1. Introduction
The cultivation of
Ulva intestinalis, a green macroalga commonly found in temperate and brackish waters, holds significant promise for sustainable aquaculture and bioremediation [
1,
2]. In the Baltic Sea region, high seawater nutrient concentrations present a challenge for sustainability [
3] but cultivating low-trophic aquaculture like fast-growing
Ulva seaweed, could offer a solution to improve the region’s environmental conditions [
4]. However, low salinity, along with significant spatial variability and temporal fluctuations in temperature, light, and nutrient levels, presents challenges for the development of this type of aquaculture [
5]. There is increasing interest in exploring how
U. intestinalis can be effectively cultivated in the Baltic Sea regions [
6]; however, there have been no real success stories to date. This species, like many others, thrives under wide range of environmental conditions, but facilitating growth in aquaculture settings has proven to be complex [
7,
8,
9].
The cultivation of
U. intestinalis involve several processes that require careful optimization [
10]. One of the most important stages is the induction of sporulation, as this determines when and how the alga releases its spores, which are essential for the formation of new thalli and their attachment to substrates [
11]. To stimulate sporulation, environmental factors such as temperature, light, and nutrient concentration must be diligently controlled [
12]. Without optimal regulation of these conditions, sporulation may either fail to initiate or occur at insufficient levels, hindering the subsequent growth phases [
12].
Another crucial stage is the growth of
U. intestinalis in the marine environment, which is often unpredictable due to competition, and losses caused by storm casts and grazers, all of which can impact its growth and survival [
13]. The high variability in environmental conditions of the Baltic Sea [
14] makes achieving stable biomass yields even more challenging [
15]. Experiences from other regions have shown that successful cultivation of
Ulva species is possible [
16,
17] but achieving the same success in the Baltic Sea has not yet been realized. This highlights the need for a deeper understanding of the efficiency of various sporulation and cultivation techniques in Baltic Sea conditions to ensure success in this region [
18]. Lessons from other seas provide a foundation for assuming that, under the right conditions, the cultivation of
U. intestinalis is possible [
12,
19,
20], but testing and refining suitable methods specific to the Baltic Sea are essential.
This study addresses key bottlenecks in the cultivation of Ulva intestinalis in the Baltic Sea by investigating:
What laboratory conditions (e.g., light, temperature, nutrients) promote sporulation and attachment to artificial substrates? Understanding the specific thresholds and interactions among environmental variables is essential for triggering reliable sporulation [
11,
12]. This includes identifying the timing and duration of exposure needed to achieve consistent spore release and settlement, which can inform standardized hatchery protocols for initiating cultivation [
19].
Can
U. intestinalis naturally attach to substrates deployed in suitable Baltic Sea environments? Field deployment of substrates will assess whether passive attachment can occur under ambient conditions and determine the spatial and temporal windows most favorable for natural recruitment. These insights could reduce dependence on controlled pre-cultivation steps and simplify large-scale operations, particularly in low-salinity areas where
Ulva is already dominant [
21].
If initial methods are ineffective, what alternative approaches could support large-scale cultivation under local conditions? Alternative strategies may include pre-conditioning substrates, modifying deployment techniques, or exploring different life stages for introduction [
18]. Identifying fallback options is crucial to adapt cultivation systems to site-specific limitations while maintaining feasibility and resilience in the face of grazing, competition, and hydrodynamic stress [
7,
15].
As the first pilot experiments in the region, they provide essential input for developing informed strategies that support more detailed trials and future scaled-up production.
2. Materials and Methods
On 26 August 2020, Ulva intestinalis (Chlorophyta) was collected from Kõiguste Bay, Gulf of Riga (58.37° N, 22.97° E). The Ulva species used in the experiments was genotyped and confirmed as U. intestinalis, as part of the SeaWheat COST Action (CA 20106—Tomorrow’s ‘Wheat of the Sea’: Ulva, a Model for an Innovative Mariculture).
An initial preliminary experiment was conducted to assess sporulation intensity under cold and warm water conditions, as blooms of
U. intestinalis are regularly observed under both scenarios in the study area. On August 28, approximately 5 g (wet weight) of live
U. intestinalis were placed in each of four Petri dishes, and 30 mL of seawater was added to each dish to simulate natural submergence shifts. Darker patches of thallus fragments exhibiting early signs of sporogenesis, identified through detailed morphological examination of reproductive structures—a standard practice in similar studies—were selected for the experiment (
Figure 1). DNA analysis to confirm ploidy (n or 2n) was not conducted. Two dishes were stored in a refrigerator at 4 °C to simulate cold marine conditions, while the other two were kept at 20 °C in a shaded indoor laboratory. All dishes were maintained at constant salinity (5.8) and exposed to a natural light cycle (12 h light:12 h dark).
Thalli in each dish were inspected daily, noting areas where zoospores were forming. Five days later, on 1 September, coverslips were placed in each dish to enable zoospore attachment and were subsequently used for regular microscopic spore counts.
On day 13 (10 September), the coverslips were transferred to four separate containers, each filled with 5 L of seawater and supplemented with 5 g of AZOFOSKA fertilizer (15% total nitrogen, 15% total phosphorus) to support algal growth. All containers were maintained at 20 °C, salinity 5.8, and a 12 h light:12 h dark regime. Aeration was continuous. For five consecutive days, five coverslips from each container (n = 5) were analyzed daily to monitor zoospore development and presence. Zoospore density was determined by directly observing the coverslips under an upright light microscope at 100× to 400× magnification. Spores were counted within randomly selected fields of view to estimate the overall density on the coverslip surface.
A two-sample t-test was conducted using R version 4.3.2 to compare zoospore densities at two temperature levels (4 °C and 20 °C). Statistical summaries and visualizations were generated using the ggplot2 and dplyr packages.
Results from Experiment 1 indicated that warmer temperatures favored zoospore production. Building on this, Experiment 2 was designed to investigate sporulation intensity in finer temperature increments: 20 °C, 22 °C, 24 °C, and 26 °C. The temperature treatments were chosen to reflect the typical summer conditions (19–25 °C) of the study area, where U. intestinalis blooms are most frequently observed.
The experiment began on 10 September 2020. Each of the four containers was filled with 10 L of seawater and supplemented with 50 g of U. intestinalis and 50 g of AZOFOSKA fertilizer to provide nutrients. Coverslips were placed in each container to enable spore attachment. All containers were maintained at a salinity of 5.8, a 12 h light:12 h dark cycle, and equipped with aeration. For nine consecutive days, coverslips (n = 5) from each temperature treatment were collected daily from each container for spore quantification.
A second-order polynomial regression was used to assess the relationship between temperature and zoospore density. The analysis and plotting were performed in R using the ggplot2, and dplyr packages.
On 23 September, Ulva thallus pieces (approximately 1 g wet weight) were placed in separate 1-L containers filled with filtered natural seawater at an indoor laboratory facility. Fertilizer AZOFOSKA was added in concentrations ranging from 1 to 10 g per liter. A control treatment without fertilizer was included. To assess sporogenesis, coverslips were placed in each container. The containers were maintained at a constant temperature (22 °C), salinity (5.8), and a natural light regime of 12 h light and 12 h dark. pH fluctuated slightly between 8.0 and 8.2. Aeration was continuous. For five consecutive days, coverslips (n = 5) were collected daily from each container for spore quantification.
A second-order polynomial regression was used to assess the relationship between nutrient concentration and zoospore density. The analysis and plotting were performed in R using the ggplot2, and dplyr packages.
On 22 July 2020, U. intestinalis material was collected from two different locations: the Kesknõmme (58.44° N, 22.06° E) and the Saaremaa harbor area (58.53° N, 22.24° E). This dual-source collection was designed to assess variations in sporogenesis based on the health and condition of the algae.
Upon microscopic examination, the material from Kesknõmme displayed poor health—thallus color ranged from light green to yellow, and cells were partially empty, indicating stress or degradation. However, some fragments showed darker spots and regions of active sporogenesis, including areas with emerged spores. In contrast, the material from the Saaremaa harbor was in excellent condition, with thalli exhibiting a dark green color and chloroplasts filling most of the cell space. Some of this material was collected with its attachment substrate to minimize disturbance to the plants.
On 23 July, the collected materials were separately housed in containers to prevent cross-contamination. The algal biomass was placed at the bottom of the containers, which were then filled with natural seawater to ensure complete coverage of the algae. Half of the containers were maintained at natural seawater nutrient levels, while the other half were enriched with AZOFOSKA fertilizer at a concentration of 5 g/L. All containers were maintained at a salinity of 5.8, a 12 h light:12 h dark cycle, and equipped with aeration. pH fluctuated between 7.6 and 8.5. The water in the containers was refreshed weekly to prevent the buildup of waste products and maintain adequate nutrient levels.
Various types of artificial substrates, including plastic floats and ropes, were introduced into the containers. The objective was to observe the attachment efficiency and growth patterns of U. intestinalis zoospores on these substrates. The condition of the algae and the development of new attachments were monitored regularly.
On 23 July, different types of artificial substrates, including various ropes and synthetic mesh, were placed into six 40 L tanks. In addition, each tank was supplemented with algal mass that had been blended to induce sporulation, a common practice in Ulva cultivation farms. To explore the effect of salinity on algal attachment, the salinity of three tanks was increased to 12 ppt, while the salinity in the remaining tanks was kept unchanged. All containers were maintained at a salinity of 5.8, a 12 h light:12 h dark cycle, and equipped with aeration. pH fluctuated between 7.6 and 8.5. The water in the containers was refreshed weekly.
Observations were conducted at intervals of 7, 14, and 20 days to assess the algae’s attachment to the substrates under the varied conditions. During these observations, notes were taken regarding the health of the algal material, including signs of degradation or color change, which could indicate adverse conditions.
On 22 July 2020, U. intestinalis attached to its natural substrate collected from Saaremaa Harbor was placed into two large containers, each with a capacity of 50 L, which were then filled with seawater. In addition, Ulva intestinalis material that had been detached from the substrate (approximately 50 g wet weight) was placed into two similar containers to compare the effects of substrate presence on the health and sporogenesis of the algae.
All containers were maintained at a salinity of 5.8, a 12 h light:12 h dark cycle, and equipped with aeration. pH fluctuated between 7.6 and 8.5. The water in these containers was exchanged weekly.
The health of the algae and the development of zoospores were closely monitored over a two-month period. Observations included the general health and color of the algae, the presence and development of zoospores on the algae thallus, and the abundance of other microscopic organisms within the container environment. This included organisms on the surface of the U. intestinalis thalli as well as on the inner walls of the containers.
On 12 May 2020, U. intestinalis attached to natural substrates was collected from Kõiguste Bay (58.37° N, 22.97° E) and were placed in containers filled with natural seawater. The next day, artificial substrates made of ropes and mesh with various surface textures were added to each container. These substrates remained in the containers for two weeks to allow for initial attachment and growth. All containers were maintained at a salinity of 5.8, a 12 h light:12 h dark cycle, and equipped with aeration. The water in these containers was exchanged weekly.
On 27 May, the substrates, now hosting U. intestinalis juveniles, were deployed in Kõiguste Bay. These substrates were periodically observed over the following months to assess growth and attachment success.
On 9 August 2021, U. intestinalis was collected from the Kõiguste Bay area. Immediately following collection, the algae were transferred to a net cage anchored in the same bay. This cage was constructed with 1 mm mesh netting to ensure adequate water flow while preventing the algae from escaping. The cage had a diameter of 1 m, and the mesh extended down to a depth of 0.5 m.
The water temperature during the experiment varied between 17 °C and 19 °C, which is considered optimal for the growth of U. intestinalis. The first week of the experiment experienced sunny weather, which likely enhanced growth conditions. However, the weather turned mostly cloudy and persistently stormy in the last two weeks, which could have impacted the growth conditions adversely.
The growth of U. intestinalis within the net cage was documented daily, with three measurements taken each day (n = 3). The growth of Ulva was measured by determining the total wet weight of the algae in the net cage, with a precision of 0.01 g. Before weighing, the seaweed was gently pressed between blotting paper to remove excess water. The entire weighing process took less than 5 min, after which the seaweed was promptly returned to the cage. At the start of the experiment, replicate measurements of the same Ulva batch were conducted to assess any potential errors in wet weight determination. Results indicated that the weighing error was less than 0.1% of the total biomass. This seaweed was then dried at 40 °C for two weeks to establish a wet-to-dry weight conversion factor, enabling daily Ulva growth to be expressed in dry weight units.
3. Results
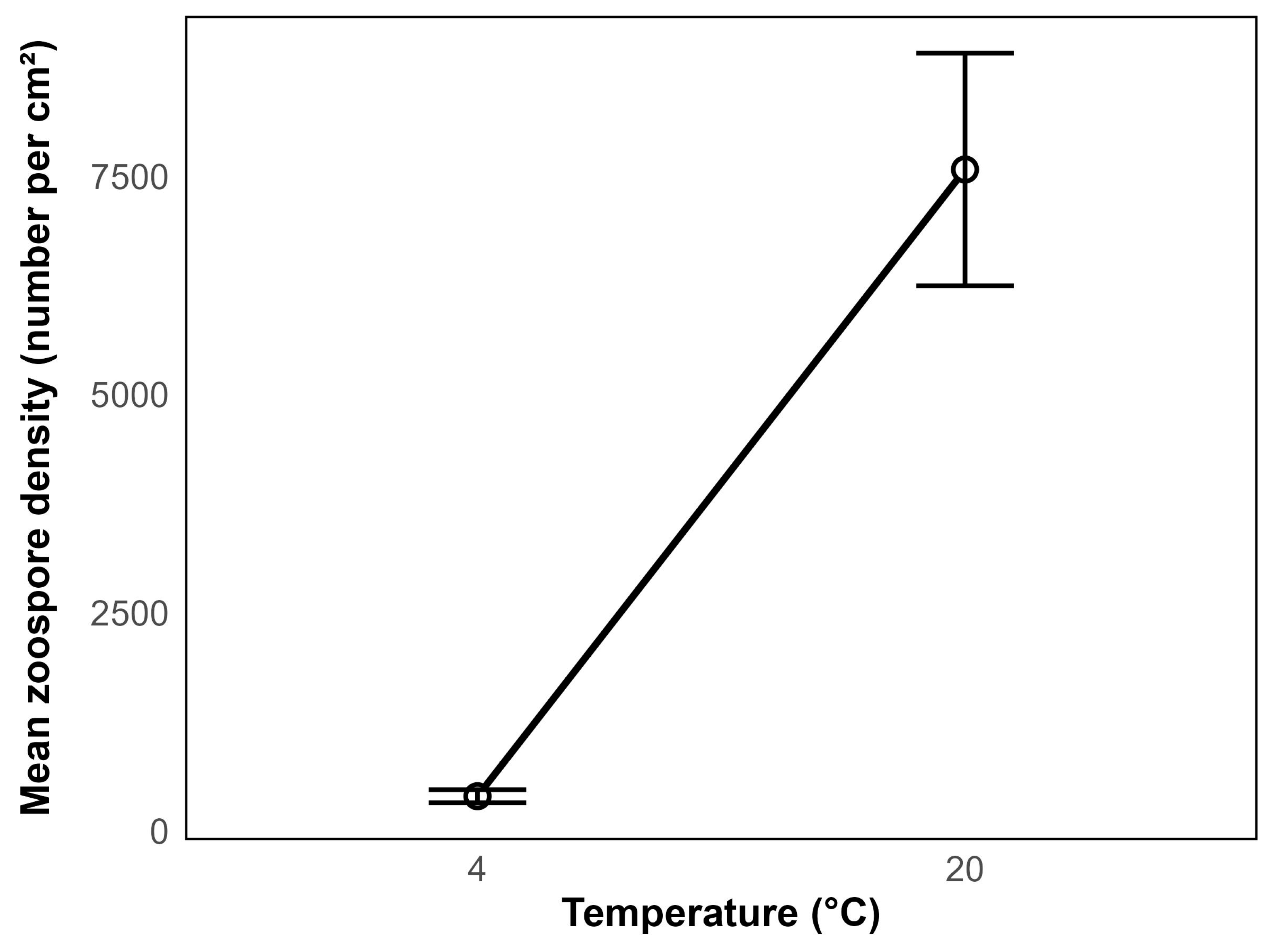
Experiments 1 demonstrated that zoospore production is highly sensitive to water temperature. The zoospore density was significantly higher at 20 °C compared to 4 °C (
p < 0.001) (
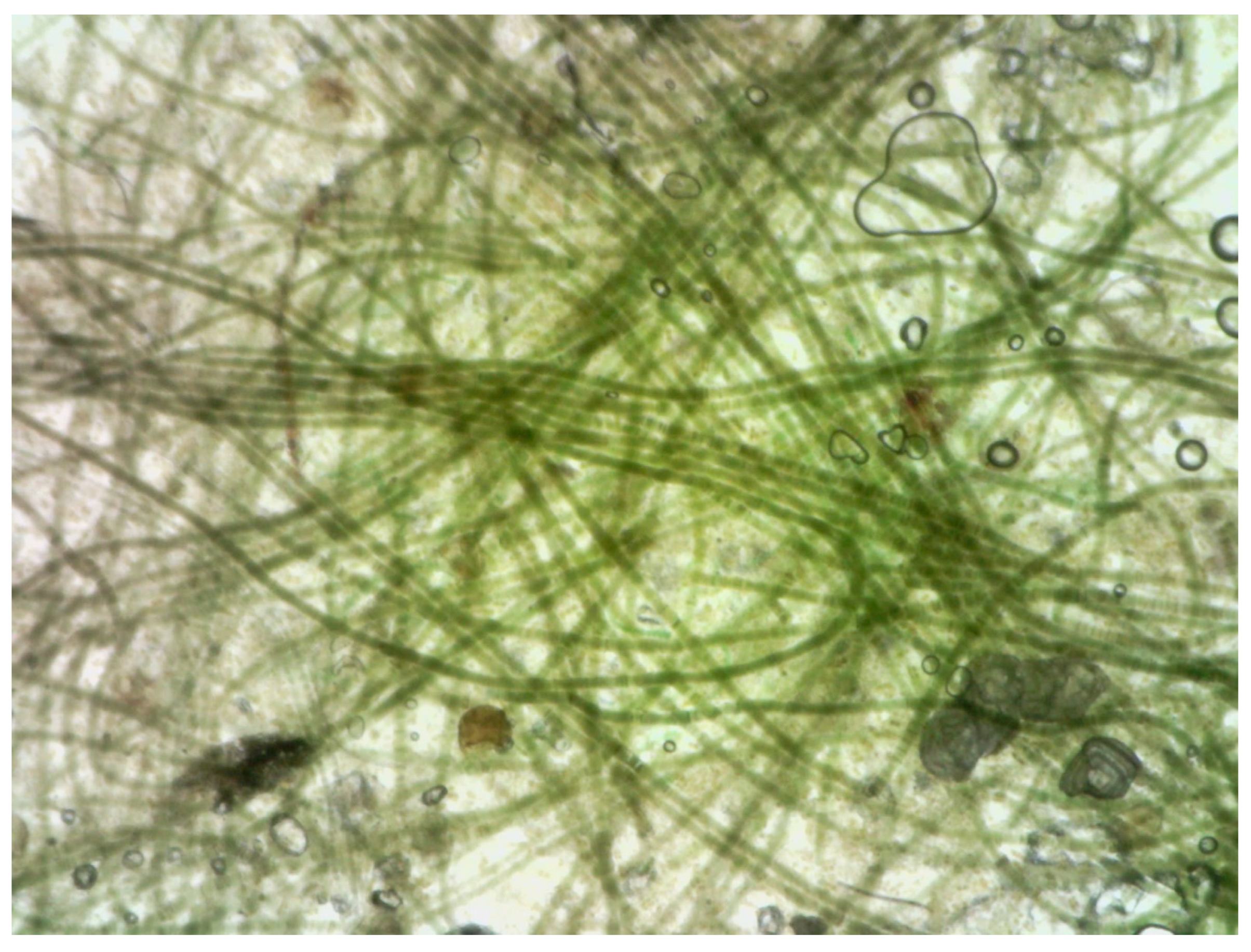
Figure 2). This suggests that increased temperature strongly enhances zoospore production under the given experimental conditions. Toward the end of the experiment, cyanobacteria (
Oscillatoria sp.) began to proliferate extensively in the aquaria, forming a fine greenish film that covered all available substrate. In the final stage of the experiment, no
Ulva plants could be found beneath the cyanobacterial layer (
Figure 3). As a result, all subsequent laboratory experiments were conducted using filtered or sterilized seawater.
Experiment 2, which focused on warmer conditions, showed that zoospore density exhibited a non-linear response to temperature, with the second-order polynomial regression revealing a peak at intermediate temperatures around 22–24 °C. Tanks with temperatures outside of this range, either higher or lower, showed reduced zoospore release (
Figure 4).
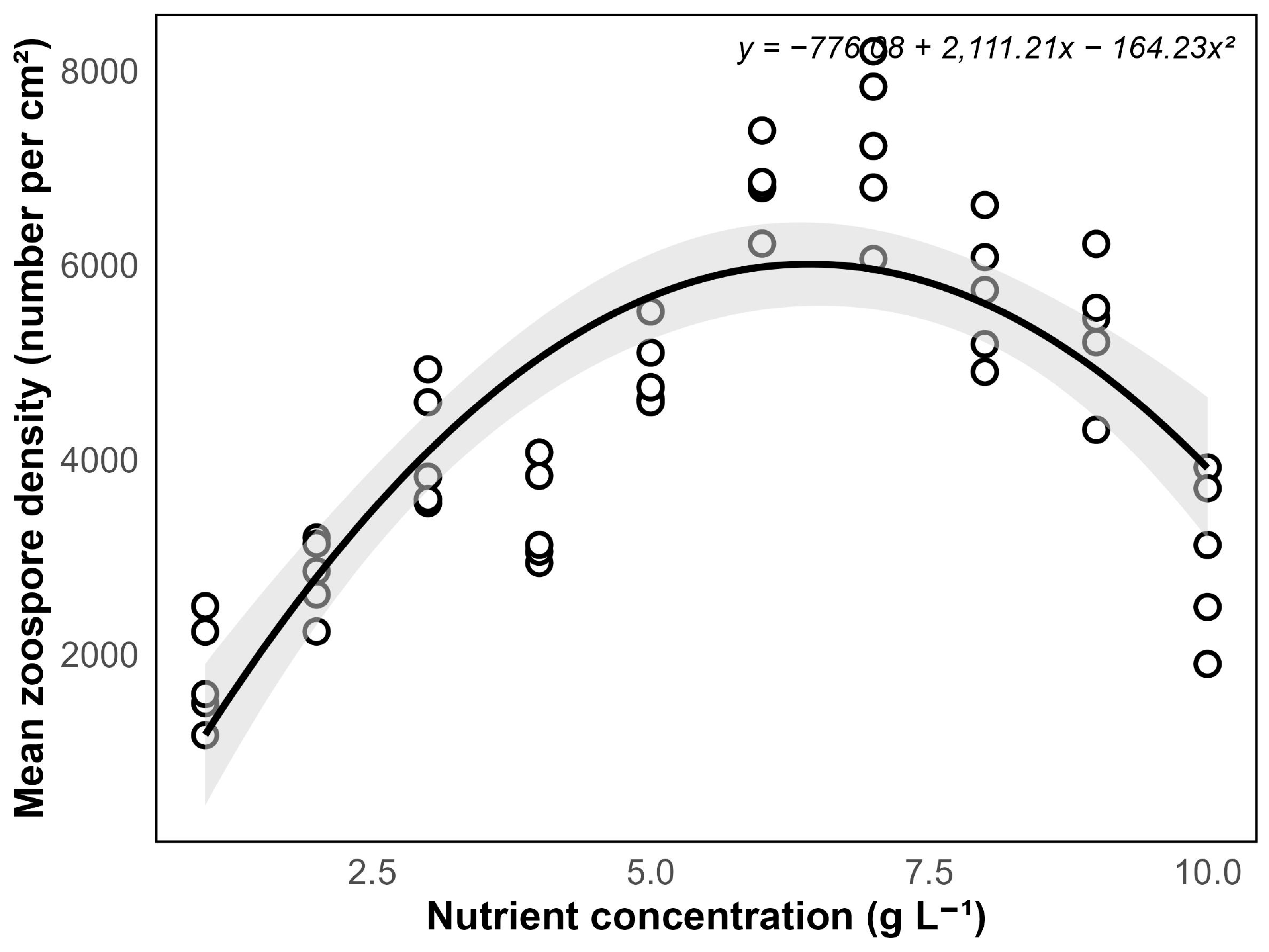
Experiment 3 demonstrated that nutrient concentration plays an important role in promoting sporogenesis. Zoospore formation was first observed within a week in containers with nutrient concentrations at 4–6 g/L. The highest zoospore production was observed around the third week, notably at a nutrient concentration of 6–7 g/L (
Figure 5). However, containers with higher nutrient concentrations of 8–10 g/L showed signs of cellular stress, including chloroplast separation and degradation, which likely impaired sporogenesis. By the fourth week, no further zoospore production was observed.
Experiments 4 and 5, conducted with various artificial substrates under controlled conditions, showed minimal initial attachment success and low zoospore production during the first week. However, the introduction of mineral and organic fertilizers significantly improved growth. Within five days of fertilization, significant green overgrowth, primarily
U. intestinalis, was observed (
Figure 6). In contrast, non-fertilized control substrates showed little to no growth. By the end of the experiment, the fertilized substrates showed extensive
Ulva coverage, confirming the importance of nutrient supplementation for successful attachment and growth. However, microbial overgrowth, particularly by diatoms and bacteria, persisted as a challenge that could interfere with the long-term sustainability of the cultivation process. Variations in salinity within the observed range had no impact on the outcome.
Experiment 6, a long-term maintenance study under controlled artificial conditions, demonstrated that U. intestinalis can remain viable without degradation for at least two months. The algal material remained stable throughout the experiment, and sporogenesis occurred at low levels. Despite the presence of zoospores, no new attachment or substantial growth was observed on the artificial substrates, suggesting that while Ulva can be maintained in artificial settings, further refinements in environmental conditions are required to stimulate more robust reproductive and attachment processes.
Experiments 7, which examined the attachment of U. intestinalis in natural marine environments, yielded mixed results. Initial attachment to substrates occurred within five days. However, this success was short-lived, as blue-green algae quickly colonized the available surfaces. This overgrowth formed a dense layer that hindered further Ulva growth and eventually obscured it from view. Further observations of the substrates confirmed limited Ulva growth, with the brown alga Pylaiella littoralis occupying most of the available space. Over time, other algal species such as Cladophora glomerata (Chlorophyta) and Ceramium tenuicorne (Rhodophyta) also colonized the substrates, leaving little room for U. intestinalis to establish itself. By the end of the experimental period, Ulva was largely absent from the artificial substrates, having been outcompeted by faster-growing and more opportunistic species. These results suggest that in mixed algal communities, Ulva struggles to compete for space and nutrients, particularly when faced with dominant species like Pylaiella and Cladophora.
Experiment 8, in which
U. intestinalis was cultivated in a mesh cage, offered a promising approach for cultivating the seaweed in open marine environments. In the initial phase,
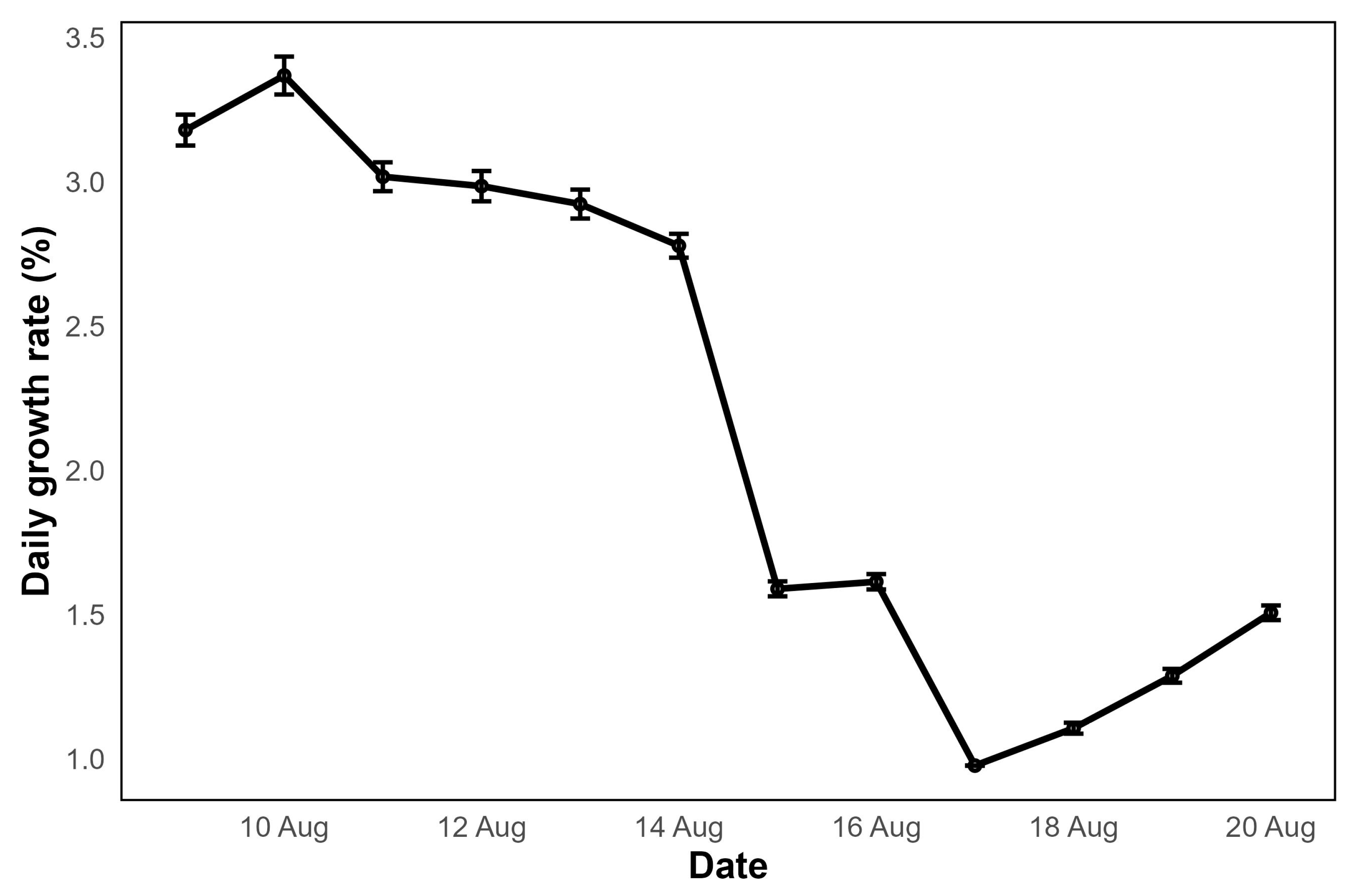
Ulva exhibited very rapid growth. However, during the second phase of the experiment, growth rates declined (
Figure 7) plausibly due to environmental factors such as water turbidity caused by frequent storms, which significantly reduced light availability.
4. Discussion
A key factor influencing
Ulva cultivation outcomes is the species’ broad environmental tolerance, which is especially important in the Baltic Sea, where strong spatial gradients and seasonal fluctuations in these environmental factors are common. Studies from the Baltic and North Seas have shown that
Ulva species (including
U. intestinalis and
U. fenestrata) thrive across salinity gradients and varying nutrient loads, maintaining growth from fully marine to mesohaline waters. However, their strong seasonality must be carefully considered in farming applications [
21,
22]. Importantly,
Ulva’s broad environmental tolerance also highlights its potential role in nutrient remediation, particularly under Baltic Sea conditions, as it can sustain growth in high-nutrient coastal waters while sequestering excess nitrogen and phosphorus. By linking these tolerances to cultivation practices, farmers can time and locate
Ulva deployments to align with optimal temperature and nutrient conditions while avoiding periods of extreme stress.
However,
Ulva’s adaptability presents the challenge of managing its sporulation patterns under changing conditions. Abrupt shifts in temperature or nutrient availability can trigger sporulation, during which
Ulva thalli release swarmers (zoids) and subsequently disintegrate, leading to sudden biomass loss. For example, in offshore cultivation trials, rising early summer temperatures and declining nutrient levels have been associated with increased fertility of
U. fenestrata fronds, evident through thallus perforation and tissue decay as the algae transition into their reproductive phase [
23]. Thus, when
Ulva experiences environmental stress or seasonal cues, such as rapid warming or nutrient depletion, it may divert energy to reproduction, leading to partial die-off of cultivated biomass. Understanding this relationship is essential for
Ulva farms in the Baltic Sea. On one hand, it can be used to promote successful sporulation and seeding of cultivation ropes. On the other hand, during rope deployment, mitigating stressors through smart site selection that favors stable microclimates can help reduce unintended sporulation and biomass loss. By fine-tuning cultivation timing and conditions, growers can keep
Ulva in its vegetative state longer, thereby sustaining higher yields and avoiding the “boom-and-bust” cycles associated with uncontrolled sporulation.
In the current study, the experiments on U. intestinalis sporogenesis, attachment and growth under varied environmental conditions highlighted challenges in its cultivation in both controlled and natural environments. Despite a long history of research in the Baltic Sea region, surprisingly little is known about the detailed ecology of Ulva species, including their temperature preferences. However, field observations indicate that Ulva blooms occur under both cold conditions (around 5 °C) and warmer conditions (around 20 °C). As our preliminary experiments suggested that sporulation intensity increases under warmer conditions, we focused on temperatures typical of the summer period in the study area, which generally range between 19 °C and 25 °C. This range guided the selection of temperature treatments for our experiment. Testing 16 °C and 18 °C was not prioritized, as these fall outside the main temperature window observed during peak bloom conditions.
Sporogenesis, although enhanced by specific nutrient concentrations (4–7 g/L) and optimal temperatures (22–24 °C), proved difficult to control. Artificial substrates showed promise in lab settings when supplemented with fertilizers; however, microbial overgrowth, particularly by diatoms and bacteria, persisted. In natural environments, Ulva had trouble attaching to substrates because faster-growing species like Pylaiella and Cladophora took over, suggesting that artificial substrates are not practical for large-scale open-water use.
Research in high-salinity marine environments, such as the Mediterranean Sea and Atlantic Ocean (salinity ~30), has demonstrated significantly higher success in
Ulva sporogenesis, attachment, and growth across varied nutrient and temperature conditions. In these environments,
Ulva species show faster growth within wider temperature and nutrient ranges, likely due to stable salinity and frequent favorable temperatures around 18–20 °C, which support their physiological processes. For example,
Ulva lactuca grown in high-salinity regions responds well to temperature shifts, achieving daily growth rates of up to 10–15% under controlled conditions, facilitating frequent sporogenesis and higher biomass yields through continuous nutrient absorption and rapid cell division [
24,
25]. Similarly, large-scale offshore sea farms in the North Sea, particularly on Sweden’s west coast, benefit from stable high salinity (~27–28) and moderate seasonal temperature fluctuations, leading to sustainable biomass production [
22]. In contrast, the Baltic Sea’s lower salinity (5–7) and pronounced seasonal temperature variability limit
Ulva’s adaptability, making cultivation more challenging and less resilient to environmental fluctuations. These conditions reduce nutrient uptake and cellular metabolism, significantly affecting growth and sporogenesis.
Thus, future studies should focus on refining the management of sporogenesis and growth under Baltic Sea conditions, potentially through controlled nutrient cycling and targeted environmental adjustments. Integrating closed-circuit fertilization or selective light filters could limit competitive species and boost
Ulva productivity [
23,
26]. Controlled experiments on seasonal temperature and nutrient fluctuations would further clarify
Ulva’s responses to the Baltic’s environmental conditions. Adapting successful high-salinity practices while addressing Baltic-specific challenges may establish sustainable
Ulva aquaculture and align with Baltic ecosystem management goals, including eutrophication mitigation [
22].
While models from the Mediterranean, North Sea, and Atlantic illustrate sustainable cultivation practices, adapting them to the Baltic’s low-salinity environment requires significant modification. Conventional techniques that succeed in higher salinities, such as open-water artificial substrates and nutrient supplementation, are less effective in the Baltic, where high nutrient levels often promote dense algal mats, increasing competition and hypoxia, which restrict
Ulva attachment and growth [
27]. Given these constraints, Baltic-specific approaches, like denser seeding to counter limited growth conditions, may prove beneficial [
22]. Moreover, adaptations for Baltic cultivation could include pre-treating seedlings in controlled hatcheries and refining nutrient supply. Testing
Ulva strains with higher tolerance to low salinity and variable conditions could further improve growth.
In practical terms, this also indicates that traditional rope-based methods (where Ulva is grown on longlines or twines) are less effective in the low-salinity waters of the Baltic Sea, partly due to weaker algal attachment and inconsistent growth. Ulva seeded on ropes often failed to form secure holdfasts or detached under wave action, reducing yield stability. These challenges are driving a shift toward cage or net cultivation systems better suited to Baltic conditions. By cultivating Ulva in enclosed net structures that either allow it to float freely within a frame or attach to substrates protected from wave force, it is possible to support its opportunistic growth habit and reduce losses from detachment or sporulation-driven disintegration.
In our study, the mesh cage cultivation method emerged as a reasonably viable approach. Initial growth rates within the cage were comparable to those in controlled environments [
21]. Although storms occasionally increased water turbidity,
Ulva demonstrated notable resilience as the algae maintained steady, albeit reduced, growth. Unlike substrates susceptible to competitive overgrowth, the cage provides an open environment that reduces direct competition, presenting a promising solution for sustainable
Ulva farming in the Baltic Sea. Despite these encouraging results, the experiment highlighted the importance of environmental stability for successful open-water cultivation and suggested that future net cage designs could be optimized to enhance growth by improving water clarity and nutrient delivery. Nevertheless, such methodological adaptations support the expansion of
Ulva farming in the Baltic Sea, ensuring that cultivation remains viable despite the region’s low salinity and reinforcing the importance of tailoring farming strategies to local environmental constraints.