Abstract
Atlantic salmon aquaculture has become an important seafood producer, contributing to the human diet. The natural productivity of Atlantic salmon populations is not sufficient to meet even a fraction of current aquaculture production, and it has not been able to do so in the past. Alternative process technologies are needed to maintain aquaculture production at current levels while mitigating the environmental impact along the coasts. Future aquaculture development must align with the UN Sustainable Development Goals. This study describes an aquaculture process vessel operating in the open sea and powered largely by renewable energy. Production conditions are fully adapted to the biology of salmon, improving production reliability, reducing coastal environmental impacts, and enabling more sustainable production. This study specifies the biological and technological aspects, provides evidence of the technical and economic feasibility, and justifies the relocation of salmon aquaculture to a large oceanic ecosystem, the North Atlantic Ocean.
1. Introduction
Atlantic salmon aquaculture (Salmo salar) experiences difficulties while still being a remarkable profitable endeavor [1]. For a long time, the major risks were assumed to be related to pricing, institutional risks, and fish diseases. The means of choice of risk management was maintaining low production costs [2]. Today global warming is not only impacting species assemblage in natural systems [3], but it also becomes an issue for coastal aquaculture as ocean warming is accelerating [4]. Temperature is a driving force on natural systems and also affects Atlantic salmon which is a cold-water anadromous species with an optimum water temperature for growth at around 14 °C [5]. This concurs with recent results on the oceanic distribution of Atlantic salmon [6]. A northward movement of the thermal niche of anadromous salmonids is foreseen, potentially resulting in decreased natural productivity and potential population extinction in southern parts [7].
A risk assessment of the environmental impact of Atlantic salmon farming in net pens point towards a moderate-to-high risk for genetic introgression from escaped salmon and a moderate-to-high risk for salmon lice infection in wild salmonids. Virus disease transfer and pollution through dissolved nutrients and organic waste underneath farms are ranked as lower risks [8]. However, results from Gracilaria chilensis showed that production close to a salmon net pen farm showed higher growth and higher nutrient content near the net pens [9], indirectly proving the potential of eutrophication. The excretion of nitrogen and phosphorus is part of the nutritional physiology of fish, and particulate organic waste formation is part of every heterotrophic aquaculture production process. However, two environmental factors must be seen critical: the increasing water temperature and the lack of dissolved oxygen [10] as a result of eutrophication.
Salmon farming is seeking alternative methods that allow production farther away from coastal ecosystems, where the absorbing water volume is much larger. The potential limitations of nearshore net cage farms have led to the development of floating offshore aquaculture facilities. These could include, for example, flexible floating fish cages, enclosed fish cages, or submersible spherical fish cages [11]. A recent study on spatial planning for salmon aquaculture along the northeast Atlantic coast from Maryland to Maine indicates lower costs for cage farms and production with increasing distance from the coast [12]. Influencing factors include environmental conditions and conflicts of interest. However, production prices are significantly higher than those shown for Norwegian salmon aquaculture [13]. The study recommends the use of renewable energy through wave-powered aquaculture farms [12], which is consistent with the objectives of our study.
Production in land-based systems also carries significant risks. The difficulties of the production of Atlantic salmon in closed systems (recirculating aquaculture systems, RASs) is often due to design flaws and poor production management [14], although the biology, the technical components, and the process chain has been examined in many publications. The logic behind the RAS project chain was recently assessed in a critical review [15] showing the potential of the technology. A potentially erroneous assumption is that RASs are more environmentally friendly. This is only the case if nutrients and waste are recycled in coupled processes and not released into the environment. The simpler the process chain [15], the higher the potential nutrient input via the waste stream, as water renewal is often used to remove waste from the recirculated water stream. The pollution potential of RAS production is evident. This also applies to fish farms at the coast using seawater in through-flow systems.
The change in sea surface temperatures due to climate change may require a relocation of salmon farming to the open sea, where suitable temperature conditions prevail. Best temperature conditions can be assumed in the natural oceanic distributional range [6]. This requires a fully maneuverable vessel shifting its operating area according to the seasons. To ensure optimal conditions for fish production, the cruising area and course of the vessel are determined based on nautical, meteorological, and hydrographic data and forecasts.
Operating an aquaculture vessel in the harsh conditions of the open sea requires a ship architecture that enables production processes in high wind speeds and rough seas (waves) [16]. The use of renewable energy through sail propulsion is an essential feature of the concept presented here: the use of fossil energy is counterproductive given the already evident challenges of climate change. Vessel size is important not only for economic reasons but also to minimize vessel movements along the transversal and longitudinal axes. In addition, the geometry of the production tanks must prevent sloshing, which is obviously less important for near-shore aquaculture vessels [17] operating in calmer hydrographic conditions. The wind-driven water flow through the production tanks must be maintained under all weather conditions, and a closed-vessel operation must allow access to inshore destinations. The process technology must be tailored to the biology of the fishes [15] so that best production conditions are maintained throughout the entire production cycle. This is also important to avoid mass mortalities. Mass mortalities in conventional aquaculture production of Atlantic salmon are huge [18] and at the end a waste of resources. The database in [18] summarizes 865,000,000 perished fish in the six major producing nations during the last decade.
A crucial aspect is the justification of open-ocean aquaculture as it is integrated into the oceanic system. Even if particulate waste could be partly removed from the effluent water, dissolved nutrients, particulate, and organic mater will be spread over the cruising area, causing nutrients and organic matter to be released into the natural biogeochemical cycle. The use of the oceans’ biogeochemistry seems possible due to the losses of chemical elements through landings from fisheries. In the North Atlantic, the total fish caught [19] were in the range from 7 to 15 million tonnes per year (Figure 1), reducing the fish biomass and the release of inorganic carbon into the biogeochemical cycle [20]. Fish are part of the marine biological carbon cycle [21]. The pelagic CaCO3 rain generated in fish [22] feeds the marine inorganic carbon cycle at the regional and global scales, supporting the regulation of atmospheric carbon dioxide [23]. The stabilization of this vastly important ecosystem service, however, would require lower fishing mortality rates than those needed to maintain a sustainable fishing yield [21] of commercial fisheries.
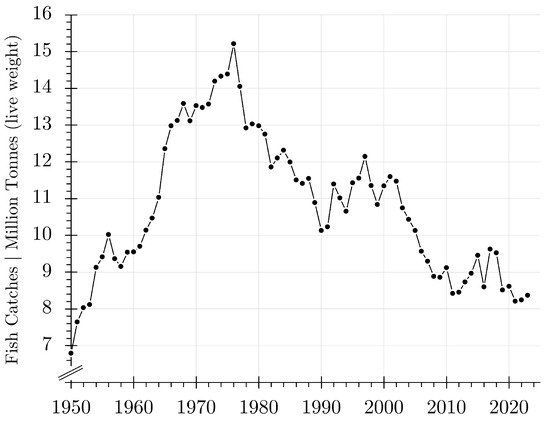
Figure 1.
Fish landings from commercial fisheries in the Northeast and Northwest Atlantic (FAO fishing areas 21 and 27) [19]. Double lines sloped towards the axes indicate an axis break.
The goal, reducing fishing mortality, will not be easy to achieve, as fisheries have long since been unable to adequately supply the world’s population. Fisheries management was not able to limit exploitation. Today, aquaculture is no longer a supplement to fishery capture [24]. It has become an indispensable part of the food supply for a growing global population. The introduction of novel sustainable methods is necessary to be able to exploit the potential of aquaculture in a responsible way in the future, i.e., without impacting the function of coastal ecosystems.
Beyond the link to global warming [23], the marine nitrogen and phosphorous pool is depleted through commercial fisheries. More than half of the total nutrient extraction is caused by fishing on mid-level trophic and pelagic organisms [25]. The consequences, unfortunately, seem not to be sufficiently known. The full picture requires looking into the mass balance between nutrient extraction through fisheries and anthropogenic nutrient inputs from land [26]. The nutrient inputs to coasts typically exceed the extraction of nutrients through fishing over the past five decades.
Coastal ecosystems are most directly affected through anthropogenic nutrient inputs [27], and their effects are anticipated to be more pronounced [28] compared to those observed inland. Coastal aquaculture, which also contributes to these nutrient fluxes, could be partly relocated to areas where fisheries had already diminished the nutrient pool and continue to diminish it.
This publication describes an alternative production process for Atlantic salmon (S. salar) that could complement existing production methods. An aquaculture production vessel carrying 15 independently operating production lines is outlined. The vessel is largely powered by renewable energy (wind power). The aquaculture processes are strictly based on the biology of the species, securing the production process.
However, this study does not provide a detailed blueprint as this would be the effort of the next step development. It summarizes a preliminary design study by proving the biological and technical feasibility. It is based on standard scientific processes carried out by specialists from the required fields of knowledge. The study includes
- -
- The architecture of the vessel as well as the aquaculture process technology, which is based on the biology of Atlantic salmon (S. salar).
- -
- Estimates are given on forces and the balance of forces of a large vessel propelled and operated with wind forces. The carbon footprint is reckoned.
- -
- Information is also provided on the cruising area and prevailing meteorological and hydrographic conditions in order to prove the feasibility of the concept of a truly ocean-going aquaculture operation.
- -
- A provisional financial scheme is provided based on sound assumptions and data.
2. Aquaculture Production in a Process Vessel
Salmon production in the process ship presented here is aligned with the current practice of conventional net cage aquaculture. The final production weight of Atlantic salmon is reckoned between a body weight (BW) of 4 and 5 kg (Figure 2). This weight is in the preferred range of the European processing industry. It is usually attained after two year in the saltwater phase in coastal net pens. This will be different in open-ocean aquaculture. The production period can be assumed to become shorter as growth depressions during cold seasons and at high summer sea surface temperatures (SST) can be avoided in a fully maneuverable process vessel.
Data from early net-pen aquaculture show quite high growth rates for Atlantic salmon at water temperature of 14 °C [29,30]. The data were used to compile a growth curve for Atlantic salmon from smolt (0.1 kg BW) to market size fish (4.5 kg BW) (Figure 2). The weight data were the base for all further calculations. It turned out that the fast growth at a constant optimum water temperature would reduce the production time from smolt to harvest weight to one year. Another reason for the fast growth is that ocean water from the North Atlantic, which comes from the natural habitat of the species, is continuously supplied throughout the entire production period.
The wind-driven aquaculture process vessel follows the isothermal 14° sea surface temperature in the North Atlantic throughout the entire production period. However, bunker ports and aquaculture facilities for smolt production finally determine the track of the aquaculture process vessel.
Production takes place in production lines comprising four production tanks of different sizes and volumes. While the tanks for cohorts 1 and 2 are six meters deep, the tanks for the larger animals in cohorts 3 and 4 are nine meters deep. This supports the size-dependent fish distribution observed in sea cages [31]. The tank diameters are 6, 8.8, 9.4, and 12.2 m, respectively. All tanks have a funnel-shaped lid with a centrally located vertical tube that acts as a riser, minimizing energy transfer at the water–air interface. This riser protrudes 1.3 m above the waterline of the tank. Each tank has a conical bottom and a waste collector in the center to collect particles and excrement. This waste collector must be emptied regularly by pumping.
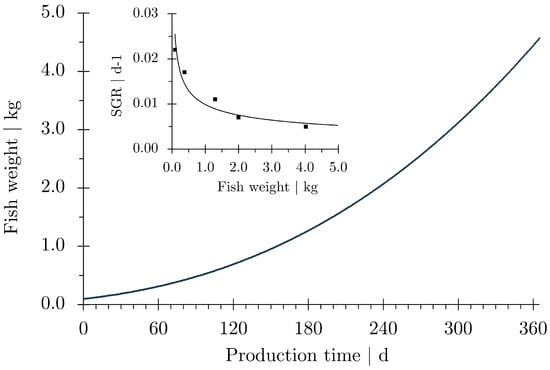
Figure 2.
Predicted growth of Atlantic salmon (Salmo salar). Weight-dependent growth rates (exponential growth rate, ) were derived from literature data shown in the inserted smaller diagram [29,30]. The weight-dependent growth rates were used to calculate body weight (wt) for consecutive days (). Calculations were repeated until a body weight of 4.5 kg was reached. The timespan from stocking of smolt (0.1 kg body weight) to harvest of market-sized fish (4.5 kg body weight) is 363 days.
Every tank holds a cohort of fish of different sizes and body weights. This is necessary to ensure the formation of schooling behavior and to suppress agonistic behavior. In doing so, pelleted feed of the required size can be supplied to each size group at the required feeding rate. The holding of fish in each process line closely follows the layout of a modern RAS to produce Atlantic salmon.
Cohorts will be transferred to the subsequent tank every three months. The transport of smolt to the aquaculture process vessel will be performed by supply vessels also transporting fish feed and supply goods. On the way back, market-sized fish are carried to processing plants. The meeting point for the vessels is as close to the coast as possible and is each time determined by weather and hydrographic forecasts.
The production capacity is , which corresponds to the typical demand of the seafood processing industry, which is a potential candidate for such a concept. It was also assumed that the seafood industry requires a continuous supply. For this reason, aquaculture production was distributed across 15 production lines that can individually be stocked and harvested according to the market demand.
Table 1 summarizes benchmarking data for a single production line. The values for initial and final body weight of fish were read from the average growth curve shown in Figure 2. Assuming a survival rate of 97.5% for the fish in every cohort and an acceptable stocking density in the fourth production cohort, the biomass at the end of each production period, as well as the final and initial biomass, was backwards computed for all cohorts. From that, the number of fish entering and harvested from a production line was calculated. The central outcome of Table 1 is that the stocking densities will remain between 36 and 62 . The steady-state production capacity of each production line is 266,400 kg yr−1, summing up to 4000 tons of living weight for the process vessel in one year.

Table 1.
Benchmarking data for one production line for Atlantic salmon (S. salar). Smolt body weights at different times were derived from Figure 2. The survival rate was assumed to be 90% for the entire production period. Cohort numbering refers to cohorts of fish of different sizes and weights kept in production tanks 1 to 4.
Particular attention is paid to the hydrodynamics of the production tanks, as continuous forced swimming at high speeds must be avoided for animal welfare reasons. The permissible swimming speed of Atlantic salmon is in the range of 0.5 body length [32,33]. Assuming a rheotactic behavior of the fish, flow velocities between 19 and 68 , reflecting the voluntary swimming speed of salmon, must not be exceeded in the production tanks (Table 2). The threshold swimming speed for RAM ventilation is approximately one-third of the critical swimming speed (Ucrit). RAM ventilation is the passive ventilation of the gills through dynamic pressure, reducing the metabolic expenditure for oxygen uptake. Thus, this specific swimming speed could be beneficial for the fishes’ metabolic energy allocation [34]. However, the seawater inflow rate into the production tanks must be sufficient to supply oxygen and remove dissolved and particulate waste products.

Table 2.
Parameters characterizing the swimming performance of Atlantic Salmon with different body weights and body lengths. Ucrit: critical swimming speed [32], Uv: voluntary swimming speed [32], and Ut: threshold swimming speed for RAM ventilation [34].
It is assumed that an inflow rate of seawater of three process tank volumes per hour will be sufficient. At this flow rate the water renewal rate in the production tanks amounts to 0.95 [35]. Seawater is supplied through dynamic pressure through a pitot pipe mounted in the vessel hull (Figure 3). To ensure even mixing of the incoming seawater, inlet nozzles are mounted vertically at different tank depths (Figure 3). The geometry of the inlet nozzles is adjusted to limit the momentum input into the production tanks to limit water flow velocity. A vertical strainer that extends across the entire tank water column is installed. A maintenance opening at the work-deck level allows the strainer to be cleaned during production without interrupting the water flow. The seawater flow rates towards the production tanks are shown in Table 1. The total seawater volume flow towards one production line sums up to .

Figure 3.
Process scheme of a production line and the seawater supply. Seawater is forced into the main seawater inlet pipe through a pitot tube mounted in the starboard wall of the hull. The seawater is distributed via manifolds. The outlet pipes collect the wastewater from the production tanks and discharge it via the main seawater outlet pipe back into the sea on the port side. Strainers are mounted in every inlet and outlet pipe. An auxiliary pump supplies seawater during standstill. Additional components, light, automated feeding, and oxygen supply, are drawn to complete the process scheme.
Flotsam and marine life are retained by screens at the forward inlet. Additional screens retain smaller objects and organisms upstream of the seawater inlet to the production tanks.
The water level in the production tanks is kept constant. Water movements (waves) are limited to a very small area of the tanks in the tapered head section. This special tank geometry prevents sloshing of the tank water [16] at the water/air interface (Figure 4). Sloshing of the tank water would likely trigger avoidance behavior in the fish and reduce the tank water volume available to the fish. At the end, this also contributes to occupational safety on board the vessel.
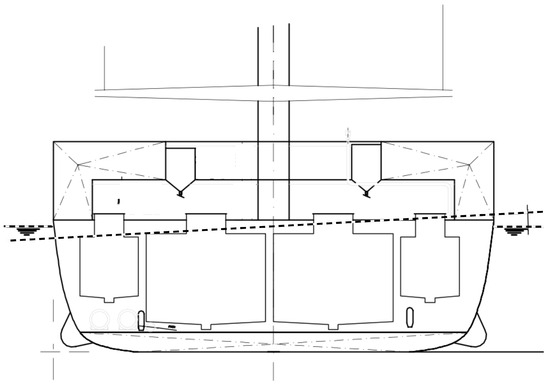
Figure 4.
Transverse section of the aquaculture process vessel. Four production tanks are shown. The special geometry of the head part minimizes the sloshing area at the water/air interface. The maximum expected roll of the vessel is shown by dashed lines.
The seawater distributor, which distributes the seawater to production lines and tanks, exhibits a design-related pressure drop. This was estimated using standard process engineering methods [36]. For this purpose, the required pipe lengths and the number of fittings and filters were taken from a preliminary design study of the naval architects. The optimal pipe diameter for the given seawater volume flows was determined iteratively, and friction values were calculated. The friction values for fittings were added from tabulated data and from calculations of filter systems. The total pressure drop that must be overcome by back pressure was calculated to be approximately 30 . The naval architects were thus able to develop a design and define the sail area to generate the forces required for ship movement and process engineering. The pressure loss of 30 can be overcome by the dynamic pressure generated at a vessel speed of 5.4 knots (kn) or . A throttle valve controls the seawater volume flow towards the fish tanks at higher vessel velocities (Figure 3).
Figure 5 shows the arrangement of the production lines and production tanks. The seawater supply for every two production lines is provided via a pitot tube mounted on one side of the vessel. The seawater outlet pipe for every two production lines is located on the opposite side of the hull. Production line fifteen is supplied by its own supply lines. Figure 4 shows the transverse section of the vessel with the production tanks.

Figure 5.
Cross-section of the aquaculture process vessel at the level of the propeller shaft. The arrangement of 15 production lines, each with four octagonal production tanks of different sizes and volumes (Table 1), is shown. The production tanks are part of the supporting structure of the sailing vessel.
For low-wind-speed situations, reducing the speed of the vessel below 5.4 kn or is required, and auxiliary seawater pumps are installed in the bypass system (Figure 3) supplying seawater at the required flow rate and pressure to every two production lines. The capacity of the electric seawater pump is at a pressure head of 0.5 m. The electric power for the auxiliary pumps is generated by electrical generators operated with combustion engines. During standstill of the vessel, an internal circulation pump can be used to maintain water flow in the production tanks.
The seawater stream flowing through the production tanks carries most of the oxygen required by fish. Atlantic salmon avoids water with low oxygen levels [37] but appears to tolerate lower oxygen saturation levels [38] well, even under production conditions. Oxygen saturation in the production tanks is maintained between 80 and 100% oxygen saturation. The oxygen concentration of the incoming seawater is increased as needed by enriching the air stream up to 80% using oxygen generators (Figure 3). Oxygen enrichment is routinely achieved using electrical power generated by the dual-mode propellers at the stern of the vessel. The oxygen generator also provides nitrogen gas to maintain an inert atmosphere in the feed stores.
The feeding system supplies the fish with feed below the water’s surface. Standard machines, such as those used in net-pen aquaculture, are used. The four-tank production scheme allows each of the four cohorts to be supplied with the appropriate feed quality. Feed distribution and mass flow throughout the day are software-controlled and coupled with automated video monitoring of fish size, activity, and feeding behavior using machine learning (AI) algorithms. Feed losses are eliminated not only for environmental reasons but also, and especially, for resource conservation. To minimize peak electrical consumption, feeding takes place sequentially in the fifteen production lines.
Feed for every four cohorts will be stored in separate feed silos, which are tanks having the capacity to store the required fish feed plus a 20% safety reserve for three months. The feed silos are located on the main deck. The nitrogen off gas from oxygen generators is used to maintain an inert atmosphere in the feed silos. The feed is bunkered from supply vessels every three months. The filling of silos will be carried out with corn lifters mounted on the upper deck of the vessel.
3. The Aquaculture Process Vessel
The aquaculture process sailing vessel was configured to allocate the necessary process conditions described in Section 2. The results of the preliminary design study presented here are based on standard algorithms and computational software [39,40].
The aquaculture process vessel will operate almost entirely at sea in the natural oceanic habitat of Atlantic salmon [6]. The vessel will be navigated to maintain the optimal temperature of 14 °C for growth [5]. It will therefore cruise in the nautical latitude near Iceland during the summer months and the nautical latitude near Portugal during the autumn and winter months.
It was assumed that wind power would provide the majority of the energy for the propulsion of the vessel, the hotel and nautical operations on board, and aquaculture production under the meteorological and hydrographic conditions on the Atlantic Ocean. The vessel was, therefore, designed for atmospheric temperatures between −20 °C and +40 °C, sea water temperatures between 0 °C and 32 °C, and salinities between 0 and 38 psu. The design was optimized to routinely operate under high wind conditions between Bft 7 (strong breeze) and Bft 9 (strong gale).
Typical wave heights during routine operation are expected to range between two and three meters, while the maximum wave height in stormy conditions may reach five meters. These sea conditions are manageable for a large sailing vessel (Table 3). The maximum roll and pitch of the ship are approximately 3° and 2°, respectively.

Table 3.
The projected dimensions of the aquaculture production vessel.
The sailing vessel is constructed from steel, with a longitudinal framing system amidships. Fore and aft ships are constructed as a transverse framing system. Ballast tanks control vessel draft mainly during fish transfer between production tanks, during maintenance of production tanks, and during harvest of fish. A passive roll damping tank stabilizes the motion of the vessel under choppy sea conditions even if it can be assumed that the size of the vessel and the sail forces at beam reach heading dampen the rolling and pitching of the vessel anyway.
The maintenance intervals (class dockings) for the aquaculture process vessel can be extended to every seven years. The service life of the process vessel is assumed to reach 35 years.
The preliminary dimensions of a vessel having an aquaculture production capacity of about 4000 are summarized in Table 3. The vessel carries 15 production lines, which are assembled along the longitudinal axis. Fourteen process lines are assembled parallel and share one seawater intake and outlet (Figure 5 and Figure 6). Process line fifteen is located in the ships’ bow and has its own seawater supply and discharge.
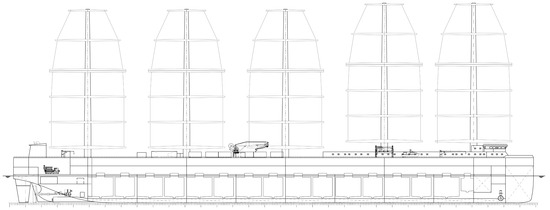
Figure 6.
Lateral view of the aquaculture process vessel showing the starboard production tanks (Table 1).
The open deck area at the upper deck level can be used to lash reefer (refrigerated) containers. Electric power supply is available, as well as a waste water discharge system that will be used during cleaning. Hydraulic cranes are provided for loading or unloading containers during rendezvous with supply vessels. Fender bars on both sides on the level of the main deck support supply vessel mooring during loading/unloading. On the main deck near the mooring system for the supply vessels, transport pipes are installed to transport smolts to the first production tank of each production line. Another transport pipe system brings the feed to the feed bins of the individual production lines on the working deck. The feed bins are blanketed with a dry nitrogen atmosphere, which is a byproduct of oxygen generation.
The aquaculture process vessel is outlined to be primarily propelled by sail power. However, to ensure maneuverability and in case of emergency, the vessel can be propelled by two dual-mode controllable-pitch propellers. The projected dual-mode propellers can operate as ancillary water turbines to generate electrical power when the speed of the vessel is high enough. The electrical energy is used for the operation of the vessel, the sail system, and the aquaculture production process. Electrical power is also required for oxygenating the water in the production tanks and for waste disposal at the bottom drain of the production tanks as well as for control and automation systems, lighting, and feeding systems (Table 4). Depending on wind intensity and vessel speed, excess electrical power can be stored in batteries.

Table 4.
Wind-powered operation of the process vessel. At wind speeds of 6.7 Bft and above, the vessel and fish production are powered exclusively by wind power. At wind speeds of 9 knots, excess electrical energy can be stored in a battery storage system. All fish processing processes are on hold. Abbreviations: ins: installed load [kW], aon: always-on electrical power [kW], eec: electrical energy consumption [], Bft: Beaufort.
In conditions where the wind force is insufficient to propel the vessel, four multi-fuel combustion engines drive electrical generators that power the propellers. The capacity of the generators is sufficient to supply energy to all consumers, as listed in Table 4. In any case, an emergency supply is officially required to ensure all essential operations.
The course of the aquaculture sailing vessel determines the angle to the wind and the propulsion force. Such a process vessel therefore requires a constantly updated shipping route that ensures sufficient wind speeds at the required sea surface temperature (14 °C). This will be achieved by simultaneously processing meteorological and hydrographic data available from continuously updated databases. Even if the ship does not have an apparent destination, route planning is an essential task of the nautical staff of the vessel. Exception include scheduled rendezvous with supply vessels every three months or possibly port visits. However, even for these exceptions, the route needs to be planned to assure a continuous production. Possible outage times when approaching coastal waters need to be minimized.
The water flow through the production lines is expected to be maintained through the dynamic pressure generated by the speed of the vessel. However, to reach a fossil-free operation, the wind forces need to deliver sufficient energy to maintain the seawater flow through the process tanks and to generate ancillary electrical energy for the operation of the vessel and for aquaculture production. A window for wind intensity (Figure 7) at sea was specified for further calculations, taking into account the near-surface wind speeds over the North Atlantic [41]. At the lower end of the range, wind intensity supports a hybrid operation; i.e., energy is supplied through wind force and additional energy generated through combustion engines. At the upper end of the range, wind intensity allows a full operation through wind forces; excess energy can be stored in batteries. Table 4, Table 5 and Table 6 summarize the energy balance for the operation of the vessel and aquaculture production for different scenarios so far, which was estimated on a limited number of data.
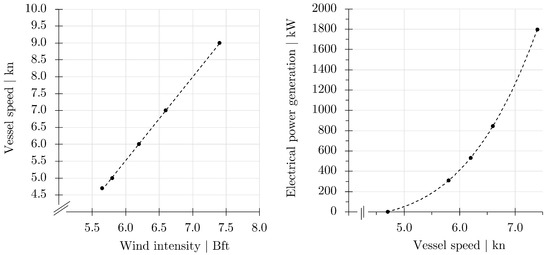
Figure 7.
Design parameter for the speed of the process vessel at different wind intensities (left). Design parameter for electrical power generation through the use of the two ship propellers as water turbines at different wind intensities (right). Double lines sloped towards the axes indicate axis breaks.

Table 5.
Hybrid operation of the process vessel, i.e. the use of wind power and electrical energy generation through combustion engines, at lower wind intensities. The electrical generators delivers additional electrical energy for the operation of the vessel and the fish production lines. All fish processing processes are on hold. Abbreviations: ins: installed load [kW], aon: always-on electrical power [kW], eec: electrical energy consumption [], Bft: Beaufort.

Table 6.
Energy allocation during regular rendezvous of supply and process vessels on the high seas. This requires operation with combustion engines. During encounters, the production vessel’s speed is zero. Electrical energy is required for positioning. Fish processing is in progress. Abbreviations: ins: installed load [kW], aon: always-on electrical power [kW], eec: electrical energy consumption [], Bft: Beaufort.
The key question that needs to be addressed is the extent to which the process vessel can be powered by wind power. This then determines the additional energy demand that must be met with fuels (preferably green fuels). Table 4 summarizes the energy distribution during pure wind operation of the process vessel. From a wind speed of 6.7 Bft, the vessel and fish production are powered exclusively by wind power. The vessel’s speed reaches approximately 7.3 knots. The auxiliary water pumps are idle. The propellers (water turbines) generate electricity and provide the energy for the operation of the vessel and sails, as well as for oxygen generation, waste disposal, control and automation, lighting, and feeding systems. Fish transfer, fish harvesting, cold storage, and internal circulation are idle during routine production at sea. The always-on electrical power summing up to 920 kW is fully supplied by wind power. Electrical energy consumption for salmon production and the operation of the vessel remains free of charge. If wind intensity increases to above 7.4 Bft, the water turbines deliver more electrical energy as demanded. The excess electrical energy is stored in batteries.
Table 5 summarizes the scenarios where the speed of the vessel is insufficient to fully operate with wind power. At wind intensities of ≥5.4 and ≈6.2 Bft, the water flow through the production tanks is maintained by the forward thrust of the vessel. The auxiliary water pumps are not in use, as well as all processes for fish transfer, harvest, cold storage, and internal circulation. The energy demand for aquaculture production and the vessel and sail operation amounts to 860 and 880 kW, respectively. The water turbines on the other hand deliver 0 and 532 kW of energy. Part of the electrical energy consumption, 1.88 and 0.76 , needs to be generated by combustion engines. Below 5.4 Bft wind intensity, the combustion engines need to generate electrical energy. Electrical energy consumption increases to 3.29 .
Table 6 summarizes the energy requirements during the regular three-month rendezvous of supply and process vessels at sea. The rendezvous last approximately 16 days per year. During these rendezvous, the internal combustion engines, preferably powered by renewable fuel, provide all energy needs for the vessel’s operation, as well as for fish transport, fishing, and cold storage. In addition, the auxiliary pumps supply seawater to the production tanks. Maneuverability, keeping the vessels in position, is maintained by propeller thrust. The electrical energy requirement during loading and unloading is estimated at 5.3 . This estimate needs to be validated at a later stage of project development.
Figure 8 shows the energy demand and the speed of the vessel at increasing wind intensities, reflecting the data in Table 4, Table 5 and Table 6. Strong winds are required for the fossil-free operation of the vessel at sea. The vessel is constructed to operate smoothly and safely at these high wind intensites.
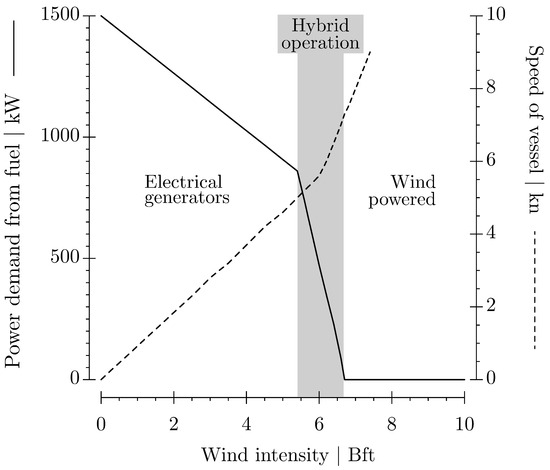
Figure 8.
Power demand and speed of the vessel at increasing wind intensities. A hybrid operation marks the transitional range of wind intensities where fossil fuel for the generators and wind power are required to supply energy. Above 6.7 Bft, the vessel is fully powered by wind forces.
Wind intensity may be insufficient for a quarter of the year. During this time, combustion engines generate electricity. The resulting carbon dioxide (CO2) emissions can be roughly estimated as follows:
- -
- The average electricity consumption for a partly wind-operated production line could be estimated at (3.29 + 1.88 + 0.76)/3 kWh·kg−1 fish production·yr−1 (Table 5, electrical energy consumption).
- -
- For a quarter of the year in which there is insufficient wind, the average electricity consumption would be about 1/4 ≈ 0.5 kWh·kg−1 of fish production.
- -
- Generating electrical energy with marine biodiesel (DMA) consumes approximately 0.2 kg DMA·kWh−1 of electrical energy.
- -
- DMA consumption would be 0.5 kWh·kg−1 fish production × 0.2 kg DMA·kWh−1 ≈ 0.1 kg DMA·kg−1 of fish production.
- -
- The carbon dioxide emission of 1 kg of DMA is 3.206 kg CO2.
- -
- The average carbon dioxide emission for a hybrid operation during one quarter of the year would then amount to 0.10 kg DMA·kg−1 of fish production × 3.2 kg CO2·kg−1 DMA ≈ 0.32 kg CO2·kg−1 of fish production.
- -
- Rendezvous will take 16 days during one year, so the electrical power consumed during the rendezvous can be reduced to 0.23 kWh·kg−1 of fish production (Table 6).
- -
- The electrical power consumption of 0.23 kWh·kg−1 would need 0.046 kg DMA·kg−1 of fish production. The CO2 emission would amount to around 0.14 kg CO2·kg−1 of fish production.
- -
- The total carbon dioxide emission for electrical power generation on board the described process vessel would sum up to 0.32 kg CO2·kg−1 of fish production + 0.14 kg CO2·kg−1 of fish production ≈ 0.46 kg CO2·kg−1 of fish production.
4. Discussion
This study presents a novel concept for the aquaculture of Atlantic salmon (Salmo salar) in its natural oceanic habitat. The concept was developed after it became clear that neither open-net pens nor recirculating systems (RASs) appeared to be able to fully address future challenges related to environmental factors and technical uncertainties [14,42]. Open-net pens and closed containment systems (RASs) typically release nutrients, the by-products of fish production, into the environment, even if technical upgrades such as anoxic denitrification, anaerobic bio-digesters [43], and integration into phototrophic production [44] could provide solutions to minimize nutrient transfer from RASs. Flow-through (hybrid) aquaculture systems for salmon production with a daily process water consumption of about 35% of the system volume [45] by no means meet the crucial requirements for sustainable production. The eutrophication of the coastal zone threatens the structure and functionality of coastal ecosystems [46]. There can be no doubt that coastal ecosystems must be maintained in a healthy, productive, and resilient state, likely forcing part of coastal aquaculture to the open sea.
Ocean warming is accelerating [4] and has severe implications for the aquaculture industry [47]. Rising sea surface temperatures along coasts will force the relocation of open-net pens towards areas with suitable temperatures. Rising temperatures over land will increase the energy required to maintain suitable temperatures for Atlantic salmon [5] in recirculating aquaculture systems. However, temperature control may be not sufficiently considered in RASs [14]. The introduction of new technologies in Atlantic salmon farming appears necessary to ensure optimal growth and high survival rates while reducing the impact on coastal areas. The concept proposed here requires the use of existing infrastructure such as hatcheries, RAS smolt production, and processing facilities. It is a complement to existing grow-out production lines. Diversification of production methods is a step toward sustainable aquaculture. Harnessing the open ocean can reduce pressure on the coasts, thus preserving space for coastal production where safe environmental conditions for Atlantic salmon production prevail. Relocating salmon farming to the natural range of the species [6] is a logical step. Efforts to protect the large Atlantic ecosystem must be made in parallel.
The presented concept can help overcome foreseeable obstacles to the future development of salmon farming. These include aspects of animal welfare, environmental protection, and resource use, as well as reducing the fossil footprint through the use of renewable energy and environmentally friendly fuels. Production takes place under conditions that correspond to those in the natural habitat of Atlantic salmon.
The underlying growth model is based on data collected four decades earlier on farms in a less disturbed environment. The growth model used in this study does, therefore, not account for genetic improvements in growth but is a solid basis for the calculations. The size of the smolts could be considered small, as the industry now prefers larger smolts. This is intended to shorten the seawater phase, which is not necessary in open-ocean aquaculture. The use of the sea allows for the maintenance of optimal growth temperatures throughout production. An aquaculture process tank follows the 14 °C isotherm, whereas an anchored cage farm cannot.
Optimal growth temperatures [5] are maintained throughout production. The seawater flow is designed to ensure that the production tanks receive abundant, top-quality seawater, which contains almost all of the dissolved oxygen required by the fish [38]. Under optimal temperature and oxygen conditions, the fish can reach the projected market size within one year.
Stocking density is maintained between 36 and 62 , which, according to literature reports, is within the upper limit for welfare and production performance in tank culture systems [48,49,50]. The conditions in the process tank are constantly maintained at optimal levels by the process technology and the water supply from the ocean so that these stocking densities can be used. Smaller fish with higher specific metabolic rates are kept at lower densities than larger fish. This could make resources more accessible to each individual fish. On the other hand, lower densities can promote food intake and growth through less competition and aggressive behavior [51]. However, the stocking densities are typical for Atlantic salmon aquaculture and are only reached shortly before transfer to the subsequent production tank.
Survival rates are expected to be high because, in addition to the water quality, the process technology is proven and state-of-the-art. A survival rate of over 85% is expected in the RAS [14]. Because water treatment, which is critical in the RAS, is not required in an aquaculture process vessel, a survival rate of 90% is expected.
One aspect is crucial for successful production: The smolts must come from biologically safe breeding and production lines to avoid the transmission of pathogens. This is essential for the protection of the marine environment. On the other hand, the ocean may be a source of pathogens. However, it can be assumed that the host density threshold [52] for salmon disease will hardly be achieved on the open sea.
It was shown that the vessel requires considerable wind forces to operate in a fossil-fuel-free manner in the Northern Atlantic. The fully wind-powered mode is possible at wind intensities above 6.7 Bft (Figure 8). The vessel speed reaches 7.3 kn, ensuring that propulsion and production processes are supplied by RAM forces and electrical energy conversion by water turbines. The speed of the vessel increases along with increasing wind intensities to 9 kn at 7.4 Bft. This condition allows the vessel to store excess electrical energy in batteries to bridge energy gaps, e.g., during short-term wind depressions or during nautical maneuvers.
Results on near-surface wind speeds over the North Atlantic [41] demonstrate that sufficient wind intensities prevail in the ship’s planned cruising area for much of the year. The contour diagrams of the long-term observations (98. percentile) show sufficient wind speeds above 14.5 m/s (6.6 Bft) in the months of January to May and September to December. Lower wind speeds prevail in June, July, and August. This provides some hints that full wind operation could be possible during at least three-quarters of the year. The aquaculture vessel will not only operate in cruising areas with suitable sea surface temperatures but will also choose a course along the annual storm tracks in the North Atlantic [41]. In this way, wind forces could possibly be used year-round.
A passive roll-damping tank stabilizes the vessel’s motion in rough seas, although the vessel’s size and sail forces on a beam reach can dampen the vessel’s roll and pitch anyway. Floating offshore facilities exposed to waves can move and deform with large amplitudes. While hydroelasticity is not a problem and can be improved [12], anchored offshore aquaculture facilities can compromise worker accessibility and safety. This is different for an aquaculture production vessel, which can navigate to safe conditions on the open sea. Rendezvous with supply vessels can take place in regions where safe working is possible.
The ability to operate in any suitable area of the North Atlantic is an important distinction from fossil-free cargo ships [53], which must stick to their route to reach their destination on time. Sailing the North Atlantic exposes vessels to changes in wind direction and wind intensity, ranging from calm to fresh gale [54], which can be used for propulsion and energy conversion by water turbines. However, an in-depth analysis of wind fields and storm tracks was not part of this study and must be conducted as part of further detailed development.
Salmon aquaculture, like every business, aims for profitability, and profits must be guaranteed. The financial performance of the proposed aquaculture operation in a sail-powered process vessel was calculated on assumptions and data known from planning (Table 7). The economic feasibility and profitability of the innovative production process is demonstrated in Table 8.

Table 7.
Assumptions and input values used for the financial estimation of Atlantic salmon production in a sea-going process vessel that is operated by wind force and supplemental electrical energy produced from fuels (preferably green fuels).

Table 8.
Estimation of costs and revenues for the production of Atlantic salmon in a sea going process vessel that is operated by wind force and supplemental electrical energy produced from fuels (preferably green fuels).
A prerequisite is a continuous and predictable production process. This is provided by the aquaculture process described above. In addition, the production in separate and independent production lines enables regular harvest of fish in quantities that avoid a drifting down in price. The production conditions allows for the expectation of excellent fish quality, and the size of the harvested fish (4.5 kg) is in the preferred range of the markets [55]. The price was set at 11 EUR·kg−1 (Table 7), corresponding to the average German import price in 2024 [56].
Most of the input values tabulated in Table 7 were obtained from various inquiries. The input data still compare well to those used in a comparison of economic performance of closed containment systems and open-net-pen farms [13].
The investment costs (Table 7) are calculated from the steel weight of the vessel (25,000 tons). Assuming a price of EUR 3500 for every ton of fabricated steel, the construction costs for the vessel hull and superstructures amounts to EUR 88 million. The process equipment for the vessel and aquaculture production lines was estimated at EUR 25 million, and the cost of the sail system was estimated at EUR 20 million. The total investment, shown as capital expenses in Table 7, is estimated at EUR 133 million. The specific investment per ton of production for the aquaculture process vessel is two to five times higher than that for open-net pens or RASs [13,14]. In contrast, the lifetime of the process vessel is two to three times longer [13]. Ultimately, the economic result shown in Table 8 counts at the end.
The payback time was set to 15 years, which is less than half the service time of the vessel. The average always-on electrical power of 980 kW was interpolated from Table 4, Table 5 and Table 6 and Figure 8. This must be seen as a provisional value as the operation will strive for a full wind-powered operation without any energy conversion from fuel. This would cut the costs by EUR 1.3 million every year.
Operating an aquaculture vessel exclusively with fossil fuels is a questionable undertaking in light of climate change. At this stage of development, it is still unclear whether full-wind operation would be feasible year-round. However, full wind operation for at least three-quarters of the year would be an acceptable level given the significantly improved living conditions for the fish, leading to higher survival rates, consistent growth, and good health.
The carbon dioxide (CO2) emission for the production process, i.e. electrical power generation through combustion engines, was estimated at 0.46 kg CO2·kg−1 of fish production. This emission would be much lower compared to the emissions calculated for RAS production (3.5 kg CO2·kg−1 of fish production) [13]. Production in open-net cages is calculated at 0.16 kg CO2·kg−1 of fish production production [13], but it has higher calculated emissions for the feed compared to the RAS due to increased feed conversion (FCR). Better feed conversion and less feed losses can be assumed for the process vessel. The increased feed consumption in net cages would increase emission by 0.5 kg CO2·kg−1 of fish production [13]. Total carbon dioxide emissions would then amount to 0.66 kg CO2·kg−1 of fish production in net cages, which is about 40% higher than the estimated emissions for production in an aquaculture process vessel. The estimated carbon dioxide emissions for Atlantic salmon production in an aquaculture process vessel will not exceed the carbon dioxide emissions for net-cage production. It will remain minor in comparison to recirculating aquaculture systems. It should not be overlooked that in addition to the energetic CO2 emissions, further CO2 emissions for feed production, cooling, packaging and transport must be added, which were considered very carefully in the cited study [13].
The financial analysis in Table 8 is based on the calculation of production costs, capital costs, and various incidental costs. The total cost per kilogram of produced salmon is EUR 9.07. This is within the projected range for salmon production on the northeast Atlantic coast for nearshore aquaculture [12] but double the costs of Norwegian salmon farming calculated a decade ago [13] and still reported by the industry today [55]. However, this could indicate that higher spending will be required in the future to ensure the protection of marine resources.
The costs of items (Table 8) add up to EUR 30.46 million·a−1. Gross profit was calculated as the difference between revenues (EUR 36.96 million·a−1) and costs. The gross profit is therefore EUR 6.50 million·a−1 or EUR 1.93 per kg of fish. Expressed as a relative value, the gross profit is 18%. The financial estimates suggests that a positive result can be achieved, even though this analysis was based on preliminary data. After 15 years of operation, another 20 years of ship service time remain, yielding a higher gross profit.
Atlantic salmon aquaculture has become an important seafood producer. Aquaculture production currently exceeds fishery landings by 2000 times (Figure 9); fishery landings have been minimal throughout the past three decades. A reversal is unlikely. Illegal, unreported, and unregulated (IUU) fishing at sea is likely the cause of the lower numbers of returning adult fish [57], although a number of other impacts have been reported. According to FAO statistical data [19], the amount of salmon caught in 2023 was 1248 . In contrast, aquaculture produced 2,714,649 tons in the same year.
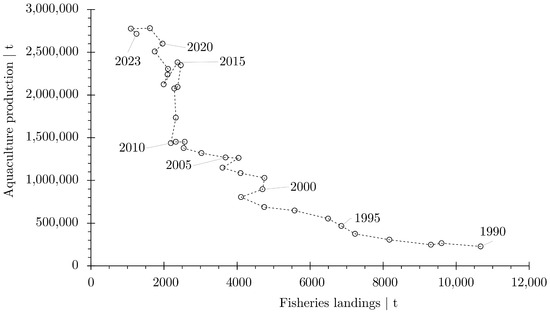
Figure 9.
Comparison of aquaculture production and fishery landings for Atlantic salmon compiled with statistical data from the FAO [19]. The diagram includes all data for Atlantic salmon (Salmo salar) for all areas and countries.
Salmon farming is an example of a well-developed aquaculture industry that provides seafood on a scale that could never otherwise be caught at sea. This distinguishes salmon farming from the aquaculture overoptimism expressed in the literature [58]. Indeed, we need to worry about declining natural populations as this is the source of genetic diversity, which is important for the resilience of populations in the wake of global changes. Sound aquaculture could continue to help protect salmon populations from critical exploitation through fisheries and environmental destruction of habitats and populations.
Salmon aquaculture is a contribution to the human supply [47] with healthy food. However, the annual growth rates for aquaculture production show decreasing trends, having negative values during 2022 and 2023 (Figure 10). The difficulties reported with open-net-pen production [18] and closed containment systems (RAS) [14] indicate that alternative production methodologies that are ready to be implemented in the existing structures of the salmon aquaculture industry are desirable. The concept presented here on an Atlantic salmon aquaculture system can be implemented worldwide.
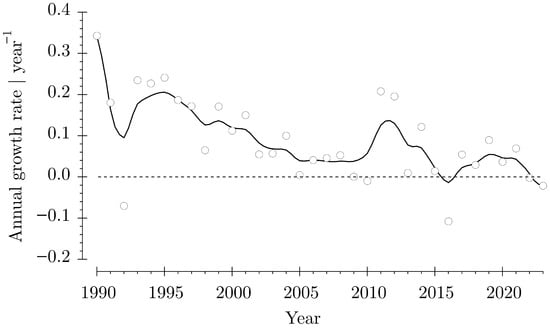
Figure 10.
Annual growth rate of Atlantic salmon aquaculture compiled with statistical data from the FAO [19]. The smoothed curve was computed with the Stineman function (KaleidaGraph 5.0, Synergie Software). The diagram includes all data for (Salmo salar) all areas and countries.
We believe that the process vessel represents a critical and timely development and complements existing salmon farming technology. Given the global impacts of land use [59] and the environmental impacts of conventional livestock farming, it appears necessary to harness the ocean for the production of healthy food. However, maintaining ecosystem functionality and minimizing resource consumption is essential. This requires that open-ocean aquaculture, like any other, be accompanied by biological research and monitoring, similar to meteorology or oceanography, to obtain data to assess the ocean’s biogeochemical status. An aquaculture process vessel could participate in this research while ensuring seafood supplies.
5. Conclusions
Aquaculture of Atlantic salmon, a cold-water species, faces challenges. Rising sea temperatures are affecting fish in open-net pens. Recirculating systems could offer an alternative, provided species-appropriate living conditions are maintained. However, this remains a challenge for the industry. Seawater in flow-through systems is not a sound environmental solution. Salmon farming must deploy innovative methods, including using the open sea, to meet future challenges and achieve globally agreed sustainability goals.
Salmon farming must relocate production far away from coastal ecosystems. The North Atlantic provides the optimal conditions for production, as it is the natural habitat of the species. This requires a fully maneuverable aquaculture process vessel operating along the 14° isotherm and shifting its operating area according to the seasons.
Open-ocean aquaculture in a process vessel is being integrated into the biogeochemistry of the ocean, as the impacts of fishing on these large ecosystems are evident. Paradoxically, the depletion of the nutrient pool through fisheries opens a niche for aquaculture. Given the ecological impacts of land use and eutrophication along coastal areas, it seems logical and timely to reconsider the use of the open ocean for food production through aquaculture.
This preliminary design study investigates the biological and technical feasibility of an aquaculture process vessel. It is based on standard scientific processes conducted in an interdisciplinary manner. However, it does not claim to be a complete description, as this would be part of the subsequent detailed design planning phase.
An aquaculture process vessel is a technically and economically viable solution that contributes to the seafood supply. The quality of the produced fish is excellent, as the fish is produced within the natural oceanic range of the species. The cost and revenue estimates result in a higher gross profit than can be achieved on average in agricultural production and the processing industry.
Atlantic salmon has become an important commodity in the food market as aquaculture has evolved into a well-structured and cooperative industry. New production technologies such as the aquaculture process vessel describe here, which considers both environmental aspects and the impacts of global change, will help stabilize production. A fully maneuverable aquaculture process vessel can be deployed across the oceans.
Author Contributions
Conceptualization, U.W.; methodology, H.J., K.K., and U.W.; software, H.J. and K.K.; validation, H.J., K.K., and U.W.; writing, U.W.; review and editing, U.W., H.J., and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Gerhard Braun and Stefan Weißkircher for their valuable contributions to this publication. Last but not least, we thank our students at the Saarland University of Applied Sciences for their interest in the project, whose questions and answers provided valuable support and motivation.
Conflicts of Interest
Author Harald Jensen is employed by the company SDC, Ship Design an Consulting GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Marine Harvest. Salmon Farming Industry Handbook. 2018. Available online: https://mowi.com/wp-content/uploads/2019/04/2018-salmon-industry-handbook-1.pdf (accessed on 8 April 2025).
- Bergfjord, O.J. Risk perception and risk management in Norwegian aquaculture. J. Risk Res. 2009, 12, 91–104. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G.W. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Cheng, L.; Abraham, J.P.; Hausfather, Z.; Trenberth, K.E. How fast are the oceans warming? Science 2019, 363, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Rikardsen, A.H.; Righton, D.A.; Strøm, J.F.; Thorstad, E.B.; Gargan, P.G.; Sheehan, T.F.; Økland, F.; Chittenden, C.M.; Hedger, R.D.; Næsje, T.F.; et al. Redefining the oceanic distribution of Atlantic salmon. Sci. Rep. 2021, 11, 12266. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef]
- Taranger, G.L.; Karlsen, Ø.; Bannister, R.J.; Glover, K.A.; Husa, V.; Karlsbakk, E.; Kvamme, B.O.; Boxaspen, K.K.; Bjørn, P.A.; Finstad, B.; et al. Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES J. Mar. Sci. 2015, 72, 997–1021. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Nilsson, A.; Buschmann, A.H.; Kautsky, N.; Kautsky, L. Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 1997, 156, 45–61. [Google Scholar] [CrossRef]
- Berntsson, E.V.; Stevik, T.K.; Bergheim, A.; Persson, D.; Stormoen, M.; Liland, K.H. Managing the Dissolved Oxygen Balance of Open Atlantic Salmon Sea Cages: A Narrative Review. Rev. Aquac. 2024, 17, e12992. [Google Scholar] [CrossRef]
- Mohapatra, S.C.; Guedes Soares, C. A Review of the Hydroelastic Theoretical Models of Floating Porous Nets and Floaters for Offshore Aquaculture. J. Mar. Sci. Eng. 2024, 12, 1699. [Google Scholar] [CrossRef]
- Ewig, G.; Hasankhani, A.; Won, E.T.; Haji, M. Marine spatial planning techniques with a case study on wave-powered offshore aquaculture farms. Renew. Energy 2025, 238, 121791. [Google Scholar] [CrossRef]
- Liu, Y.; Rosten, T.W.; Henriksen, K.L.; Hognes, E.S.; Summerfelt, S.T.; Vinci, B. Comparative economic performance and carbon footprint of two farming models for producing Atlantic salmon (Salmo salar): Land-based closed containment system in freshwater and open net pen in seawater. Aquac. Eng. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Golfand, I. Economics of growing salmon in recirculating aquaculture systems (RAS). J. Aquac. Mar. Biol. 2023, 12, 99–102. [Google Scholar] [CrossRef]
- Waller, U. A Critical Assessment of the Process and Logic Behind Fish Production in Marine Recirculating Aquaculture Systems. Fishes 2024, 9, 431. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, R.; Gu, J.; Wei, Q.; Hu, F.; Xu, X.; Zhang, Z.; Li, Z. Sloshing Response of an Aquaculture Vessel: An Experimental Study. J. Mar. Sci. Eng. 2023, 11, 2122. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Yin, F.; Liu, H.; Cui, M. Aquaculture in an Offshore Ship: An On-Site Test of Large Yellow Croaker (Larimichthys crocea). J. Mar. Sci. Eng. 2023, 11, 101. [Google Scholar] [CrossRef]
- Singh, G.G.; Sajid, Z.; Mather, C. Quantitative analysis of mass mortality events in salmon aquaculture shows increasing scale of fish loss events around the world. Sci. Rep. 2024, 14, 3763. [Google Scholar] [CrossRef]
- FAO. FishStat: Global capture production 1950–2023. In FishStatJ; FAO: Rome, Italy, 2025; Available online: www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 28 March 2025).
- ICES. Workshop on Assessing the Impact of Fishing on Oceanic Carbon (WKFISHCARBON; outputs from 2023 meeting). ICES Sci. Rep. 2004, 6, 1–63. [Google Scholar] [CrossRef]
- Jennings, S.; Rod, W. Wilson. Fishing impacts on the marine inorganic carbon cycle. J. Appl. Ecol. 2009, 46, 976–982. [Google Scholar] [CrossRef]
- Walsh, P.J.; Blackwelder, P.L.; Gill, K.A.; Danulat, E.; Mommsen, T.P. Carbonate deposits in marine fish intestines: A new source of biomineralization. Limnol. Oceanogr. 1991, 36, 1227–1232. [Google Scholar] [CrossRef]
- Cavan, E.L.; Hill, S.L. Commercial fishery disturbance of the global ocean biological carbon sink. Glob. Change Biol. 2021, 28, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.B.; Clark, B.; York, R.; Jorgenson, A.K. Aquaculture and the displacement of fisheries captures. Conserv. Biol. 2019, 33, 832–841. [Google Scholar] [CrossRef] [PubMed]
- González Ortiz, A.A.; Walsworth, T.E.; Hammill, E.; Palomares, M.L.D.; Pauly, D.; Atwood, T.B. Fisheries disrupt marine nutrient cycles through biomass extraction. Commun. Earth Environ. 2025, 6, 277. [Google Scholar] [CrossRef]
- Maranger, R.; Caraco, N.F.; Duhamel, J.; Amyot, M. Nitrogen transfer from sea to land via commercial fisheries. Nat. Geosci. 2008, 1, 111–112. [Google Scholar] [CrossRef]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Cosby, A.G.; Lebakula, V.; Smith, C.N.; Wanik, D.W.; Bergene, K.; Rose, A.N.; Swanson, D.; Bloom, D.E. Accelerating growth of human coastal populations at the global and continent levels: 2000–2018. Sci. Rep. 2024, 14, 22489. [Google Scholar] [CrossRef] [PubMed]
- Austreng, E.; Storebakken, T.; Åsgård, T. Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture 1987, 60, 157–160. [Google Scholar] [CrossRef]
- Einen, O.; Roem, A.J. Dietary protein/energy ratios for Atlantic salmon in relation to fish size: Growth, feed utilization and slaughter quality. Aquac. Nutr. 1997, 3, 115–126. [Google Scholar] [CrossRef]
- Folkedal, O.; Stien, L.H.; Nilsson, J.; Torgersen, T.; Fosseidengen, J.E.; Oppedal, F. Sea caged Atlantic salmon display size-dependent swimming depth. Aquat. Living Resour. 2012, 25, 143–149. [Google Scholar] [CrossRef]
- Hvas, M.M.; Folkedal, O.; Oppedal, F. Fish welfare in offshore salmon aquaculture. Rev. Aquac. 2021, 13, 836–852. [Google Scholar] [CrossRef]
- Timmerhaus, G.; Lazado, C.C.; Cabillon, N.A.R.; Reiten, B.K.M.; Johansen, L.-H. The optimum velocity for Atlantic salmon post-smolts in RAS is a compromise between muscle growth and fish welfare. Aquaculture 2021, 532, 736076. [Google Scholar] [CrossRef]
- Farrell, A.P.; Steffensen, J.F. An analysis of the energetic cost of the branchial and cardiac pumps during sustained swimming in trout. Fish Physiol. Biochem. 1987, 4, 73–79. [Google Scholar] [CrossRef]
- Kraul, S.; Szyper, J.P.; Burke, B. Practical Formulas for Computing Water Exchange Rates. Progress.-Fish-Cult. 1985, 47, 69–70. [Google Scholar] [CrossRef]
- Bohl, W.; Elmendorf, W. Technische Strömungslehre, 15th ed.; Vogel Verlag: Wuerzburg, Germany, 2014; 504p. [Google Scholar]
- Stehfest, K.M.; Carter, C.G.; Mcallister, J.D.; Ross, J.D.; Semmens, J.M. Response of Atlantic salmon Salmo salar to temperature and dissolved oxygen extremes established using animal-borne environmental sensors. Sci. Rep. 2017, 7, 4545. [Google Scholar] [CrossRef]
- Remen, M.; Sievers, M.; Torgersen, T.; Oppedal, F. The oxygen threshold for maximal feed intake of Atlantic salmon post-smolts is highly temperature-dependent. Aquaculture 2016, 464, 582–592. [Google Scholar] [CrossRef]
- van Lammeren, W.P.A.; van Manen, J.D.; Oosterveld, M.W.C. The Wageningen B-Screw Series. In Proceedings of the Society of Naval Architects ans Marine Engineers, Annual Meeting, New York, NY, USA, 12 November 1969. [Google Scholar]
- Anonymous. Software and Data Services for Ship Design and Operation. Available online: http://www.napa.fi (accessed on 10 June 2025).
- Laurila, T.K.; Sinclair, V.A.; Gregow, H. Climatology, variability, and trends in near-surface wind speeds over the North Atlantic and Europe during 1979–2018 based on ERA5. Int. J. Climatol. 2020, 41, 2253–2278. [Google Scholar] [CrossRef]
- Brown, A.R.; Wilson, R.W.; Tyler, C.R. Assessing the Benefits and Challenges of Recirculating Aquaculture Systems (RAS) for Atlantic Salmon Production. Rev. Fish. Sci. Aquac. 2024, 33, 380–401. [Google Scholar] [CrossRef]
- Yogev, U.; Sowers, K.R.; Mozes, N.; Gross, A. Nitrogen and carbon balance in a novel near-zero water exchange saline recirculating aquaculture system. Aquaculture 2017, 467, 118–126. [Google Scholar] [CrossRef]
- Waller, U.; Buhmann, A.K.; Ernst, A.; Hanke, V.; Kulakowski, A.; Wecker, B.; Orellana, J.; Papenbrock, J. Integrated multi-trophic aquaculture in a zero-exchange recirculation aquaculture system for marine fish and hydroponic halophyte production. Aquac. Int. 2015, 23, 1473–1489. [Google Scholar] [CrossRef]
- Misund, A.; Thorvaldsen, T.; Strand, A.V.; Oftebro, T.L.; Dahle, S.W. Opportunities and challenges in new production systems for salmon farming in Norway—Industry perspective. Mar. Policy 2024, 170, 106394. [Google Scholar] [CrossRef]
- Kennish, M.J. Eutrophication of Estuarine and Coastal Marine Environments: An Emerging Climatic-Driven Paradigm Shift. Open J. Ecol. 2025, 15, 289–324. [Google Scholar] [CrossRef]
- Reid, G.H.; Gurney-Smith, H.; Flaherty, M.S.; Garber, A.; Forster, I.; Brewer-Dalton, K.; Knowler, D.; Marcogliese, D.; Chopin, T.; Moccia, R.; et al. Climate change and aquaculture: Considering adaptation potential. Aquac. Environ. Interact. 2019, 11, 603–624. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Wang, X. The effect of stocking density on growth and seven physiological parameters with assessment of their potential as stress response indicators for the Atlantic salmon (Salmo salar). Mar. Freshw. Behav. Physiol. 2015, 48, 177–192. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Sun, G. Effects of stocking density on growth performance and welfare-related physiological parameters of Atlantic salmon Salmo salar L. in recirculating aquaculture system. Aquac. Res. 2015, 48, 2133–2144. [Google Scholar] [CrossRef]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbesson, L.O.; Pedrosa, C.; Fivelstad, S.; Hosfeld, A.D.; Stefansson, S.O.; Terjesen, B.F.; Takle, H.; et al. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) emphasis on production performance and welfare. Aquaculture 2017, 468, 363–370. [Google Scholar] [CrossRef]
- Reid, D.E.; Armstrong, J.D.; Metcalfe, N.B. Estimated standard metabolic rate interacts with territory quality and density to determine the growth rates of juvenile Atlantic salmon. Funct. Ecol. 2011, 25, 1360–1367. [Google Scholar] [CrossRef]
- Krkošek, M. Host density thresholds and disease control for fisheries and aquaculture. Aquac. Environ. Interact. 2010, 1, 21–32. [Google Scholar] [CrossRef]
- Julià, E.; Tillig, F.; Ringsberg, J.W. Concept Design and Performance Evaluation of a Fossil-Free Operated Cargo Ship with Unlimited Range. Sustainability 2020, 12, 6609. [Google Scholar] [CrossRef]
- Atkinson, G.; Nguyen, H.D.; Binns, J. Considerations regarding the use of rigid sails on modern powered ships. Cogent Eng. 2018, 5, 1543564. [Google Scholar] [CrossRef]
- Mowi Salmon Farming Industry Handbook. 2024. Available online: https://mowi.com/wp-content/uploads/2024/05/2024-Salmon-Industry-Handbook.pdf (accessed on 8 April 2025).
- Bundesanstalt für Landwirtschaft und Ernährung, Referat 531—Fischereimanagement. Bericht über die Fischerei und die Marktsituation für Fischereierzeugnisse in der Bundesrepublik Deutschland. 2024. Available online: https://www.ble.de/SharedDocs/Downloads/DE/Fischerei/Fischwirtschaft/Monatsbericht2024/Monatsbericht_24_12.pdf?__blob=publicationFile&v=2 (accessed on 9 August 2024).
- Dadswell, M.J.; Spares, A.D.; Reader, J.M.; Mclean, M.F.; Mcdermott, T.; Samways, K.M.; Lilly, J. The Decline and Impending Collapse of the Atlantic Salmon (Salmo salar) Population in the North Atlantic Ocean: A Review of Possible Causes. Rev. Fish. Sci. Aquac. 2021, 30, 215–258. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Pierruci, A.; Oyinlola, M.A.; Cannas, R.; Froese, R.; Glaser, S.; Jacquet, J.; Kaiser, B.A.; Issifu, I.; Micheli, F.; et al. Aquaculture over-optimism? Front. Mar. Sci. 2022, 9, 984354. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.S.; Asner, G.P.; Barford, C.; Bonan, G.B.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Coe, M.T.; Daily, G.C.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).