Following the Food: Dynamic, Seasonal Changes in the Fine-Scale Distribution of Foraging Minke Whales Within a Scottish Marine Protected Area (MPA)
Abstract
1. Introduction
2. Materials and Methods
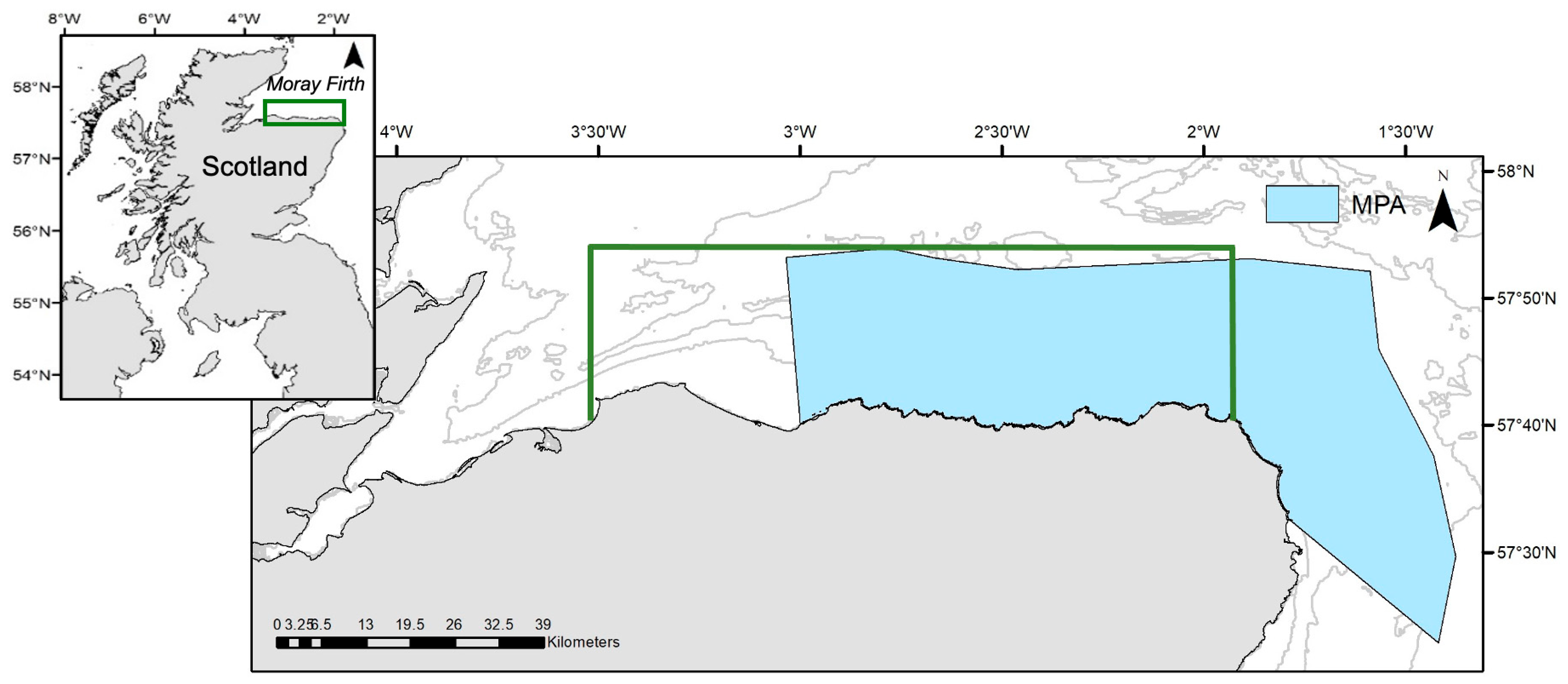
2.1. Data Collection and Processing
2.2. Statistical Analysis
2.3. Model Validation and Selection
3. Results
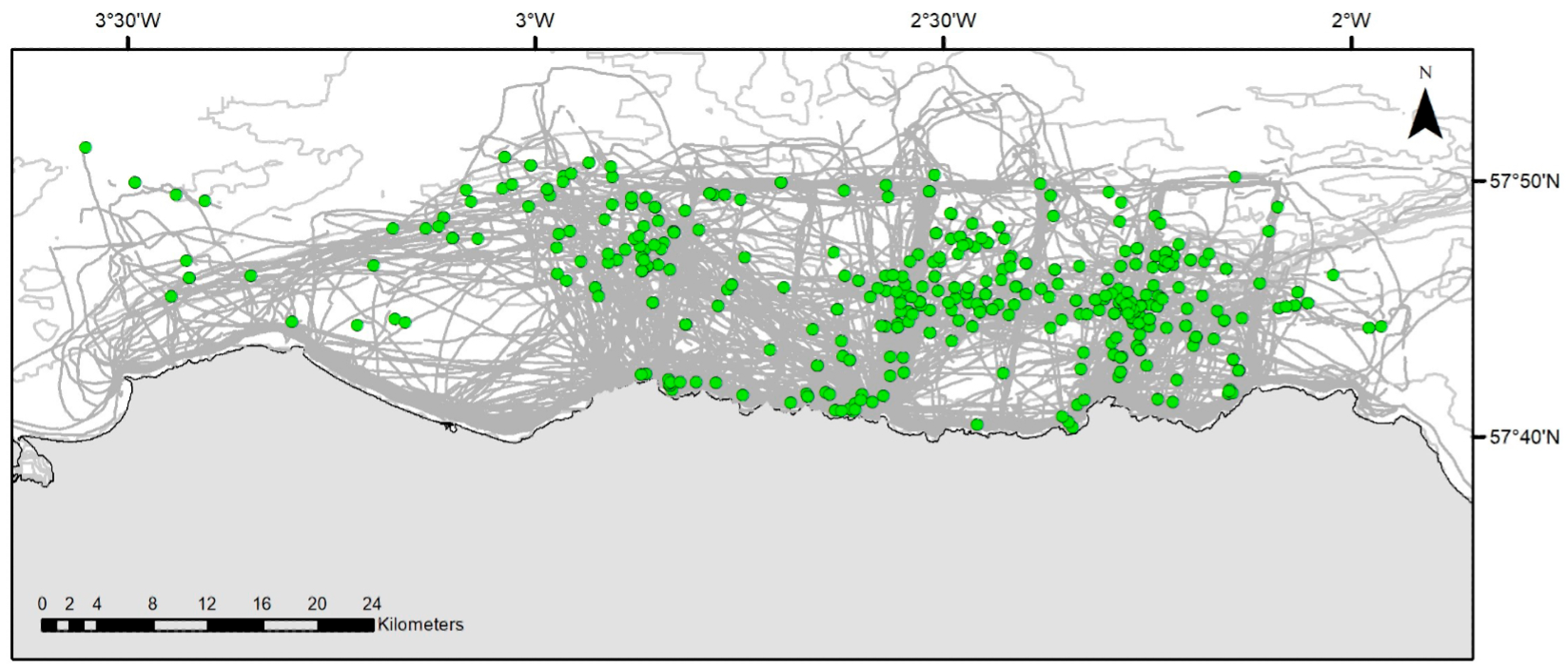
3.1. Sightings Data
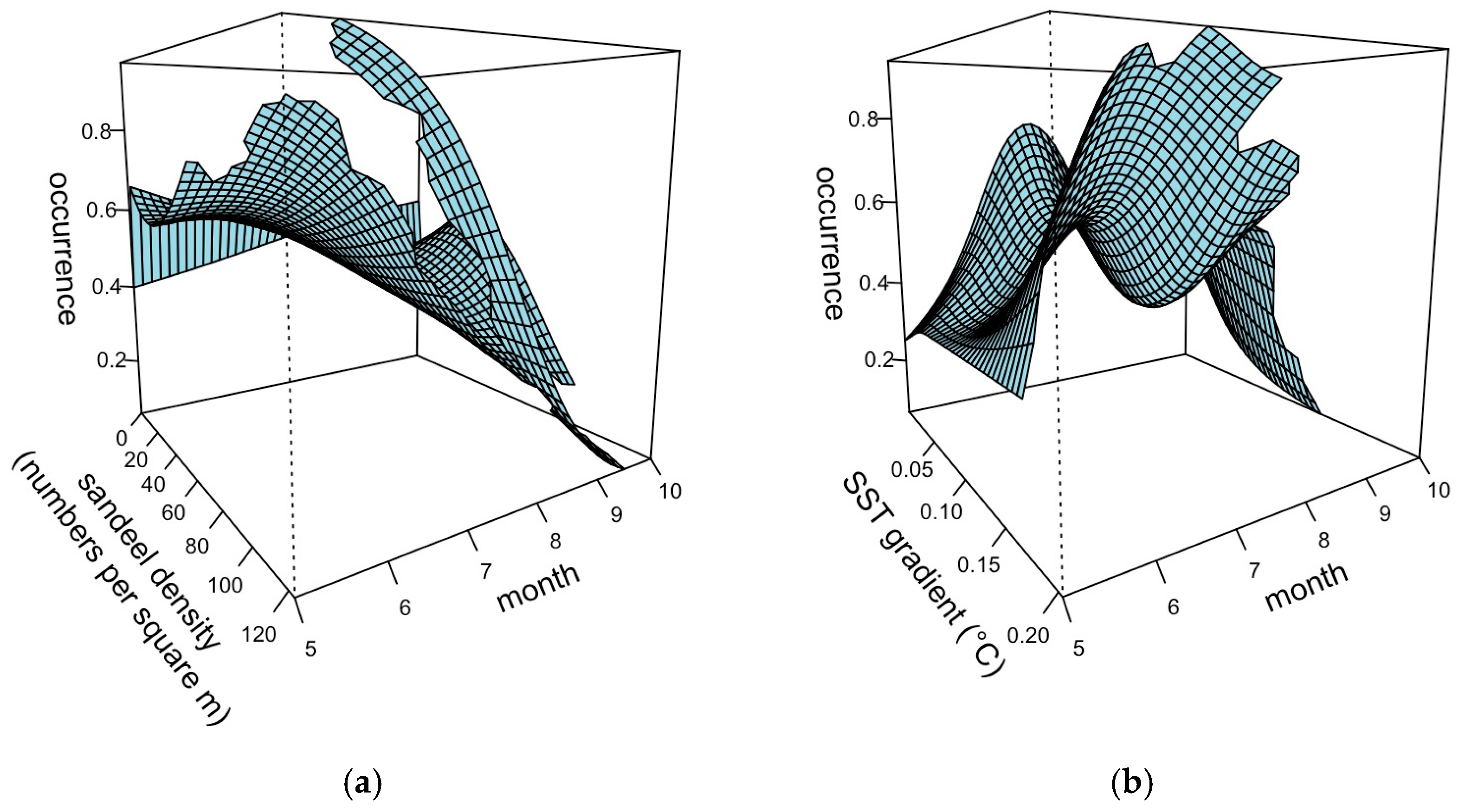
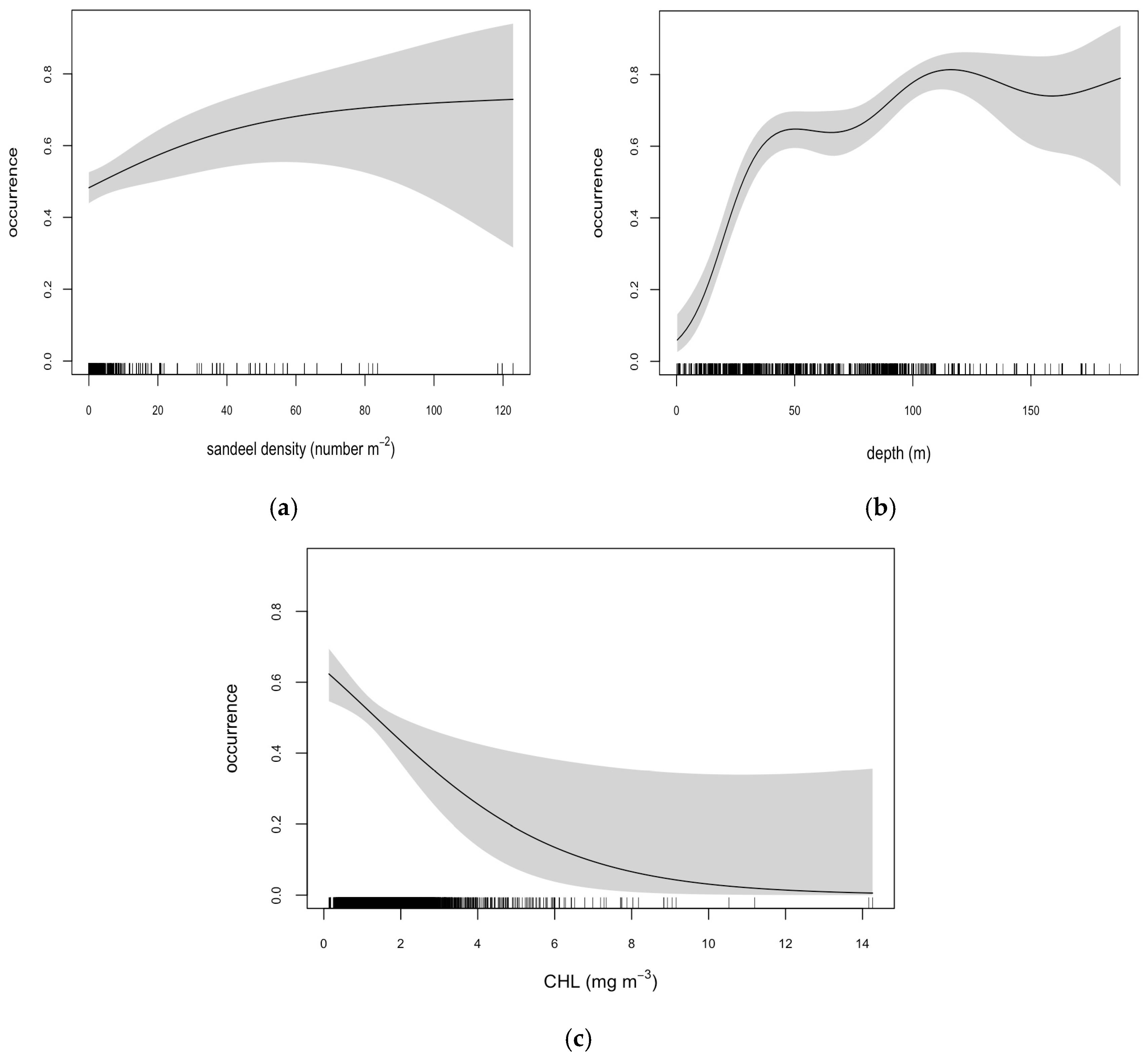
3.2. Model Selection and Outputs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, H.; Thompson, P.M. Using Marine Mammal Habitat Modelling to Identify Priority Conservation Zones within a Marine Protected Area. Mar. Ecol. Prog. Ser. 2009, 378, 279–287. [Google Scholar] [CrossRef]
- Cañadas, A.; Sagarminaga, R.; García-Tiscar, S. Cetacean Distribution Related with Depth and Slope in the Mediterranean Waters off Southern Spain. Deep Sea Res. I Oceanogr. Res. Pap. 2002, 49, 2053–2073. [Google Scholar] [CrossRef]
- Franks, P. Sink or Swim, Accumulation of Biomass at Fronts. Mar. Ecol. Prog. Ser. 1992, 82, 1–12. [Google Scholar] [CrossRef]
- Panigada, S.; Sciara, G.N.D.; Panigada, M.Z.; Airoldi, S.; Borsani, J.F.; Jahoda, M. Fin Whales (Balaenoptera physalus) Summering in the Ligurian Sea: Distribution, Encounter Rate, Mean Group Size and Relation to Physiographic Variables. J. Cetacean Res. Manage. 2005, 7, 137–145. [Google Scholar] [CrossRef]
- Macleod, K.; Fairbairns, R.; Gill, A.; Fairbairns, B.; Gordon, J.; Blair-Myers, C.; Parsons, E. Seasonal Distribution of Minke Whales Balaenoptera acutorostrata in Relation to Physiography and Prey off the Isle of Mull, Scotland. Mar. Ecol. Prog. Ser. 2004, 277, 263–274. [Google Scholar] [CrossRef]
- Kimura, S.; Kasai, A.; Nakata, H.; Sugimoto, T.; Simpson, J.H.; Cheok, J.V.S. Biological Productivity of Meso-Scale Eddies Caused by Frontal Disturbances in the Kuroshio. ICES J. Mar. Sci. 1997, 54, 179–192. [Google Scholar] [CrossRef]
- Griffin, R.B. Sperm Whale Distributions and Community Ecology Associated with a Warm-Core Ring Off Georges Bank. Mar. Mammal Sci. 1999, 15, 33–51. [Google Scholar] [CrossRef]
- Scales, K.L.; Miller, P.I.; Hawkes, L.A.; Ingram, S.N.; Sims, D.W.; Votier, S.C. REVIEW: On the Front Line: Frontal Zones as Priority at-Sea Conservation Areas for Mobile Marine Vertebrates. J. Appl. Ecol. 2014, 51, 1575–1583. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Hazen, E.L.; Lewison, R.L.; Dunn, D.C.; Bailey, H.; Bograd, S.J.; Briscoe, D.K.; Fossette, S.; Hobday, A.J.; Bennett, M.; et al. Dynamic Ocean Management: Defining and Conceptualizing Real-Time Management of the Ocean. Mar. Policy 2015, 58, 42–50. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Gjerde, K.M.; Conners, M.G.; Crowder, L.B. Mobile Protected Areas for Biodiversity on the High Seas. Science 2020, 367, 252–254. [Google Scholar] [CrossRef]
- Hoyt, E. Marine Protected Areas for Whales, Dolphins and Porpoises: A World Handbook for Cetacean Habitat Conservation, 2nd ed.; Earthscan: Oxon, UK, 2011; ISBN 978-1-84407-763-2. [Google Scholar]
- Camphuysen, K.C.; Shamoun-Baranes, J.; Bouten, W.; Garthe, S. Identifying Ecologically Important Marine Areas for Seabirds Using Behavioural Information in Combination with Distribution Patterns. Biol. Conserv. 2012, 156, 22–29. [Google Scholar] [CrossRef]
- Horton, T.W.; Palacios, D.M.; Stafford, K.M.; Zerbini, A.N. Baleen Whale Migration. In Ethology and Behavioral Ecology of Mysticetes; Clark, C.W., Garland, E.C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 71–104. ISBN 978-3-030-98449-6. [Google Scholar]
- Lee, S.H.; Schell, D.M.; McDonald, T.L.; Richardson, W.J. Regional and Seasonal Feeding by Bowhead Whales Balaena Mysticetus as Indicated by Stable Isotope Ratios. Mar. Ecol. Prog. Ser. 2005, 285, 271–287. [Google Scholar] [CrossRef]
- Konishi, K.; Tamura, T.; Isoda, T.; Okamoto, R.; Hakamada, T.; Kiwada, H.; Matsuoka, K. Feeding Strategies and Prey Consumption of Three Baleen Whale Species Within the Kuroshio-Current Extension. J. Northwest Atl. Fish. Sci. 2009, 42, 27–40. [Google Scholar] [CrossRef]
- Bentaleb, I.; Martin, C.; Vrac, M.; Mate, B.; Mayzaud, P.; Siret, D.; de Stephanis, R.; Guinet, C. Foraging Ecology of Mediterranean Fin Whales in a Changing Environment Elucidated by Satellite tracking and Baleen Plate Stable Isotopes. Mar. Ecol. Prog. Ser. 2011, 438, 285–302. [Google Scholar] [CrossRef]
- Mitani, Y.; Bando, T.; Takai, N.; Sakamoto, W. Patterns of Stable Carbon and Nitrogen Isotopes in the Baleen of Common Minke Whale Balaenoptera Acutorostrata from the Western North Pacific. Fish. Sci. 2006, 72, 69–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Nong, Z.; Chen, M.; Hao, Y.; Wang, J.; Wang, K.; Wang, D.; Mei, Z. The First Baleen Whale Marine Protected Area Proposed for Bryde’s Whales in the Beibu Gulf, China. Mar. Mammal Sci. 2024, 40, e13082. [Google Scholar] [CrossRef]
- Ashe, E.; Noren, D.P.; Williams, R. Animal Behaviour and Marine Protected Areas: Incorporating Behavioural Data into the Selection of Marine Protected Areas for an Endangered Killer Whale Population. Anim. Conserv. 2010, 13, 196–203. [Google Scholar] [CrossRef]
- NatureScot. Minke Whale. Available online: https://www.nature.scot/plants-animals-and-fungi/mammals/marine-mammals/minke-whale (accessed on 24 January 2025).
- Robinson, K.P.; Tetley, M.J.; Mitchelson-Jacob, E.G. The Distribution and Habitat Preference of Coastally Occurring Minke Whales (Balaenoptera acutorostrata) in the Outer Southern Moray Firth, Northeast Scotland. J. Coast. Conserv. 2009, 13, 39–48. [Google Scholar] [CrossRef]
- Robinson, K.P.; MacDougall, D.A.I.; Bamford, C.C.G.; Brown, W.J.; Dolan, C.J.; Hall, R.; Haskins, G.N.; Russell, G.; Sidiropoulos, T.; Sim, T.M.C.; et al. Ecological Habitat Partitioning and Feeding Specialisations of Coastal Minke Whales (Balaenoptera acutorostrata) Using a Recently Designated MPA in Northeast Scotland. PLoS ONE 2023, 18, e0246617. [Google Scholar] [CrossRef]
- Paxton, C.; Scott-Hayward, L.; Rexstad, E. Statistical Approaches to Aid the Identification of Marine Protected Areas for Minke Whale, Risso’s Dolphin, White-Beaked Dolphin and Basking Shark; Scottish Natural Heritage: Inverness, Scotland, 2014.
- Robinson, K.P.; Tetley, M.J. Behavioural Observations of Foraging Minke Whales (Balaenoptera acutorostrata) in the Outer Moray Firth, North-East Scotland. J. Mar. Biol. Assoc. UK 2007, 87, 85–86. [Google Scholar] [CrossRef]
- Tetley, M.J.; Mitchelson-Jacob, E.G.; Robinson, K.P. The Summer Distribution of Coastal Minke Whales (Balaenoptera acutorostrata) in the Southern Outer Moray Firth, NE Scotland, in Relation to Co-Occurring Mesoscale Oceanographic Features. Remote Sens. Environ. 2008, 112, 3449–3454. [Google Scholar] [CrossRef]
- Boyse, E.; Robinson, K.P.; Beger, M.; Carr, I.M.; Taylor, M.; Valsecchi, E.; Goodman, S.J. Environmental DNA Reveals Fine-Scale Spatial and Temporal Variation of Marine Mammals and Their Prey Species in a Scottish Marine Protected Area. Environ. DNA 2024, 6, e587. [Google Scholar] [CrossRef]
- Wilson, B.; Reid, R.J.; Grellier, K.; Thompson, P.M.; Hammond, P.S. Considering the Temporal When Managing the Spatial: A Population Range Expansion Impacts Protected Areas-Based Management for Bottlenose Dolphins. Anim. Conserv. Forum 2004, 7, 331–338. [Google Scholar] [CrossRef]
- Hooker, S.K.; Cañadas, A.; Hyrenbach, K.D.; Corrigan, C.; Polovina, J.J.; Reeves, R.R. Making Protected Area Networks Effective for Marine Top Predators. Endanger. Species Res. 2011, 13, 203–218. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; QGIS: London, UK, 2024. [Google Scholar]
- Tepsich, P.; Rosso, M.; Halpin, P.; Moulins, A. Habitat Preferences of Two Deep-Diving Cetacean Species in the Northern Ligurian Sea. Mar. Ecol. Prog. Ser. 2014, 508, 247–260. [Google Scholar] [CrossRef]
- Robinson, K.P. Minke Whale Sightings. Cetacean Research and Rescue Unit. 2025. Available online: https://crru.org.uk/research/minke-whales (accessed on 1 November 2024).
- Forney, K.A.; Becker, E.A.; Foley, D.G.; Barlow, J.; Oleson, E.M. Habitat-Based Models of Cetacean Density and Distribution in the Central North Pacific. Endanger. Species Res. 2015, 27, 1–20. [Google Scholar] [CrossRef]
- Gilles, A.; Viquerat, S.; Becker, E.A.; Forney, K.A.; Geelhoed, S.C.V.; Haelters, J.; Nabe-Nielsen, J.; Scheidat, M.; Siebert, U.; Sveegaard, S.; et al. Seasonal Habitat-Based Density Models for a Marine Top Predator, the Harbor Porpoise, in a Dynamic Environment. Ecosphere 2016, 7, e01367. [Google Scholar] [CrossRef]
- Laidre, K.L.; Heide-Jørgensen, M.P.; Heagerty, P.; Cossio, A.; Bergström, B.; Simon, M. Spatial Associations between Large Baleen Whales and Their Prey in West Greenland. Mar. Ecol. Prog. Ser. 2010, 402, 269–284. [Google Scholar] [CrossRef]
- Pendleton, D.E.; Holmes, E.E.; Redfern, J.; Zhang, J. Using Modelled Prey to Predict the Distribution of a Highly Mobile Marine Mammal. Divers. Distrib. 2020, 26, 1612–1626. [Google Scholar] [CrossRef]
- Zerbini, A.N.; Friday, N.A.; Palacios, D.M.; Waite, J.M.; Ressler, P.H.; Rone, B.K.; Moore, S.E.; Clapham, P.J. Baleen Whale Abundance and Distribution in Relation to Environmental Variables and Prey Density in the Eastern Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 134, 312–330. [Google Scholar] [CrossRef]
- Pickens, B.; Taylor, J.; Campbell, M.; Driggers, W. Offshore Snapper and Shark Distributions Are Predicted by Prey and Area of Nearby Estuarine Environments in the Gulf of Mexico, USA. Mar. Ecol. Prog. Ser. 2022, 682, 169–189. [Google Scholar] [CrossRef]
- Schick, R.S.; Lutcavage, M.E. Inclusion of Prey Data Improves Prediction of Bluefin Tuna (Thunnus thynnus) Distribution. Fish. Oceanogr. 2009, 18, 77–81. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Halpin, P.N.; Qian, S.S.; Lawson, G.L.; Wiebe, P.H.; Thiele, D.; Read, A.J. Whale Distribution in Relation to Prey Abundance and Oceanographic Processes in Shelf Waters of the Western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2006, 317, 297–310. [Google Scholar] [CrossRef]
- van der Kooij, J.; Scott, B.E.; Mackinson, S. The Effects of Environmental Factors on Daytime Sandeel Distribution and Abundance on the Dogger Bank. J. Sea Res. 2008, 60, 201–209. [Google Scholar] [CrossRef]
- Langton, R.; Boulcott, P.; Wright, P.J. A Verified Distribution Model for the Lesser Sandeel Ammodytes Marinus. Mar. Ecol. Prog. Ser. 2021, 667, 145–159. [Google Scholar] [CrossRef]
- RStudio Development Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Gelman, A.; Su, Y.-S.; Yajima, M.; Hill, J.; Pittau, M.G.; Kerman, J.; Zheng, T.; Dorie, V. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Conners, M.G.; Sisson, N.B.; Agamboue, P.D.; Atkinson, P.W.; Baylis, A.M.M.; Benson, S.R.; Block, B.A.; Bograd, S.J.; Bordino, P.; Bowen, W.D.; et al. Mismatches in Scale between Highly Mobile Marine Megafauna and Marine Protected Areas. Front. Mar. Sci. 2022, 9, 897104. [Google Scholar] [CrossRef]
- Anderwald, P.; Evans, P.; Dyer, R.; Dale, A.; Wright, P.; Hoelzel, A. Spatial Scale and Environmental Determinants in Minke Whale Habitat Use and Foraging. Mar. Ecol. Prog. Ser. 2012, 450, 259–274. [Google Scholar] [CrossRef]
- De Boer, M. Spring Distribution and Density of Minke Whale Balaenoptera acutorostrata along an Offshore Bank in the Central North Sea. Mar. Ecol. Prog. Ser. 2010, 408, 265–274. [Google Scholar] [CrossRef]
- Pierce, G.J.; Santos, M.B.; Reid, R.J.; Patterson, I.A.P.; Ross, H.M. Diet of Minke Whales Balaenoptera acutorostrata in Scottish (UK) Waters with Notes on Strandings of This Species in Scotland 1992–2002. J. Mar. Biol. Assoc. UK 2004, 84, 1241–1244. [Google Scholar] [CrossRef]
- Greenstreet, S.P.R.; McMillan, J.A.; Armstrong, E. Seasonal Variation in the Importance of Pelagic Fish in the Diet of Piscivorous Fish in the Moray Firth, NE Scotland: A Response to Variation in Prey Abundance? ICES J. Mar. Sci. 1998, 55, 121–133. [Google Scholar] [CrossRef][Green Version]
- Winslade, P. Behavioural Studies on the Lesser Sandeel Ammodytes marinus (Raitt) III. The Effect of Temperature on Activity and the Environmental Control of the Annual Cycle of Activity. J. Fish Biol. 1974, 6, 587–599. [Google Scholar] [CrossRef]
- Pedersen, S.A.; Lewy, P.; Wright, P. Assessments of the Lesser Sandeel (Ammodytes marinus) in the North Sea Based on Revised Stock Divisions. Fish. Res. 1999, 41, 221–241. [Google Scholar] [CrossRef]
- Robinson, B.W.; Wilson, D.S. Optimal Foraging, Specialization, and a Solution to Liem’s Paradox. Am. Nat. 1998, 151, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Maravelias, C.; Reid, D. Identifying the Effects of Oceanographic Features and Zooplankton on Prespawning Herring Abundance Using Generalized Additive Models. Mar. Ecol. Prog. Ser. 1997, 147, 1–9. [Google Scholar] [CrossRef]
- Maravelias, C.; Reid, D.; Swartzman, G. Seabed Substrate, Water Depth and Zooplankton as Determinants of the Prespawning Spatial Aggregation of North Atlantic Herring. Mar. Ecol. Prog. Ser. 2000, 195, 249–259. [Google Scholar] [CrossRef]
- Van Haren, J.J.M.; Joordens, J.C.A. Observations of Physical and Biological Parameters at the Transition between the Southern and Central North Sea. Neth. J. Sea Res. 1990, 25, 351–364. [Google Scholar] [CrossRef]
- Gissel, T.N.; Munk, P. Zooplankton Diversity and the Predatory Impact by Larval and Small Juvenile Fish at the Fisher Banks in the North Sea. J. Plankton Res. 1998, 20, 2313–2332. [Google Scholar] [CrossRef]
- Elliott, A.J.; Clarke, T. Seasonal Stratification in the Northwest European Shelf Seas. Cont. Shelf Res. 1991, 11, 467–492. [Google Scholar] [CrossRef]
- Van Haren, H.; Howarth, M.J.; Jones, K.; Ezzi, I. Autumnal Reduction of Stratification in the Northern North Sea and Its Impact. Cont. Shelf Res. 2003, 23, 177–191. [Google Scholar] [CrossRef]
- Doniol-Valcroze, T.; Berteaux, D.; Larouche, P.; Sears, R. Influence of Thermal Fronts on Habitat Selection by Four Rorqual Whale Species in the Gulf of St. Lawrence. Mar. Ecol. Prog. Ser. 2007, 335, 207–216. [Google Scholar] [CrossRef]
- Yan, H.F.; Kyne, P.M.; Jabado, R.W.; Leeney, R.H.; Davidson, L.N.K.; Derrick, D.H.; Finucci, B.; Freckleton, R.P.; Fordham, S.V.; Dulvy, N.K. Overfishing and Habitat Loss Drive Range Contraction of Iconic Marine Fishes to near Extinction. Sci. Adv. 2021, 7, eabb6026. [Google Scholar] [CrossRef] [PubMed]
- Northridge, S.; Cargill, A.; Coram, A.; Mandleberg, L.; Calderan, S.; Reid, B. Entanglement of Minke Whales in Scottish Waters; an Investigation into Occurrence, Causes and Mitigation; Sea Mammal Research Unit: Fife, Scotland, 2010. [Google Scholar]
- Leaper, R.; MacLennan, E.; Brownlow, A.; Calderan, S.; Dyke, K.; Evans, P.; Hartny-Mills, L.; Jarvis, D.; McWhinnie, L.; Philp, A.; et al. Estimates of Humpback and Minke Whale Entanglements in the Scottish Static Pot (Creel) Fishery. Endanger. Species Res. 2022, 49, 217–232. [Google Scholar] [CrossRef]
- Robinson, K.P. Minke Whale Catalogue. Cetacean Research and Rescue Unit. 2025. Available online: https://crru.org.uk/research/catalogues?catalogue=minke-whale&page=1 (accessed on 1 November 2024).
- NatureScot. Conservation and Management Advice: Southern Trench MPA; NatureScot: Inverness, Scotland, 2024.
- Wright, P.J.; Begg, G.S. A Spatial Comparison of Common Guil lemots and Sandeels in Scottish Waters. ICES J. Mar. Sci. 1997, 54, 578–592. [Google Scholar] [CrossRef][Green Version]
- Carroll, M.J.; Bolton, M.; Owen, E.; Anderson, G.Q.A.; Mackley, E.K.; Dunn, E.K.; Furness, R.W. Kittiwake Breeding Success in the Southern North Sea Correlates with Prior Sandeel Fishing Mortality. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1164–1175. [Google Scholar] [CrossRef]
- MacLeod, C.D.; Santos, M.B.; Reid, R.J.; Scott, B.E.; Pierce, G.J. Linking Sandeel Consumption and the Likelihood of Starvation in Harbour Porpoises in the Scottish North Sea: Could Climate Change Mean More Starving Porpoises? Biol. Lett. 2007, 3, 185–188. [Google Scholar] [CrossRef]
- Wanless, S.; Harris, M.P.; Greenstreet, S.P.R. Summer Sandeel Consumption by Seabirds Breeding in the Firth of Forth, South-East Scotland. ICES J. Mar. Sci. 1998, 55, 1141–1151. [Google Scholar] [CrossRef]
- Pommier, M.; O’Donnell, C.; Barile, C.; McGill, R.; Berrow, S.; O’Brien, J. Exploring Environmental and Biological Drivers of Cetacean Occurrence in the Cross-Border Region of the Malin Shelf Using Data from a European Fishery Survey. Front. Mar. Sci. 2023, 10. [Google Scholar]
- Scales, K.L.; Miller, P.I.; Embling, C.B.; Ingram, S.N.; Pirotta, E.; Votier, S.C. Mesoscale Fronts as Foraging Habitats: Composite Front Mapping Reveals Oceanographic Drivers of Habitat Use for a Pelagic Seabird. J. R. Soc. Interface 2014, 11, 20140679. [Google Scholar] [CrossRef]
- Miller, P.I.; Scales, K.L.; Ingram, S.N.; Southall, E.J.; Sims, D.W. Basking Sharks and Oceanographic Fronts: Quantifying Associations in the North-East Atlantic. Funct. Ecol. 2015, 29, 1099–1109. [Google Scholar] [CrossRef]

| Model | Equation | AIC |
|---|---|---|
| Full | 3104.575 | |
| Alternative 1 | 3102.965 | |
| Alternative 2 | 3103.725 |
| Smooth Terms | Effective Degrees of Freedom | Reference Degrees of Freedom | Chi-Square | p |
|---|---|---|---|---|
| BSD | 1.611 | 1.988 | 8.390 | <0.05 * |
| ti (BSD, month) | 4.448 | 5.376 | 25.177 | <0.001 * |
| te (SST-SD, month) | 14.050 | 16.666 | 78.470 | <0.001 * |
| Depth | 5.777 | 6.522 | 133.689 | <0.001 * |
| CHL | 1.020 | 1.039 | 10.070 | <0.05 * |
| Slope | 1.709 | 2.088 | 1.113 | 0.537 |
| Parametric Terms | Estimate | Standard Error | Z | p |
| SST | −0.196 | 0.130 | −1.504 | 0.133 |
| Year (2009 as reference) | ||||
| 2010 | 0.515 | 0.292 | 1.766 | 0.077 |
| 2011 | 0.130 | 0.377 | 0.035 | 0.972 |
| 2012 | −0.235 | 0.357 | −0.658 | 0.510 |
| 2013 | 0.651 | 0.278 | 2.341 | <0.05 * |
| 2014 | −0.251 | 0.530 | −0.474 | 0.636 |
| 2015 | 0.597 | 0.299 | 1.995 | <0.05 * |
| 2016 | 0.595 | 0.286 | 2.081 | <0.05 * |
| 2017 | 0.358 | 0.292 | 1.227 | 0.220 |
| 2018 | 1.962 | 0.252 | 7.793 | <0.001 * |
| 2019 | 0.143 | 0.304 | 0.472 | 0.637 |
| 2020 | 1.046 | 0.302 | 3.465 | <0.001 * |
| 2021 | 2.449 | 0.251 | 9.749 | <0.001 * |
| 2022 | 0.987 | 0.268 | 3.688 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDougall, D.A.I.; Robinson, K.P. Following the Food: Dynamic, Seasonal Changes in the Fine-Scale Distribution of Foraging Minke Whales Within a Scottish Marine Protected Area (MPA). Oceans 2025, 6, 18. https://doi.org/10.3390/oceans6010018
MacDougall DAI, Robinson KP. Following the Food: Dynamic, Seasonal Changes in the Fine-Scale Distribution of Foraging Minke Whales Within a Scottish Marine Protected Area (MPA). Oceans. 2025; 6(1):18. https://doi.org/10.3390/oceans6010018
Chicago/Turabian StyleMacDougall, Duncan A. I., and Kevin P. Robinson. 2025. "Following the Food: Dynamic, Seasonal Changes in the Fine-Scale Distribution of Foraging Minke Whales Within a Scottish Marine Protected Area (MPA)" Oceans 6, no. 1: 18. https://doi.org/10.3390/oceans6010018
APA StyleMacDougall, D. A. I., & Robinson, K. P. (2025). Following the Food: Dynamic, Seasonal Changes in the Fine-Scale Distribution of Foraging Minke Whales Within a Scottish Marine Protected Area (MPA). Oceans, 6(1), 18. https://doi.org/10.3390/oceans6010018







