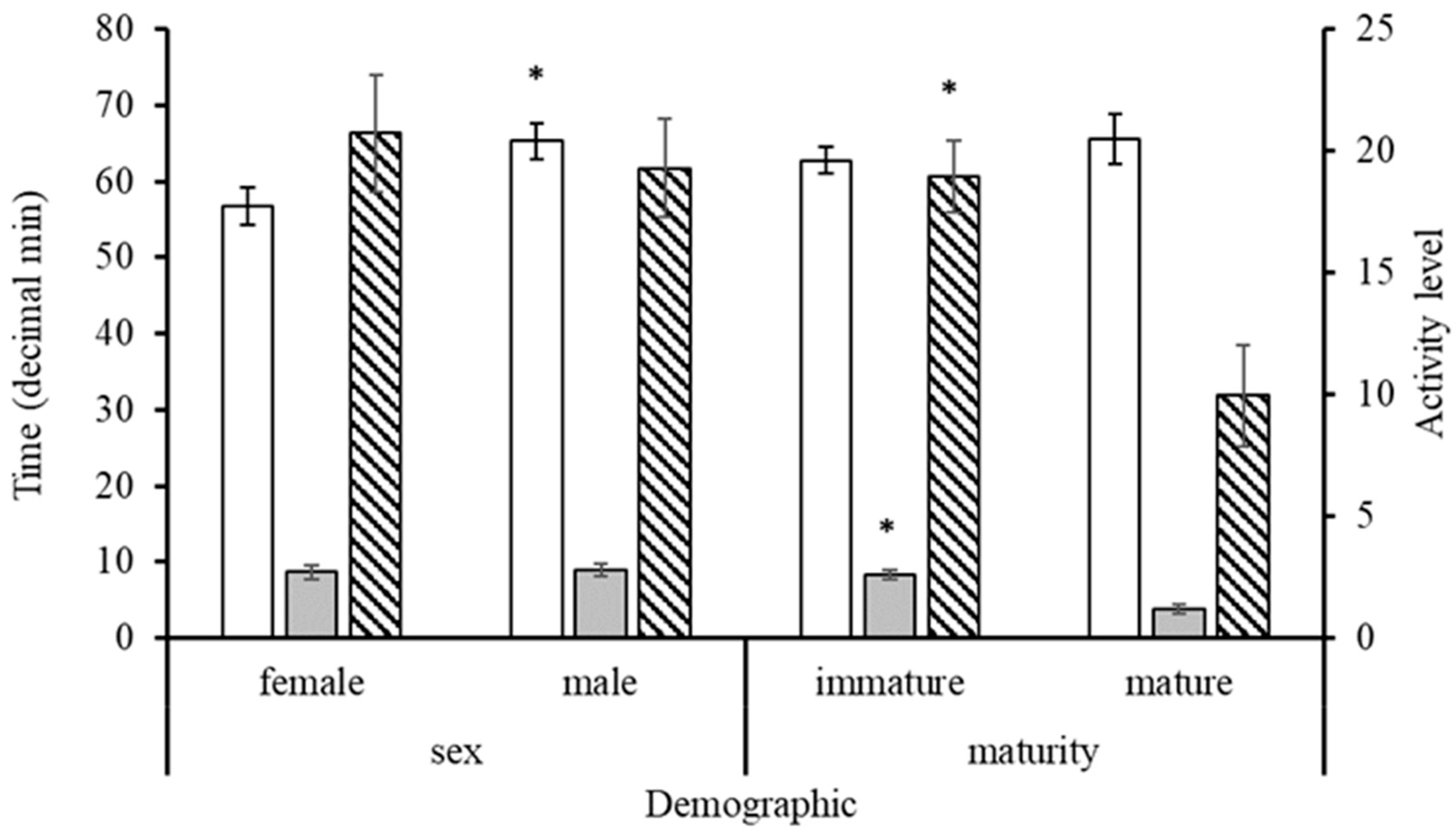
3.3. Behaviour of Sharks on BRUVs
4. Discussion
4.1. Adequate BRUV Recording Time
4.2. Comparison of Abundance Metrics
4.3. Observed Behaviour of Sharks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E.; et al. Global status and conservation potential of reef sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Ward-Paige, C.A.; Mora, C.; Lotze, H.K.; Pattengill-Semmens, C.; McClenachan, L.; Arias-Castro, E.; Myers, R.A. Large-Scale Absence of Sharks on Reefs in the Greater- Caribbean: A Footprint of Human Pressures. PLoS ONE 2010, 5, e11968. [Google Scholar] [CrossRef] [PubMed]
- Clementi, G.; Babcock, E.; Valentin-Albanese, J.; Bond, M.; Flowers, K.; Heithaus, M.R.; Whitman, E.; Bergmann, M.V.Z.; Guttridge, T.; O’sHea, O.; et al. Anthropogenic pressures on reef-associated sharks in jurisdictions with and without directed shark fishing. Mar. Ecol. Prog. Ser. 2021, 661, 175–186. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Amon, D.J.; Bervoets, T.; Shipley, O.N.; Hammerschlag, N.; Sims, D.W. The Caribbean needs big marine protected areas. Science 2020, 367, 749–750. [Google Scholar] [CrossRef] [PubMed]
- O’Bryhim, J.R.; Parsons, E.C.M.; Gilmore, M.P.; Lance, S.L. Evaluating support for shark conservation among artisanal fishing communities in Costa Rica. Mar. Policy 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Ormond, R.; Gore, M.; Bladon, A.; Dubock, O.; Kohler, J.; Millar, C. Protecting Cayman Island sharks: Monitoring, movement and motive. In Proceedings of the 69th Gulf Caribb Fish Inst, Grand Cayman, Cayman Islands, 7–11 November 2016; pp. 14–27. [Google Scholar]
- Talwar, B.S.; Anderson, B.; Avalos-Castillo, C.G.; Blanco-Parra, M.d.P.; Briones, A.; Cardeñosa, D.; Carlson, J.K.; Charvet, P.; Cotton, C.F.; Crysler, Z.; et al. Extinction risk, reconstructed catches and management of chondrichthyan fishes in the Western Central Atlantic Ocean. Fish Fish. 2022, 23, 1150–1179. [Google Scholar] [CrossRef]
- Finucci, B.; Cheok, J.; Ebert, D.A.; Herman, K.; Kyne, P.M.; Dulvy, N.K. Ghosts of the deep—Biodiversity, fisheries, and extinction risk of ghost sharks. Fish Fish. 2021, 22, 391–412. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Krueck, N.C.; Udyawer, V.; Heupel, M.R.; Chapman, D.; Pratt, H.L.; Garla, R.; Simpfendorfer, C.A. Individual and Population Benefits of Marine Reserves for Reef Sharks. Curr. Biol. 2020, 30, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.E.; Valentin-Albanese, J.; Babcock, E.A.; Hussey, N.E.; Heithaus, M.R.; Chapman, D.D. The trophic ecology of Caribbean reef sharks (Carcharhinus perezi) relative to other large teleost predators on an isolated coral atoll. Mar. Biol. 2018, 165, 67. [Google Scholar] [CrossRef]
- Roff, G.; Doropoulos, C.; Rogers, A.; Bozec, Y.-M.; Krueck, N.C.; Aurellado, E.; Priest, M.; Birrell, C.; Mumby, P.J. The Ecological Role of Sharks on Coral Reefs. Trends Ecol. Evol. 2016, 31, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Harborne, A.R. Non-consumptive effects in fish predator—Prey interactions on coral reefs. Coral Reefs 2020, 39, 867–884. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Barley, S.C.; Irschick, D.J.; Meeuwig, J.J.; Nelson, E.R.; Meekan, M.G. Predator declines and morphological changes in prey: Evidence from coral reefs depleted of sharks. Mar. Ecol. Prog. Ser. 2018, 586, 127–139. [Google Scholar] [CrossRef]
- Rasher, D.B.; Hoey, A.S.; Hay, M.E. Cascading predator effects in a Fijian coral reef ecosystem. Sci. Rep. 2017, 7, 15684. [Google Scholar] [CrossRef]
- Bond, M.E.; Babcock, E.A.; Pikitch, E.K.; Abercrombie, D.L.; Lamb, N.F.; Chapman, D.D. Reef Sharks Exhibit Site-Fidelity and Higher Relative Abundance in Marine Reserves on the Mesoamerican Barrier Reef. PLoS ONE 2012, 7, e32983. [Google Scholar] [CrossRef]
- Jaiteh, V.F.; Lindfield, S.J.; Mangubhai, S.; Warren, C.; Fitzpatrick, B.; Loneragan, N.R. Higher Abundance of Marine Predators and Changes in Fishers’ Behavior Following Spatial Protection within the World’s Biggest Shark Fishery. Front. Mar. Sci. 2016, 3, 43. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Worm, B. Global evaluation of shark sanctuaries. Glob. Environ. Chang. 2017, 47, 174–189. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- MacKeracher, T.; Diedrich, A.; Simpfendorfer, C.A. Sharks, rays and marine protected areas: A critical evaluation of current perspectives. Fish Fish. 2019, 20, 255–267. [Google Scholar] [CrossRef]
- Brooks, E.J.; Sloman, K.A.; Sims, D.W.; Danylchuk, A.J. Validating the use of baited remote underwater video surveys for assessing the diversity, distribution and abundance of sharks in the Bahamas. Endanger. Species Res. 2011, 13, 231–243. [Google Scholar] [CrossRef]
- Stobart, B.; Díaz, D.; Álvarez, F.; Alonso, C.; Mallol, S.; Goñi, R. Performance of baited underwater video: Does it underestimate abundance at high population densities? PLoS ONE 2015, 10, e0127559. [Google Scholar] [CrossRef]
- Meekan, M.G.; Cappo, M. Non-Destructive Techniques for Rapid Assessment of Shark Abundance in Northern Australia; Australian Institute of Marine Science: Townsville, Australia, 2004; pp. 1–29. [Google Scholar]
- Shipley, O.N.; Brownscombe, J.W.; Danylchuk, A.J.; Cooke, S.J.; O’Shea, O.R.; Brooks, E.J. Fine-scale movement and activity patterns of Caribbean reef sharks (Carcharhinus perezi) in the Bahamas. Environ. Biol. Fishes 2018, 101, 1097–1104. [Google Scholar] [CrossRef]
- Kohler, J.; Gore, M.; Ormond, R.; Austin, T. First estimates of population size and home range of Caribbean reef and nurse sharks using photo-identification and BRUVS. Front. Mar. Sci. 2023, 10, 1230896. [Google Scholar] [CrossRef]
- Gore, M.; Kohler, J.; Ormond, R.; Gallagher, A.; Fernandes, T.; Austin, T.; Pattengill-Semmens, C. Renewed occurrence of schooling scalloped hammerhead (Sphyrna lewini) and of great hammerhead (S. mokarran) sharks in the Cayman Islands. Front. Mar. Sci. 2024, 11, 1347285. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Meeuwig, J.J. Drifting baited stereo-videography: A novel sampling tool for surveying pelagic wildlife in offshore marine reserves. Ecosphere 2015, 6, 137. [Google Scholar] [CrossRef]
- Letessier, T.B.; Mouillot, D.; Bouchet, P.J.; Vigliola, L.; Fernandes, M.C.; Thompson, C.; Boussarie, G.; Turner, J.; Juhel, J.-B.; Maire, E. Remote reefs and seamounts are the last refuges for marine predators across the Indo-Pacific. PLoS Biol. 2019, 17, e3000366. [Google Scholar] [CrossRef]
- Roskar, G.; Mccallister, M.P.; Ajemian, M.J. Performance of Two Survey Gears Targeting Elasmobranchs in a Shallow, Subtropical Estuary. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2020, 12, 50–63. [Google Scholar] [CrossRef]
- Sebastian, P.; Stean, S.J.; Erawan, A.I.R.; Gotama, R.; Swarya, I.N.; Sparks, L.D.; Prasetijo, R.; Prasetyo, A.P. Quantifying the influence of environmental factors on elasmobranch distribution and abundance in a high-use marine protected area. Mar. Environ. Res. 2025, 210, 107317. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Marrero, D.; de la Cruz-Modino, R.; Smith, A.N.H.; Salinas-de-León, P.; Pawley, M.D.M.; Anderson, M.J. Understanding human attitudes towards sharks to promote sustainable coexistence. Mar. Policy 2018, 91, 122–128. [Google Scholar] [CrossRef]
- Goetze, J.S.; Langlois, T.J.; McCarter, J.; Simpfendorfer, C.A.; Hughes, A.; Leve, J.T.; Jupiter, S.D. Drivers of reef shark abundance and biomass in the Solomon Islands. PLoS ONE 2018, 13, e0200960. [Google Scholar] [CrossRef]
- Tickler, D.M.; Letessier, T.B.; Koldewey, H.J.; Meeuwig, J.J. Drivers of abundance and spatial distribution of reef-associated sharks in an isolated atoll reef system. PLoS ONE 2017, 12, e0177374. [Google Scholar] [CrossRef] [PubMed]
- Cappo, M.; Speare, P.; De’Ath, G. Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 2004, 302, 123–152. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Peters, J.R.; McCloskey, R.M.; Hinder, S.L. Optimising stereo baited underwater video for sampling fish and invertebrates in temperate coastal habitats. Estuar. Coast. Shelf Sci. 2014, 150, 281–287. [Google Scholar] [CrossRef]
- Willis, T.J.; Millar, R.B.; Babcock, R.C. Detection of spatial variability in relative density of fishes: Comparison of visual census, angling, and baited underwater video. Mar. Ecol. Prog. Ser. 2000, 198, 249–260. [Google Scholar] [CrossRef]
- Bruns, S.; Henderson, A.C. A baited remote underwater video system (BRUVS) assessment of elasmobranch diversity and abundance on the eastern Caicos Bank (Turks and Caicos Islands); an environment in transition. Environ. Biol. Fishes 2020, 103, 1001–1012. [Google Scholar] [CrossRef]
- Whitmarsh, S.K.; Fairweather, P.G.; Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish. Biol. Fish. 2017, 27, 53–73. [Google Scholar] [CrossRef]
- Campbell, M.D.; Pollack, A.G.; Gledhill, C.T.; Switzer, T.S.; Devries, D.A. Comparison of relative abundance indices calculated from two methods of generating video count data. Fish. Res. 2015, 170, 125–133. [Google Scholar] [CrossRef]
- Kilfoil, J.P.; Wirsing, A.J.; Campbell, M.D.; Kiszka, J.J.; Gastrich, K.R.; Heithaus, M.R.; Zhang, Y.; Bond, M.E. Baited Remote Underwater Video surveys undercount sharks at high densities: Insights from full-spherical camera technologies. Mar. Ecol. Prog. Ser. 2017, 585, 113–121. [Google Scholar] [CrossRef]
- Sherman, C.S.; Chin, A.; Heupel, M.R.; Simpfendorfer, C.A. Are we underestimating elasmobranch abundances on baited remote underwater video systems (BRUVS) using traditional metrics? J. Exp. Mar. Biol. Ecol. 2018, 503, 80–85. [Google Scholar] [CrossRef]
- Gore, M.; Ormond, R.; Clarke, C.; Kohler, J.; Millar, C.; Brooks, E. Application of Photo-Identification and Lengthened Deployment Periods to Baited Remote Underwater Video Stations (BRUVS) Abundance Estimates of Coral Reef Sharks. Oceans 2020, 1, 274–299. [Google Scholar] [CrossRef]
- Castro, A.L.F.; Rosa, R.S. Use of natural marks on population estimates of the nurse shark, Ginglymostoma cirratum, at Atol das Rocas Biological Reserve, Brazil. Environ. Biol. Fishes 2005, 72, 213–221. [Google Scholar] [CrossRef]
- Domeier, M.L.; Nasby-Lucas, N. Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar. Biol. 2007, 150, 977–984. [Google Scholar] [CrossRef]
- Dudgeon, C.L.; Noad, M.J.; Lanyon, J.M. Abundance and demography of a seasonal aggregation of zebra sharks Stegostoma fasciatum. Mar. Ecol. Prog. Ser. 2008, 368, 269–281. [Google Scholar] [CrossRef]
- Lester, E.; Meekan, M.; Barnes, P.; Raudino, H.; Rob, D.; Waples, K.; Speed, C. Multi-year patterns in scarring, survival and residency of whale sharks in Ningaloo Marine Park, Western Australia. Mar. Ecol. Prog. Ser. 2020, 634, 115–126. [Google Scholar] [CrossRef]
- Harasti, D.; Lee, K.A.; Laird, R.; Bradford, R.; Bruce, B. Use of stereo baited remote underwater video systems to estimate the presence and size of white sharks (Carcharodon carcharias). Mar. Freshw. Res. 2016, 68, 1391–1396. [Google Scholar] [CrossRef]
- Stoffers, T.; de Graaf, M.; Winter, H.; Nagelkerke, L. Distribution and ontogenetic habitat shifts of reef-associated shark species in the northeastern Caribbean. Mar. Ecol. Prog. Ser. 2021, 665, 145–158. [Google Scholar] [CrossRef]
- Espinoza, M.; Cappo, M.; Heupel, M.R.; Tobin, A.J.; Simpfendorfer, C.A. Quantifying shark distribution patterns and species-habitat associations: Implications of Marine Park zoning. PLoS ONE 2014, 9, e106885. [Google Scholar] [CrossRef]
- Gladstone, W.; Lindfield, S.; Coleman, M.; Kelaher, B. Optimisation of baited remote underwater video sampling designs for estuarine fish assemblages. J. Exp. Mar. Biol. Ecol. 2012, 429, 28–35. [Google Scholar] [CrossRef]
- Harasti, D.; Malcolm, H.; Gallen, C.; Coleman, M.A.; Jordan, A.; Knott, N.A. Appropriate set times to represent patterns of rocky reef fishes using baited video. J. Exp. Mar. Biol. Ecol. 2015, 463, 173–180. [Google Scholar] [CrossRef]
- Thompson, G.G.; Withers, P.C. Effect of species richness and relative abundance on the shape of the species accumulation curve. Austral Ecol. 2003, 28, 355–360. [Google Scholar] [CrossRef]
- De Vos, L.; Götz, A.; Winker, H.; Attwood, C.G. Optimal BRUVs (baited remote underwater video system) survey design for reef fish monitoring in the Stilbaai Marine Protected Area. Afr. J. Mar. Sci. 2014, 36, 1–10. [Google Scholar] [CrossRef]
- White, J.; Simpfendorfer, C.A.; Tobin, A.J.; Heupel, M.R. Application of baited remote underwater video surveys to quantify spatial distribution of elasmobranchs at an ecosystem scale. J. Exp. Mar. Biol. Ecol. 2013, 448, 281–288. [Google Scholar] [CrossRef]
- Baremore, I.E.; Polanco-Vásquez, F.; Hacohen-Domené, A.; Castellanos, D.W.; Graham, R.T. Short-term movement of a night shark (Carcharhinus signatus) in the western Caribbean with notes on the species’ distribution and threats in the region. Environ. Biol. Fishes 2019, 102, 519–526. [Google Scholar] [CrossRef]
- Bond, M.E.; Valentin-Albanese, J.; Babcock, E.A.; Heithaus, M.R.; Grubbs, R.D.; Cerrato, R.; Peterson, B.J.; Pikitch, E.K.; Chapman, D.D. Top predators induce habitat shifts in prey within marine protected areas. Oecologia 2019, 190, 375–385. [Google Scholar] [CrossRef]
- Flowers, K.I.; Babcock, E.A.; Papastamatiou, Y.P.; Bond, M.E.; Lamb, N.; Miranda, A.; Nuñez, R.; Valentin-Albanese, J.; Clementi, G.M.; Kelley, M.C.; et al. Varying reef shark abundance trends inside a marine reserve: Evidence of a Caribbean reef shark decline. Mar. Ecol. Prog. Ser. 2022, 683, 97–107. [Google Scholar] [CrossRef]
- Leurs, G.; De Graaf, M.; Hassell-Knijff, D.; Izioka, L.A.K.; Van Looijengoed, W.; Schlochtern, M.P.M.Z.; Bervoets, T.; Nagelkerke, L.A.J.; Winter, H.V. An integrated baseline assessment of reef-associated sharks around Saba (Dutch Caribbean), combining three methods: Stereo-BRUVs, telemetry and citizen science. R. Soc. Open Sci. 2025, 12, 241754. [Google Scholar] [CrossRef] [PubMed]
- Maljković, A.; Côté, I.M. Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark. Biol. Conserv. 2011, 144, 859–865. [Google Scholar] [CrossRef]
- Phenix, L.M.; Tricarico, D.; Quintero, E.; Bond, M.E.; Brandl, S.J.; Gallagher, A.J. Evaluating the effects of large marine predators on mobile prey behavior across subtropical reef ecosystems. Ecol. Evol. 2019, 9, 13740–13751. [Google Scholar] [CrossRef]
- Priede, I.G.; Merrett, N.R. Estimation of abundance of abyssal demersal fishes; a comparison of data from trawls and baited cameras. J. Fish Biol. 1996, 49, 207–216. [Google Scholar] [CrossRef]
- Ghazilou, A.; Shokri, M.R.; Gladstone, W. Animal v. plant-based bait: Does the bait type affect census of fish assemblages and trophic groups by baited remote underwater video (BRUV) systems? J. Fish Biol. 2016, 88, 1731–1745. [Google Scholar] [CrossRef]
- Wraith, J.; Lynch, T.; Minchinton, T.E.; Broad, A.; Davis, A.R. Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef]
- Bassett, D.K.; Montgomery, J.C. Investigating nocturnal fish populations in situ using baited underwater video: With special reference to their olfactory capabilities. J. Exp. Mar. Biol. Ecol. 2011, 409, 194–199. [Google Scholar] [CrossRef]
- Coghlan, A.R.; Mclean, D.L.; Harvey, E.S.; Langlois, T.J. Does fish behaviour bias abundance and length information collected by baited underwater video? J. Exp. Mar. Biol. Ecol. 2017, 497, 143–151. [Google Scholar] [CrossRef]
- Devine, B.M.; Wheeland, L.J.; Fisher, J.A.D. First estimates of Greenland shark (Somniosus microcephalus) local abundances in Arctic waters. Sci. Rep. 2018, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Cappo, M.; Stowar, M.; Syms, C.; Johansson, C.; Cooper, T. Fish-habitat associations in the region offshore from James Price Point—A rapid assessment using Baited Remote Underwater Video Stations (BRUVS). J. R. Soc. West. Aust. 2011, 94, 303–321. Available online: https://researchonline.jcu.edu.au/21403/ (accessed on 27 July 2025).
- Schobernd, Z.H.; Bacheler, N.M.; Conn, P.B. Examining the utility of alternative video monitoring metrics for indexing reef fish abundance. Can. J. Fish. Aquat. Sci. 2014, 71, 464–471. [Google Scholar] [CrossRef]
- Smale, D.A.; Barnes, D.K.A.; Fraser, K.P.P.; Mann, P.J.; Brown, M.P. Scavenging in Antarctica: Intense variation between sites and seasons in shallow benthic necrophagy. J. Exp. Mar. Biol. Ecol. 2007, 349, 405–417. [Google Scholar] [CrossRef]
- Kohler, J.; Gore, M.; Ormond, R.; Austin, T.; Olynik, J. The Sharklogger Network–monitoring Cayman Islands shark populations through an innovative citizen science program. PLoS ONE 2025, 20, e0319637. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Shipley, O.N.; De Silva, C.; Kohler, J.K.; Fernandes, T.F.; Austin, T.; Ormond, R.F.; Gore, M.A. First records of the blurred lantern shark Etmopterus bigelowi from the Cayman Islands, Western Atlantic. Front. Mar. Sci. 2023, 10, 1165207. [Google Scholar] [CrossRef]
- Dixon, O.F.L.; Aldridge, S.E.; Kohler, J.K.; Veeder, A.; Chin, P.; Fernandes, T.F.; Austin, T.; Ormond, R.F.; Gore, M.A.; Vaz, D.F.B.; et al. First records of the roughskin dogfish Centroscymnus owstonii in the greater Antilles, central Caribbean Sea, Western Atlantic Ocean. J. Fish Biol. 2024, 106, 980–986. [Google Scholar] [CrossRef]
- Cayman Islands Government. The National Conservation Law, 2013 (Law 24 of 2013). Supplement No. 1 Published with Extraordinary Gazette No. 9. 5 February 2014. Available online: https://doe.ky/wp-content/uploads/2015/01/NationalConservationLaw-Es052014_web.pdf (accessed on 18 July 2025).
- Coelho, V.R.; Manfrino, C. Coral community decline at a remote Caribbean island: Marine no-take reserves are not enough. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, 666–685. [Google Scholar] [CrossRef]
- Ebanks-Petrie, G. Assessment of Coastal Management on Grand Cayman, B.W.I. Master’s Thesis, University of Stirling, Stirling, UK, 1993. [Google Scholar]
- Novelo-Casanova, D.A.; Suárez, G. Natural and man-made hazards in the Cayman Islands. Nat. Hazards 2010, 55, 441–466. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Realised ecosystem functions transcend biogeographic locations. Coral Reefs 2020, 39, 203–214. [Google Scholar] [CrossRef]
- Shi, W.; Liu, G. Potential mechanisms underpinning the impacts of ocean acidification on marine animals. In Ocean Acidification and Marine Wildlife; Academic Press: Cambridge, MA, USA, 2021; pp. 155–192. [Google Scholar] [CrossRef]
- Bernard, A.M.; Horn, R.L.; Chapman, D.D.; Feldheim, K.A.; Garla, R.C.; Brooks, J.; Gore, M.A.; Shivji, M.S. Genetic connectivity of a coral reef ecosystem predator: The population genetic structure and evolutionary history of the Caribbean reef shark (Carcharhinus perezi). J. Biogeogr. 2017, 44, 2488–2500. [Google Scholar] [CrossRef]
- Espinoza, M.; Heupel, M.R.; Tobin, A.J.; Simpfendorfer, C.A. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar. Biol. 2014, 162, 343–358. [Google Scholar] [CrossRef]
- Roberts, H.H. Reefs and Lagoons of Grand Cayman. In The Cayman Islands: Natural History and Biogeography; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 75–104. [Google Scholar]
- Burgess, G.H.; Smith, S.H.; Lane, E.D. Fishes of the Cayman Islands. In The Cayman Islands: Natural History and Biogeography; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 199–228. [Google Scholar]
- Logan, A. Reefs and Lagoons of Cayman Brac and Little Cayman. In The Cayman Islands: Natural History and Biogeography; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 105–124. [Google Scholar]
- Van Horn, C.J.; Candelmo, A.C.; Heppell, S.A.; McCoy, C.R.M.; Pattengill-Semmens, C.V.; Waterhouse, L.; Cherubin, L.M.; Taylor, J.C.; Michaels, W.; Locascio, J.; et al. Hydrophone placement yields high variability in detection of Epinephelus striatus calls at a spawning site. Ecol. Appl. 2025, 35, e70081. [Google Scholar] [CrossRef]
- Wilson, K.C.; Semmens, B.X.; Gittings, S.R.; McCoy, C.; Pattengill-Semmens, C.V.; Širović, A. Grouper source levels and aggregation dynamics inferred from passive acoustic localization at a multispecies spawning site. J. Acoust. Soc. Am. 2022, 151, 3052–3065. [Google Scholar] [CrossRef]
- Aldridge, S.E.; Dixon, O.F.L.; de Silva, C.; Kohler, J.K.; Shipley, O.N.; Phillips, B.T.; Fernandes, T.F.; Austin, T.; Ormond, R.F.; Gore, M.A.; et al. Depth Range Extension for the Misty Grouper Hyporthodus mystacinus Documented via Deep-Sea Landers throughout the Greater Caribbean. Fishes 2024, 9, 114. [Google Scholar] [CrossRef]
- McCoy, C.; Dromard, C.R.; Turner, J.R. An Evaluation of Grand Cayman MPA Performance: A Comparative Study of Coral Reef Fish Communities. In Proceedings of the 62nd Gulf Caribb Fish Inst, Cumana, Venezuela, 2–6 November 2009; pp. 337–345. [Google Scholar]
- Whitney, N.M.; Lear, K.O.; Gaskins, L.C.; Gleiss, A.C. The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre). J. Exp. Mar. Biol. Ecol. 2016, 477, 40–46. [Google Scholar] [CrossRef]
- Ebert, D.A.; Fowler, S.; Compagno, L. Sharks of the World. A Fully Illustrated Guide; Wild Nature Press: Plymouth, UK, 2013; p. 528. [Google Scholar]
- Humann, P.; DeLoach, N. Reef Fish Identification: Florida, Caribbean, Bahamas, 4th ed.; New World Publications: Jacksonville, FL, USA, 2014; p. 548. [Google Scholar]
- Currey-Randall, L.M.; Cappo, M.; Simpfendorfer, C.A.; Farabaugh, N.F.; Heupel, M.R. Optimal soak times for Baited Remote Underwater Video Station surveys of reef-associated elasmobranchs. PLoS ONE 2020, 15, e0231688. [Google Scholar] [CrossRef]
- Jabado, R.W.; Al Hameli, S.M.; Grandcourt, E.M.; Al Dhaheri, S.S. Low abundance of sharks and rays in baited remote underwater video surveys in the Arabian Gulf. Sci. Rep. 2018, 8, 15597. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Pierce, S.J. The use and abuse of photographic identification in sharks and rays. J. Fish Biol. 2012, 80, 1361–1379. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Salman, A.; Malik, M.I.; Shafait, F.; Mian, A.; Shortis, M.R.; Harvey, E.S. Automatic fish species classification in underwater videos: Exploiting pre-trained deep neural network models to compensate for limited labelled data. ICES J. Mar. Sci. 2018, 75, 374–389. [Google Scholar] [CrossRef]
- McConville, A.J.; Keane, I.A.; Coulson, T.; Bekenov, A.B.; Milner-Gulland, E.J. Reconstructing the observation process to correct for changing detection probability of a critically endangered species. Endanger. Species Res. 2009, 6, 231–237. [Google Scholar] [CrossRef]
- Bradley, D.; Papastamatiou, Y.P.; Caselle, J.E. No persistent behavioural effects of SCUBA diving on reef sharks. Mar. Ecol. Prog. Ser. 2017, 567, 173–184. [Google Scholar] [CrossRef]
- Southall, E.J.; Sims, D.W.; Witt, M.J.; Metcalfe, J.D. Seasonal space-use estimates of basking sharks in relation to protection and political-economic zones in the North-east Atlantic. Biol. Conserv. 2006, 132, 33–39. [Google Scholar] [CrossRef]
- Merrett, N.R.; Domanski, P.A. Observations on the Ecology of Deep-Sea Bottom-Living Fishes Collected off Northwest Africa: II. The Moroccan Slope (27°–34°N), with Special Reference to Synaphobranchus kaupi. Biol. Oceanogr. 1985, 3, 349–399. [Google Scholar] [CrossRef]
- Ward-Paige, C.; Flemming, J.M.; Lotze, H.K. Overestimating fish counts by non-instantaneous visual censuses: Consequences for population and community descriptions. PLoS ONE 2010, 5, e11722. [Google Scholar] [CrossRef]
- Gilmour, M.E.; Adams, J.; Block, B.A.; Caselle, J.E.; Friedlander, A.M.; Game, E.T.; Hazen, E.L.; Holmes, N.D.; Lafferty, K.D.; Maxwell, S.M.; et al. Evaluation of MPA designs that protect highly mobile megafauna now and under climate change scenarios. Glob. Ecol. Conserv. 2022, 35, e02070. [Google Scholar] [CrossRef]
- Kohler, J. The Comparative Abundance and Behaviour of Sharks in the Cayman Islands (BWI). Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 2022. Available online: https://hdl.handle.net/10399/4769 (accessed on 27 July 2025).
- Kohler, J.; Gore, M.; Ormond, R.; Johnson, B.; Austin, T. Individual residency behaviours and seasonal long-distance movements in acoustically tagged Caribbean reef sharks in the Cayman Islands. PLoS ONE 2023, 18, e0293884. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Thums, M.; Meeuwig, J.J.; Vianna, G.M.S.; Stevens, J.; McAuley, R.; Meekan, M.G. Crossing Latitudes-Long-Distance Tracking of an Apex Predator. PLoS ONE 2015, 10, e0116916. [Google Scholar] [CrossRef]
- Castro, J.I. The Biology of the Nurse Shark, Ginglymostoma cirratum, Off the Florida East Coast and the Bahama Islands. Environ. Biol. Fishes 2000, 58, 1–22. [Google Scholar] [CrossRef]
- Bernal, D.; Carlson, J.K.; Goldman, K.J.; Lowe, C.G. Energetics, metabolism, and endothermy in sharks and rays. In Biology of Sharks and Their Relatives, 2nd ed.; Carrier, J.C., Musick, J.A., Heithaus, M.C., Eds.; Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2012; pp. 211–237. [Google Scholar]
- Carlson, J.K.; Goldman, K.J.; Lowe, C.G. Metabolism, energetic demand, and endothermy. In Biology of Sharks and Their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 203–224. [Google Scholar]
- Dorman, S.R.; Harvey, E.S.; Newman, S.J. Bait effects in sampling coral reef fish assemblages with stereo-BRUVs. PLoS ONE 2012, 7, e41538. [Google Scholar] [CrossRef]
- Doan, M.D.; Kajiura, S.M. Adult blacktip sharks (Carcharhinus limbatus) use shallow water as a refuge from great hammerheads (Sphyrna mokarran). J. Fish Biol. 2020, 96, 1530–1533. [Google Scholar] [CrossRef]
- Springer, S. Social organization of shark populations. In Sharks, Skates and Rays; Mathewson, R., Rall, D., Eds.; Gilbert PW Johns Hopkins Press: Baltimore, MD, USA, 1967; pp. 149–174. [Google Scholar]
- Cortes, E. Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 1999, 56, 707–717. [Google Scholar] [CrossRef]
- Roff, G.; Brown, C.J.; Priest, M.A.; Mumby, P.J. Decline of coastal apex shark populations over the past half century. Commun. Biol. 2018, 1, 223. [Google Scholar] [CrossRef] [PubMed]
- Simpfendorfer, C.A.; Goodreid, A.B.; McAuley, R.B. Size, sex and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environ. Biol. Fishes 2001, 61, 37–46. [Google Scholar] [CrossRef]
- Chapman, D.D.; Pikitch, E.K.; Babcock, E.A.; Shivji, M.S. Deep-diving and diel changes in vertical habitat use by Caribbean reef sharks Carcharhinus perezi. Mar. Ecol. Prog. Ser. 2007, 344, 271–275. [Google Scholar] [CrossRef]
- Schlaff, A.M.; Heupel, M.R.; Udyawer, V.; Simpfendorfer, C.A. Sex-based differences in movement and space use of the blacktip reef shark, Carcharhinus melanopterus. PLoS ONE 2020, 15, e0231142. [Google Scholar] [CrossRef] [PubMed]
- Bolger, D.T.; Morrison, T.A.; Vance, B.; Lee, D.; Farid, H. A computer-assisted system for photographic mark-recapture analysis. Methods Ecol. Evol. 2012, 3, 813–822. [Google Scholar] [CrossRef]
- Hiby, L.; Lovell, P. A note on an automated system for matching the callosity patterns on aerial photographs of southern right whales. J. Cetacean Res. Manag. 2001, 2, 291–295. [Google Scholar] [CrossRef]

| Behaviour Metric | Mean (±SE) | Range |
|---|---|---|
| Tarrive (min) | 62.84 (±1.53) | 1.23–164.12 |
| Tvisit (min) | 6.92 (±0.48) | 0.07–84.23 |
| Activity level 1 | 16.08 (±1.19) | 1–176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohler, J.; Gore, M.; Ormond, R.; Mason, K.; Veeder, A.; Austin, T. Combining Adequate BRUV Deployment Times with Individual Photo-Identification Improves Monitoring of Shark Populations in the Caribbean. Oceans 2025, 6, 70. https://doi.org/10.3390/oceans6040070
Kohler J, Gore M, Ormond R, Mason K, Veeder A, Austin T. Combining Adequate BRUV Deployment Times with Individual Photo-Identification Improves Monitoring of Shark Populations in the Caribbean. Oceans. 2025; 6(4):70. https://doi.org/10.3390/oceans6040070
Chicago/Turabian StyleKohler, Johanna, Mauvis Gore, Rupert Ormond, Katherine Mason, Anne Veeder, and Timothy Austin. 2025. "Combining Adequate BRUV Deployment Times with Individual Photo-Identification Improves Monitoring of Shark Populations in the Caribbean" Oceans 6, no. 4: 70. https://doi.org/10.3390/oceans6040070
APA StyleKohler, J., Gore, M., Ormond, R., Mason, K., Veeder, A., & Austin, T. (2025). Combining Adequate BRUV Deployment Times with Individual Photo-Identification Improves Monitoring of Shark Populations in the Caribbean. Oceans, 6(4), 70. https://doi.org/10.3390/oceans6040070








