Abstract
Microplastics are ubiquitous in marine environments and have been documented across all ocean compartments, especially surface waters, across the world. Even though several studies identify the presence of microplastics in the world’s five oceans, there remains an overt problem of large inconsistencies in their sampling, extraction, and consequent quantification. Despite the complexity of these methodologies, researchers have tried to explore microplastic abundance in ocean surface waters. Using a systematic review approach, a dataset was derived from 73 primary studies undertaken since the year 2010 following the Oslo and Paris Conventions (OSPAR) guidelines to monitor and harmonise marine debris. The results showed differences in the abundance and distribution of microplastics in surface waters across oceans. The overall concentration of microplastics in all five oceans ranged between 0.002 and 62.50 items/m3, with a mean abundance of 2.76 items/m3. The highest mean concentration of microplastics was found in the Atlantic (4.98 items/m3), while the least was observed in the Southern Ocean (0.04 items/m3). While challenging, this paper recommends harmonisation of the sampling, separation, and identification methods across the globe to aid in the design of the appropriate mitigation strategies for reducing marine plastic pollution.
1. Introduction
Plastic debris of all sizes has been recognised as a major global threat to the environment [1,2,3]. Globally, the economic cost of marine plastic pollution was estimated at USD 6–19 billion in 2018 and is projected to be USD 100 billion by 2040 [2]. Highly abundant and widely dispersed plastics introduce massive amounts of plastics into oceans on a global scale [4,5].
Microplastics, commonly classified as plastic particles less than 5 mm in diameter, have been found across all ocean matrices [6], coastal beaches [7], in the water column [8], and ocean floors [9]. Globally, studies have documented the presence of microplastics in all areas of the marine environment, from estuarine, nearshore, offshore, open ocean, in the water column, sediment, and biota [10,11,12,13,14]. Microplastics can either be primary in origin, manufactured directly by industries as raw material for other products, or a secondary fragmentation of larger plastics due to environmental factors, such as ultraviolet solar radiation, oxidation, and biodegradation, among others [1,15,16].
The evidence of microplastics and their hazardous effects on coastal and marine environments has been increasing progressively since the 1970s [17,18,19]. Microplastics constitute over 95% of the plastic debris that accumulates and spreads over all ocean matrices [20,21]. For instance, [22] estimated that microplastics constitute more than 92% of floating plastic debris across all five sub-tropical gyres, but also noted that the estimate was lower than expected due to challenges in sampling and identification methods. At present, microplastic debris is one of the environmental concerns with diverse detrimental impacts across all marine environments, owing to the persistent and ubiquitous nature of plastics [2,23,24]. According to a 2019 report by the United Nations Industrial Development Organization [25], the magnitude of plastic pollution is largely dependent on the economic status of the country, with high-income nations, specifically G20 countries, producing an estimated two-thirds of global plastic debris. The gravity of the negative consequences is compounded by the fact that, even if the entry of plastic debris into the marine environment is halted, the abundance of microplastics is expected to increase due to the continuous degradation of the already present plastic debris in the world’s oceans [26,27].
Owing to their small sizes and ubiquity in the oceans, microplastics pose a greater, immediate risk to marine biota (mainly through ingestion of toxic substances) and humans (mainly through seafood contamination) than macro-plastics (particles greater than 5 mm) [28,29]. In recent years, researchers have been progressively evaluating the impacts of microplastics on the blue economy, and warning of a global ecological crisis emanating from plastic pollution in the oceans [30]. This has led to a gain in institutional recognition across different countries, evidenced by legislation, such as the ban of microbeads (e.g., Canada, Australia, Sweden, Denmark, and the United Kingdom) and the establishment of extended producer responsibility to reduce microplastic pollution [31,32,33].
The ocean surface has been the primary focus of microplastic research among all ocean compartments, particularly due to the challenges associated with sampling microplastics from the water column and ocean floor [34]. Even though there has been a rapid expansion in microplastic research in ocean surface waters, there remains an overt problem of large inconsistencies due to non-standardised sampling, extraction, and quantification approaches, as well as a formal definition of the size ranges [35,36]. These approaches are often inadequately defined, resulting in studies that are neither comparable nor reproducible. Despite the call for standardised approaches by researchers [37], a huge range of different techniques, each championed by different research groups, continue to be used [38,39,40].
The purpose of this study is to conduct a thematic systematic review to assess the concentration of microplastics across the surface waters of the world’s five oceans, to contribute to the ongoing intensive research and discussions on the need for standardised approaches to inform a worldwide monitoring and mitigation system for marine plastic pollution. According to [26], there is an urgent need for comprehensive and practical strategies to mitigate the detrimental impacts of microplastic pollution, which has prompted leading researchers, policymakers, and environmental activists to take action as microplastics in oceans continue to expand and infiltrate the food chain [41].
In this current study, our primary objective is estimating the global concentration of microplastics for all five of the world’s oceans, using clearly defined systematic review guidelines to cover a wide range of sampling and identification methods used between 2010 and 2023. This paper adds valuable information to the marine plastic literature, by summarising physical properties and temporal trends in the abundance of microplastics across the world’s five oceans and then highlighting the extent of relative comparability of studies. To effectively address the problem of microplastics in marine environments, data on the abundance, distribution, and composition of microplastics are essential [10,42].
The findings from this study show differences in the sizes, shapes, polymers, concentration, and distribution of microplastics across the surface waters of the world’s five oceans. The findings indicate that the Atlantic Ocean has the highest concentration of microplastics, followed by the Arctic, Indian, Pacific, and Southern Oceans, respectively. Across the five oceans, the overall concentration of microplastics ranged between 0.002 and 22 items/m3, with a mean abundance of 1.21 items/m3 and a median of 0.135 items/m3, suggesting a skewed distribution. The first quartile and third quartiles were between 0.01 and 1.09 items/m3, respectively, with the highest mean concentration in the Atlantic Ocean (2.58 items/m3) and the lowest in the Southern Ocean (0.04 items/m3). The dominant colours, shapes, and polymers (black and blue, fragments and films, polystyrene (PS) and polyethylene terephthalate (PET)) across these oceans suggest secondary microplastics (e.g., dispersal and deterioration of single-use plastics and fishing gear) compared to primary sources from industries or cosmetic products. The wide variation in the sampling, identification, and quantification methods used in the reviewed studies reveals the need for standardisation to minimise estimation errors.
In the next section, the paper presents a background to the problem and the methods and materials employed, including the search terms, inclusion criteria, and data collection. Section 3 provides the results and discussion, supported by related literature and, finally, Section 4 presents the conclusion and recommendations.
2. Materials and Methods
2.1. Literature Sources and Search Terms
An extensive systematic literature search was carried out on Google Scholar, Web of Science, Science Direct, Scopus, and OpenGrey, following the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Materials). The systematic review across all specified databases (Google Scholar, Web of Science, Science Direct, Scopus, and OpenGrey) was conducted between 1 October 2023 and 31 January 2024 to identify all studies reporting the concentration of microplastics across the world’s five oceans. All the keywords were interchangeably paired with the Boolean connectors and the truncations to identify studies between 1 January 2010 and 31 December 2023. Over the years, the term ‘microplastic’ has largely been used interchangeably with other synonymous terms by different authors [43]. Therefore, to identify all relevant studies that assess the abundance of microplastics on ocean surface waters, a series of Boolean search operators (‘and’, ‘or’) and the use of synonyms for ‘microplastic’ were developed. To account for different forms of search words, ‘*’ truncation and quotation marks were used to allow the search for exact phrases. The search terms constituted a combination of the following keywords: microplastics, microplastic, microliter, micro-plastic, micro-litter, microparticle, micro debris, plastic particle, and micro and nano plastics. For concentration, the terms used were concentration, quantity, amount, number, abundance, and measure. Terms used to identify the oceans were marine, ocean, Pacific, Atlantic, Arctic, Indian, Southern, or Antarctic oceans. A systematic search was conducted for studies and samples collected.
2.2. Inclusion and Exclusion Criteria
The comprehensive literature search yielded a total of 7870 pertinent studies retrieved from 5 distinct databases, as follows: 951 in Google Scholar, 6541 in Web of Science, 183 in Science Direct, 185 in Scopus and 10 studies in OpenGrey. From each database, the studies were exported to Endnote 20 for further data management and the initial removal of duplicates. The software package, Covidence 2024, was used for screening the remaining studies for any duplicates and relevance based on their titles and abstracts, as well as full screening using detailed inclusion and exclusion criteria. The process, involving three reviewers, started with the screening interface, where the reviewers independently screened the titles and abstracts to identify the relevant studies using predefined filter words, such as ‘microplastics’, ‘micro-plastics’, ‘concentration’, ‘abundance’, ‘quantity’, and ‘number’, to ensure efficiency. The titles and abstracts that received conflicting votes among the reviewers were flagged for a consensus discussion. The relevant studies were subjected to the full-text screening interface, where the reviewers conducted a detailed assessment based on the following inclusion and exclusion criteria:
Studies had to be undertaken in the year 2010 and beyond because the first attempt to develop guidelines to monitor and harmonise data collection for marine debris was developed in 2010 by OSPAR.
Studies had to report the estimate of the concentration or abundance of microplastics, and this estimate had to be sampled from the ocean surface waters in the Pacific, Atlantic, Arctic, Indian, Southern, or Antarctic Ocean. The Southern Ocean is internationally recognized as the fifth ocean by the International Hydrographic Organization (IHO), which refers to the ocean waters surrounding Antarctica. In adherence to the international literature, this study uses the terminology ‘Southern Ocean’.
The surface zone represents the ocean’s least dense water and generally extends to about 150 m in depth [44]. To maintain relatively acceptable inter-research comparability, we defined the ocean surface water as the matrix from the surface to 10 m deep; thus, studies included had to adhere to this definition.
Microplastics from water samples are reported in different formats and units, such as items per volume, area, or mass [45]. In this review, only studies reporting the concentration of microplastics using items/m3 and items/km2 were included. Furthermore, studies using items/km2 had to report the sampling depth to allow conversion into items/m3 for broader comparisons.
If a study reported the concentration of microplastics in ocean surface waters as items/km2, the data were converted to items/m3 whenever the net height and submersion depth of the sampling net mouth were provided, and the net had a rectangular shape [46]. The formula used was as follows:
where is the calculated concentration of microplastics in items/m3, is the given concentration of microplastics in items/km2, and is the submersion depth of a sampling device during the sampling process.
Any discrepancies among the reviews during the full-text screening were resolved through a discussion for transparency and to ensure only studies of sufficient quality that met the predefined criteria were included in the final analysis to reduce the study-selection bias. After a thorough selection process, 73 studies were extracted and included in the final qualitative analysis. Figure 1 provides a detailed summary of the selection process and inclusion and exclusion criteria used to obtain the number of studies included in the review using a PRISMA flow diagram.
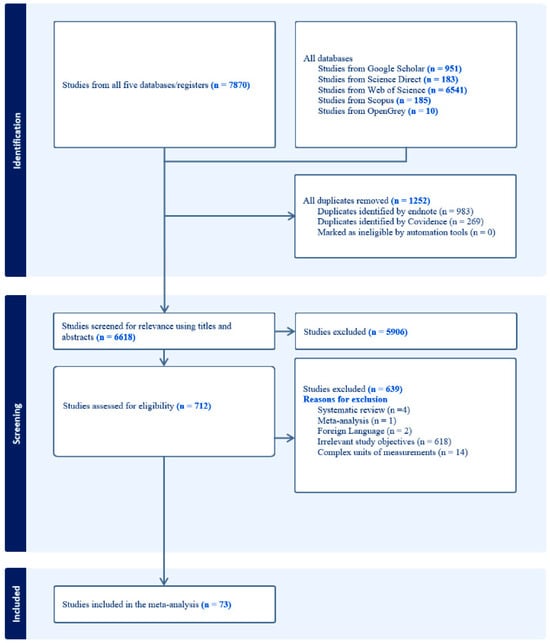
Figure 1.
A PRISMA flow diagram (source: adapted from [47]).
2.3. Data Collection Process
The 73 studies, with 105 observations, met our criteria to conduct the qualitative analysis. The studies included in the review are summarised in Table A1 in the Appendix A. This table contains the list of authors, the number of observations for microplastic concentrations obtained from each study, and the year of publication. The three reviewers were independently involved in the data collection. Data were extracted into Excel spreadsheets. We extracted data pertaining to the primary objective, the mean concentration of microplastics, a quantitative measure indicating the microplastic items or particles per unit volume (all items per squared kilometre were converted into items per cubic metres) in ocean surface waters to estimate the variation among the world’s five oceans. Out of the 73 studies, 17 reported the concentration of microplastics in items per kilometre squared. To facilitate the conversion of the concentration of microplastics in items per kilometre into items per cubic metre, data points for the height and submersion depth of the sampling device during sampling were also extracted. Additional related data points corresponding to this review were also extracted, including the year of data collection, year of publication, physical properties (colour, shape, and polymer types), sampling techniques (manta net, neuston net, plankton net, stainless-steel sieve, and underway system), separation techniques (sieving, filtration, and chemical), identification methods (visual, Raman, microscopy, stereo, and Fourier transform infrared spectroscopy (FTIR)), and microplastic sizes. During data extraction, the reviewers ensured that any studies bearing ambiguous data were excluded to reduce reporting bias unless a reasonable explanation was requested and received by email from the corresponding author of the specific article. All data points were collected ensuring consistency and uniformity in the definition of units to allow standardisation of data across studies. The reviewers verified the data points extracted from each study to ensure the data collected were reliable and reproducible to minimise bias and errors.
3. Results
3.1. General Findings from the Literature
The abundance and distribution of microplastics show considerable spatial variability across the different ocean matrices [48,49]. Modelling the transmission of microplastics is difficult since it involves physical, chemical, and biological processes [50]. More precisely, the spatial distribution of microplastics across the distinct ocean matrices varies extensively depending on biotic and abiotic environmental factors and processes, as well as the chemical structure of the plastic materials, resulting in substantial variation in their concentration profiles in both time and space [51,52]. Therefore, it is important to note that global estimates for floating microplastics also vary geographically, and depending on time and environmental conditions, the estimates may account for only 1% or less of the quantity of plastic debris annually entering into the oceans [53,54]. Furthermore, the physical properties of microplastics, including their colour, size, shape, and the polymers used, influence their mobility and distribution [55,56]. The composition of microplastics in the oceans reflects the different types of commonly used polymers. For example, more than 50% of manufactured plastic polymers have a buoyant density, causing them to largely dominate ocean surface waters [57]. However, some polymers undergo processes such as biofouling that force them to sink to the ocean floor [52]. Therefore, ocean surface waters may not be the final destination for all buoyant microplastics.
The lack of a standardised approach for collecting, pretreatment, and detecting microplastics in various environmental media makes the horizontal comparison of research findings difficult, posing a challenge to our comprehensive understanding of the mechanisms underlying microplastic pollution [58,59]. In recent years, there have been calls for the standardisation of microplastic sampling methods to facilitate data comparability [38,39]. For instance, the collection of floating microplastics on ocean surface waters may consist of discrete sampling, such as with a Niskin bottle [60], or continuous sampling, such as with a manta or neuston trawls [61] or underway pump systems [62], all with varying error rates and sampling efficiencies. Comparisons are further complicated by variations in laboratory procedures, such as methods for digesting biotic material, subsampling, identification, and polymeric analysis [13,63,64]. An effort to standardise the data collection and analysis protocols for marine debris was largely initiated by interdisciplinary and international collaborative research projects. Foremost, the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) commission in 2010, and the JPI-Oceans BASEMAN project [65] delineated baselines and standards for the analysis of microplastics in European waters through a framework to address the multifaceted challenges posed by microplastic pollution. The methodological inconsistencies identified by the two research projects are related to differences in the lower and upper size limits implemented, the sensitivity of the applied extraction technique, differences in sampling techniques leading to a wide variety of reporting units, and inconsistent microplastic classifications.
There are several protocols and guidelines for reporting microplastics in water samples (e.g., Group of Experts on the Scientific Aspects of Marine Environmental Protection [37,44,66,67]; however, no standard protocol has been approved for the sampling, separation, and identification techniques used in different laboratories/countries, making comparisons challenging [17]. The variation throughout the literature also makes the spatial and temporal distribution of microplastics difficult. The lack of standardised protocols leads to unreliable or incomparable data on microplastic concentrations at national, regional, and global levels [3,68]. Moreover, the diverse research goals in the rapidly expanding field of microplastics limit the goal of a universal solution to capture the diversity in oceans [37,69].
The absolute volumes of plastic debris across different marine environments remain largely underestimated or unknown due to the lack of standardised monitoring, sampling, and quantification methods [20,64,70,71]. Despite the increase in the number of microplastics in the oceans [72], to date, only a few systematic reviews have estimated the concentration of microplastics specifically on the ocean surface compartment to generate datasets. These include [22], who used data on 680 surface tows to estimate microplastic abundance between 2007 and 2013, van Sebille, who synthesised microplastic data between 1979 and 2013 using published and unpublished literature, and [73], who estimated microplastic abundance using pelagic (upper ocean and water column) ocean samples. However, these systematic reviews differ in their scope and approach to analysis.
The management of marine plastic pollution is highly dependent on information about the physical properties, temporal trends, spatial distribution, and quantification of microplastics to determine the extent and severity of the problem in the context of past, present, and future projections, as well as inform laboratory studies by providing data on relevant concentrations of microplastics to which biota are exposed [15,17,30]. Therefore, even though it is challenging to evaluate and compare marine plastic data across different ocean compartments at local, national, and global levels, developing reliable standardised protocols will be crucial in the decision-making process for creating sustainable and effective mitigating measures. This information could potentially be used to assess the effectiveness of existing legislation and regulations for marine pollution control. The overall purpose is to reduce the amounts of plastic debris entering the coastal and marine environments. Integrating observation data from multiple ocean basins to analyse microplastic abundance can assist in bridging the gap between laboratory-based studies and real-world problems. Real data on microplastic abundance in the oceans is necessary to validate the accuracy of numerical models [37,73].
3.2. Data Analysis
Statistical descriptive analyses for the 73 studies (Table A1 in Appendix A) were conducted using Excel and STATA version 17. Normality was assessed using the Shapiro–Wilk test, guiding the choice between parametric and non-parametric statistical analyses. Non-parametric Kruskal–Wallis H tests were applied to evaluate group differences when data deviated from normal distributions. These tests are important to ensure that the statistical methods align with data distribution to avoid misinterpretation of data and subsequent analytical bias, thereby increasing the credibility of the results. Therefore, non-parametric statistics were applied in the qualitative data analysis using measures of central tendency. The data analysis was categorised into subgroups, including physical properties, methods of microplastic analysis, mean sizes, and mean concentrations of microplastics. Each of the subgroups was compared across the world’s five oceans to explain the heterogeneity among the studies. For the mean size and mean concentration of microplastics, the outliers were excluded to attain a Gaussian distribution.
Number of Studies and Their Distribution over the Years
Figure 2a illustrates the number of studies reviewed from each ocean. Out of the 105 observations, 42% (n = 44) were conducted for the Pacific Ocean, 32% (n = 34) for the Atlantic Ocean, 12% (n = 13) for the Indian Ocean, 10% (n = 10) for the Arctic Ocean, and 4% (n = 4) Southern Ocean. A Kruskal–Wallis H test for assessing non-normality provided evidence to reject the null hypothesis of no significant differences among the studies at α = 5% (χ2 (4) =11.530; p-value = 0.0212). Figure 2b shows the distribution and frequency of the reviewed studies across the years of publication from the year 2010 to 2023. Among the studies included in the review, no studies were published in the years 2011 and 2012. More than half (57%) of the studies were published in the years 2014, 2020, and 2022. The number of studies published has been increasing since 2018, with a high number of published papers in 2020 and 2022. A non-parametric Spearman test between the year of publication and the number of studies showed a positive correlation (r = 0.192, p-value = 0.0496).
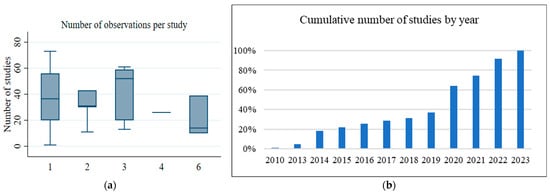
Figure 2.
(a) Number of observations per study. (b) Distribution of studies over the years of publication between 2010 and 2023.
3.3. Physical Properties
The identification of microplastic characteristics in marine and coastal environments is essential for comprehending the behaviour and fate of plastic debris [4,44,74]. Below, we discuss the physical properties of the ocean surface microplastics, including shapes, colour, and polymers. The physical features and oceanic dynamics exhibited substantial variability, which primarily influenced the concentrations of microplastics, resulting in an uneven distribution of microplastics in the ocean surface waters [43].
3.3.1. Shape
Microplastics can be categorised based on their morphology to provide insights into their origin and polymer composition [16,75]. Despite a lack of standardised methodology in morphology classifications, microplastics are mainly categorised as fragments, solid (hard) plastics and foamed plastics, fibres, spheres, films, sheets, lines, filaments, and pellets [44,76]. Microplastic shapes are known to influence their sinking and transportation behaviour and may also influence ingestion and clearance rates by aquatic species [56]. Theoretical and laboratory evidence has shown that irregular shapes exhibit slower rising or sinking velocities than spheres for the same polymer type, and fibres consistently align themselves horizontally [77]. A particle with a larger density but a less spherical shape sinks more slowly [56,78]. The knowledge of microplastic shape morphology can be used to determine their plausible origins [76]. Pellets and beads, for instance, are primary microplastics that are industrially pre-produced from personal care products, whereas secondary microplastics, such as fibres, are typically derived from fishing nets and synthetic textiles, while film/fragments are thought to originate from hard plastics through fragmentation of rigid plastic materials. Across the collected studies, we observed a variety of microplastic shapes, including fragments, foams, fibres, films, lines, pellets, beads, filaments, and spheres.
Figure 3a illustrates the diverse types of microplastic shapes in the oceans. Overall, out of the 105 observations reported by the 73 studies, with each study reporting more than 2 shapes in their sample, the overall predominant shapes in the ocean surface waters were fragments, fibres, and films, which accounted for more than half, followed by lines, foams, pellets, filaments, and beads, and the lowest was spheres. The prevalent shapes (fragments, fibres, and films) imply that secondary microplastics are the most prevalent in ocean surface waters, and according to [44], these are mainly hard plastics that generally originate from plastic bags, packaging materials, and fishing gear. Line and foam shapes are mainly microplastics from textiles and fishing nets [76,79]. This could confirm that 80% of plastics in oceans are from land-based sources [80]. The least dominant shapes (pellets and beads) indicate less industry-manufactured primary microplastics in ocean surface waters. Filaments are associated with fishing lines, while spheres are related to prolonged fragmentation of microplastics.
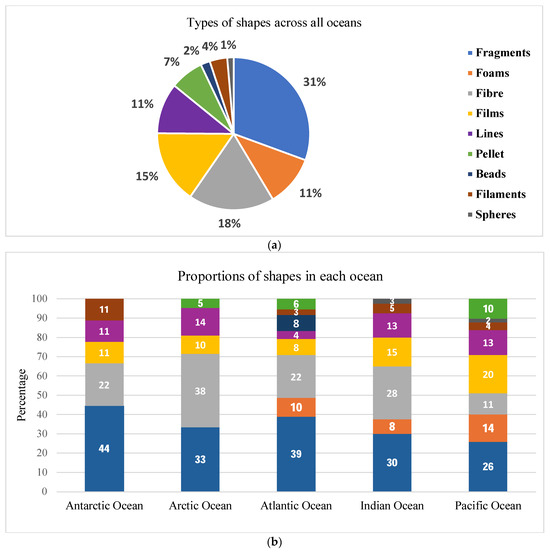
Figure 3.
(a) Proportions of types of shapes. (b) Proportions of types of shapes across the five oceans.
Figure 3b expounds on the proportions of the different shapes in each ocean. Fragments, fibres, films, and lines were found in all oceans. Fragments were dominant in the Southern and Atlantic Oceans. Fibres were abundant in the Arctic, Indian, Southern, and Atlantic Oceans, while lines were highly dominant in the Arctic Ocean, followed by the Pacific and Indian Oceans. Films were highly abundant in the Pacific and Indian Oceans. Foams were found in three oceans: highly prevalent in the Pacific, followed by the Atlantic and Indian Oceans. Pellets were found in three oceans: highly dominant in the Pacific Ocean, Atlantic Ocean, and least in the Arctic Ocean.
Filaments were found in all oceans except the Arctic and were dominant in the Southern and Indian Oceans. Spheres were found in two oceans, the Pacific and the Indian. Finally, beads were only found in the Atlantic Ocean. In summary, the presence of fragments, fibres, and films in all oceans indicates the presence of both primary and secondary microplastics, mainly emanating from single-use plastics and abandoned fishing gear. This is valuable information to further assess the origin and sources of microplastics.
3.3.2. Colour
Currently, a standard scheme for colour designation is lacking [44]. Considering the infinite spectra of colours, the 12 basic colour terms of the Inter-Society Colour Council National Bureau of Standards (ISCC-NBS) System of Colour Designation is often recommended [81]. Colour provides useful information for the potential source of plastic debris. For instance, transparent microplastics may be derived from single-use containers and packaging polymers, such as polypropylene [75,82]. Coloured microplastics are believed to emanate from consumer goods, such as cosmetics and textiles made of plastics [45,83,84], while blue microplastics have been linked to the fragmentation of fishing gear during aquaculture and fishing activities [85]. Colour has also been identified as a good predictor of ocean surface residence time and weathering [86]. For example, the yellowing or darkening of microplastics is largely due to the extent of aging and degradation [35,87].
In this study, seven colours were identified, including transparent, red, white, green, blue, yellow, black, and white. Overall, out of the 105 observations and with each study reporting more than 2 colours in their respective samples, the prevailing colour emerged as black, constituting 18% of the total, followed by blue (17%), transparent (16%), white (14%), red (13%), and green (12%), while yellow exhibited the lowest prevalence at 10% (Figure 4a). The heightened prevalence of the black colour may suggest the potential presence of microplastics undergoing prolonged aging within ocean surface waters [35].
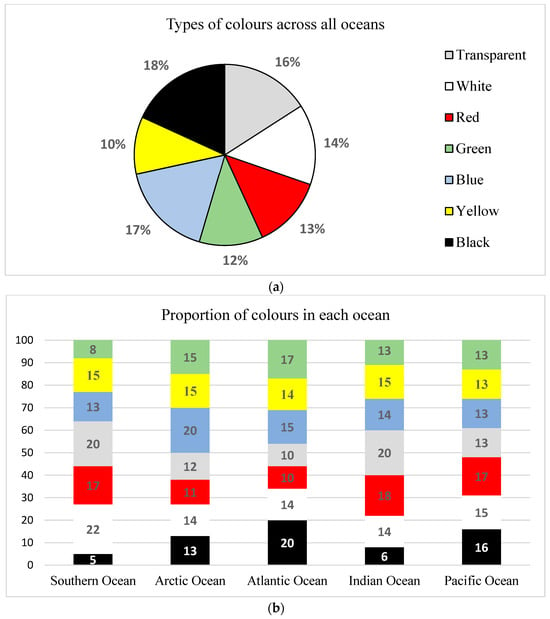
Figure 4.
(a) Types of colours found in the five oceans. (b) Proportions of types of colours across the five oceans.
As depicted in Figure 4b, the seven identified colours of microplastics were consistently observed in each of the five oceans. In the Pacific Ocean, red, transparent, and white hues predominated, with the remaining colours exhibiting relatively comparable proportions. In the Atlantic Ocean, transparent, black, and blue were most prevalent, with the least being red and green. Green and red were the most dominant in the Indian Ocean and transparent was the least. In the Arctic Ocean, blue emerged as the most dominant colour, whereas green exhibited the lowest prevalence. White was observed to be the most prevalent colour in the Southern Ocean, with transparent being the least prevalent.
The prevalence of white and transparent colours in most oceans may suggest prolonged exposure of vibrant plastics to sunlight, leading to fading owing to ultraviolet radiation. This process results in a transformation from their initial colours to the predominant white and transparent colours [59]. In addition, the dominance of transparent colour in the Atlantic and Pacific Oceans could indicate the prevalence of single-use plastics, especially in the subtropical gyres [88]. The blue, green, and red microplastics could indicate the diversity of both secondary and primary microplastics across all ocean surface waters, probably emanating from single-use plastics, textiles, and abandoned fishing nets [83]. The high dominance of black and yellow in all oceans could potentially implicate the presence of old and highly fragmented microplastics [89].
Polymer
Based on production volumes, the main polymers for both domestic and industrial purposes are polyethylene terephthalate (PET), polypropylene (PP), low- and high-density polyethylene (LDPE and HDPE), polyvinyl chloride (PVC), polystyrene (PS), and polyurethane (PU) [16,90]. However, a wide range of polymers is also produced commercially, including polyamide, polymethyl methacrylate, bioplastics, and polyethylene-vinyl acetate. Globally and over time, the market is dominated by six classes of plastics: polyethylene (PE, high and low density), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS, including expanded EPS), polyurethane (PUR), and polyethylene terephthalate (PET) [16,75].
In this study, different studies identified more than one type of polymer. Five polymers were identified, including PE, PP, PET, PP, and polyphthalamide (PPA). Overall, out of the 105 observations, PS, PET, and PPA were dominant across the five oceans, with the least prevalent being PP and PE, respectively (Figure 5a). In each ocean (Figure 5b), the five polymers had consistent prevalence, with comparable proportions across all oceans (except the least dominant PPA in the Arctic Ocean), which could be attributed to their buoyant density as well as their usage in day-to-day plastic products (such as plastic containers and bottles) across the globe [42].
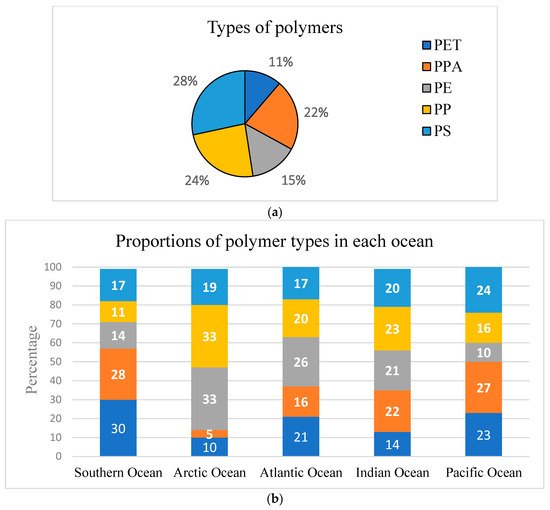
Figure 5.
(a) Proportions of types of polymers. (b) Proportions of types of polymers across the five oceans.
Globally, studies [58,91] have identified PE, PP, and PS to be predominant in surface waters due to their lower densities of between 0.90 and 0.97, 0.91 and 0.92, and 1.04 and 1.10 g/cm3 respectively. A possible explanation for the polymers with higher-density PET (1.37–1.45 g/cm3) and PPA (1.11–1.20 g/cm3) on surface waters is the influence of other complex environmental factors, including particle size and shape, ocean circulation, and sediment resuspension [91,92]. In addition, the density of microplastics is further influenced by environmental factors through biofouling, which influences the fate of microplastics [93]. For instance, [94] reported an increase in the final density of PP from 0.92 to 1.20 due to microalgae growth on the surface of the polymer.
3.4. Methods of Microplastics’ Analysis
The procedure for the analysis of microplastics in water samples follows several steps, including sampling, extraction, separation, identification, and quantification [95,96,97].
3.4.1. Sampling Methods
In this review, five sampling methods were identified across the accumulated literature. Overall, most studies reviewed used neuston nets and manta nets to sample surface waters, with fewer studies using plankton nets, stainless-steel sieves, and underway pump systems, respectively (Figure 6a). Most studies used more than one sampling method. The mesh sizes ranged from 25 to 500 µm, with 330 µm being the most common across all the sampling methods. The complexity in comparing studies on microplastics, as highlighted by [98], is primarily attributed to the diverse range of sampling methodologies employed, particularly in terms of mesh size and reported particle size.
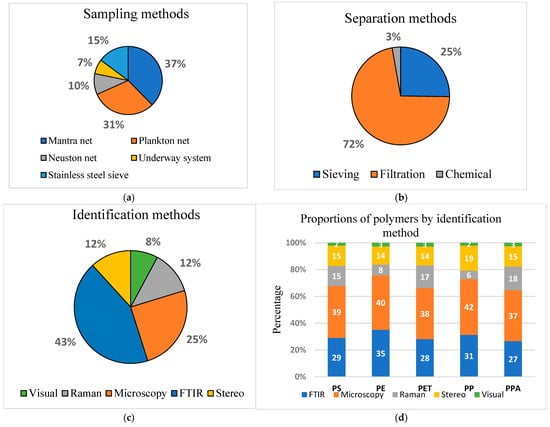
Figure 6.
(a) Types of separation methods. (b) Types of sampling methods. (c) Identification methods. (d) Proportions of polymers by identification methods.
3.4.2. Separation Methods
After sampling, microplastics are extracted from the water samples for further quantification and identification. The four commonly used extraction techniques are filtration (extraction by size), sieving, density separation (extraction by density difference), and chemical digestion (extraction by chemical reaction), as alluded by [35,99,100]. The separation methods used in the reviewed studies were sieving, filtration, and chemical digestion. In this study, sieving was used by most studies, followed by filtration, and the least used method was chemical digestion (Figure 6b). This finding reflects a methodological emphasis on physical separation techniques, particularly sieving and filtration. This approach allows researchers to capture a comprehensive range of microplastic sizes, enhancing the precision and applicability of their findings in understanding the dynamics of microplastics in ocean surface waters [101].
3.4.3. Identification Methods
The methods of identification and characterisation of microplastics range from visual identification to spectroscopy [44,101]. Most of the reviewed studies used more than one identification method. Fourier transform infrared spectroscopy (FTIR) and microscopy were predominantly utilised, with the least used being visual identification (Figure 6c). Comparisons of the identification methods and polymer characterisation (Figure 6d) showed that all five methods had been used in identifying the five polymers. The most common polymers identified by the visual methods were PE, PPA, and PET; PE, PP, and PS by FTIR; PP, PPA, and PS by stereo; PPA and PET by Raman, and PP and PE by microscopy.
The results of this analysis suggest that the studies that were examined employed a deliberate choice of methodologies, which reflected an equilibrium between precision, efficiency, and the specific features of the microplastics under investigation. Researchers appear to have opted for a combination of techniques to enhance the robustness and reliability of their findings, acknowledging the complementary strengths and pitfalls of each method [28] in addressing the complexities inherent in microplastic identification and characterisation.
3.5. Mean Sizes and Concentrations of the Microplastics
3.5.1. Mean Sizes of the Microplastics
There is no consensus on the upper and lower bound sizes of microplastics [102,103], and the classifications range from particles smaller than 1 mm [104,105] to less than 5 mm [102]. The upper size limits used in research range from 500 μm to either 1 mm or 5 mm at the upper limit [106]. However, this study used a more universally recommended classification by [16,107,108], who further classified the microplastics into three categories, including large (1–5 mm), small (<1–0.001 mm), and nano (<1 µm). The ocean litter sinking rate and rising velocity are influenced by the size of the microplastics [12,56,109].
The overall mean size for microplastics in all oceans ranged between 0.15 mm and 5 mm, with a mean and median of 2.50 mm and 2.63 mm, respectively, a first quartile (Q1) of 1.5 mm, and a third quartile (Q3) of 3.25 mm (Figure 7a). The highest microplastic size, with a mean of 2.5 mm and median of 4.39 mm (Q1 = 3.79 mm, Q2 = 5.00 mm, and Q3 = 5.00 mm), was found in the Southern Ocean. The Arctic Ocean showed a mean size of 3.55 mm (Q1 = 2.65 mm, Q2 = 3.49 mm, and Q3 = 5.00 mm), while the Pacific Ocean had a mean value of 2.54 mm (Q1 = 1.73 mm, Q2 = 2.49 mm, and Q3 = 3 mm). The Atlantic Ocean had a mean size of 2.34 mm (Q1 = 0.58 mm, Q2 = 3.49 mm, and Q3 = 3.59 mm), while the lowest MP mean size of 1.566 mm (Q1 = 0.37 mm, Q2 = 2.41 mm, and Q3 = 2.67 mm) was found in the Indian Ocean (Figure 7a).
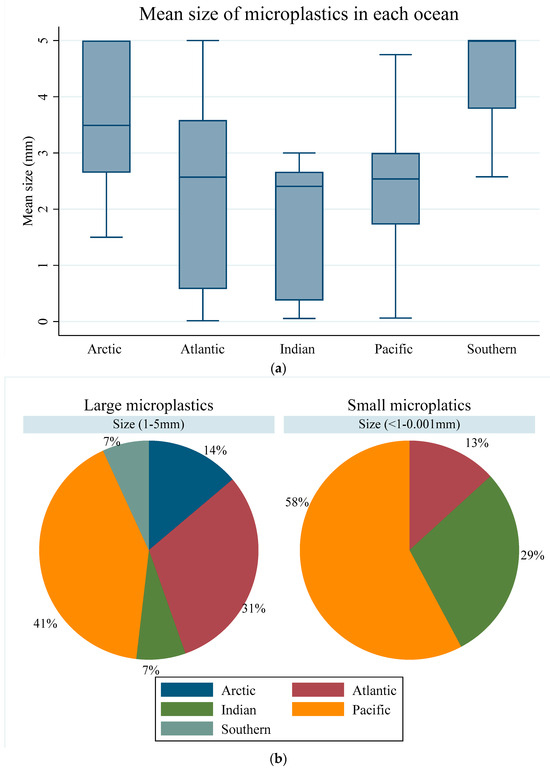
Figure 7.
(a) Mean size of microplastics over all oceans. (b) Classification of mean sizes of microplastic across all oceans.
A Shapiro–Wilk normality test conducted on the mean sizes (W = 0.95; p = 0.009) provided evidence to reject the null hypothesis of normality for the mean sizes. Due to the heterogeneous nature of the microplastic data, we further employed a non-parametric Kruskal–Wallis H test to examine the statistical differences for the mean sizes across the oceans. The results indicated significant differences and failed to reject the null hypothesis of no significant differences at alpha = 5% (Chi (4) = 14.53; p < 0.006). Furthermore, a Kruskal–Wallis H test to assess any changes in the mean size over the years of publication (2010 to 2023) showed no significant difference at alpha = 5% (Chi (2) = 18.52; p = 0.070).
When categorised into large (1–5 mm), small (<1–0.001 mm), and nano (<1 µm), the microplastics ranged between 0.15 and 5 mm. Consequently, only two classes of microplastics, large and small, were identified in our sample. Out of the total sample, large microplastics constituted 79%, with a mean of 3.09 and a median of 2.71 mm (Q1 = 2.51; Q3 = 3.77), while the small microplastics (21%) had a mean of 0.27 and the median was 0.21 (Q1 = 0.03; Q3 = 0.45).
Across the five oceans, large microplastics exhibited varying prevalence, with higher predominance observed in the Pacific, Atlantic, and Arctic Oceans, and comparably lower prevalence in the Indian and Southern Oceans (Figure 7b). Small microplastics, on the other hand, were identified in three oceans, with the Pacific Ocean having the highest proportion, followed by the Indian Ocean, and the Atlantic Ocean exhibiting the least (Figure 7b). A Kruskal–Wallis H test to assess the statistical differences between the two classes of microplastics across the five oceans showed evidence to reject the null hypothesis groups (Chi = 51.89; p < 0.001), implying significant differences among the groups.
The nuanced distribution of different sizes of microplastics across the world’s oceans is influenced by a myriad of interconnected factors, such as the multifaceted nature of their origin, human activities, transport pathways, and environmental interactions, emphasizing the need for comprehensive understanding to address the global issue of microplastic pollution. For instance, the formation of large microplastics is often linked to anthropogenic activities near the coastlines, such as maritime activities, industrial processes, and improper disposal of plastic waste [98,109,110]. In contrast, smaller microplastics may arise from the fragmentation and degradation of larger microplastic debris through atmospheric factors, such as ocean currents [111]. Both large and small microplastics may exhibit widespread distribution, being transported over long distances depending on the intensity of atmospheric processes and ocean currents. For example, small microplastics are often found in remote ocean areas, indicating the far-reaching impact of human activities on global microplastic dispersion [109].
3.5.2. Mean Concentration of Microplastics
Out of the 105 observations, 69% (n = 72) reported the mean concentration of microplastics in items/m3, while 31% (n = 33) reported in items/km2. Equation (1) in Section 2.2 was employed in the unit conversions. To compare the mean concentrations and sizes of microplastics across the oceans, a Shapiro–Wilk normality test was conducted, and the results showed non-normality for the mean concentration (W = 0.47; p = 0.054). Based on this outcome, a non-parametric Kruskal–Wallis H test was used to examine the statistical differences for the mean concentrations across the five oceans. The Kruskal–Wallis H test results (Chi (4) = 19.98; p < 0.001) failed to reject the null hypothesis of no significant difference at 1%, indicating significant differences in mean concentrations across the five oceans. A Shapiro–Wilk normality test was also conducted on the mean sizes, and the results (W = 0.97; p = 0.098) failed to reject the null hypothesis of normal distribution for the mean sizes at alpha = 5%. The abundance of microplastics tends to increase with decreasing size [112,113]; therefore, in any comparison of abundance, it is critical to consider the lower bound of microplastic size for sampling and detection. Due to the highly heterogeneous nature of microplastics in the oceans, which generally translates to non-normality of data, we reported the means and central tendency (median), as well as the first (Q1) and third quartile (Q3) ranges for both the concentrations and sizes of microplastics [80].
The overall concentrations of microplastics in all five oceans ranged between 0.002 and 62.50 items/m3, with a mean abundance of 2.76 items/m3, a median of 0.28 items/m3, and the first quartile (Q1) and third quartiles (Q3) were between 0.03 and 1.77 items/m3. In each ocean, the highest concentration of microplastics, with a mean of 4.98 (Q1 = 0.12, Q2 = 0.99, and Q3 = 4.08), was found in the Atlantic Ocean. This was followed by the Indian Ocean, with a mean value of 3.170 (Q1 = 0.01, Q2 = 1.25, and Q3 = 3.10), the Pacific Ocean, with a mean of 1.49 (Q1 = 0.01, Q2 = 0.06, and Q3 = 0.22), while the Arctic Ocean had a mean of 1.35 (Q1 = 0.34, Q2 = 1.01, and Q3 = 2.68). The lowest microplastic concentration mean and median of 0.04 (Q1 = 0.01, Q2 = 0.02, and Q3 = 0.07) was observed in the Southern Ocean (Figure 8a).
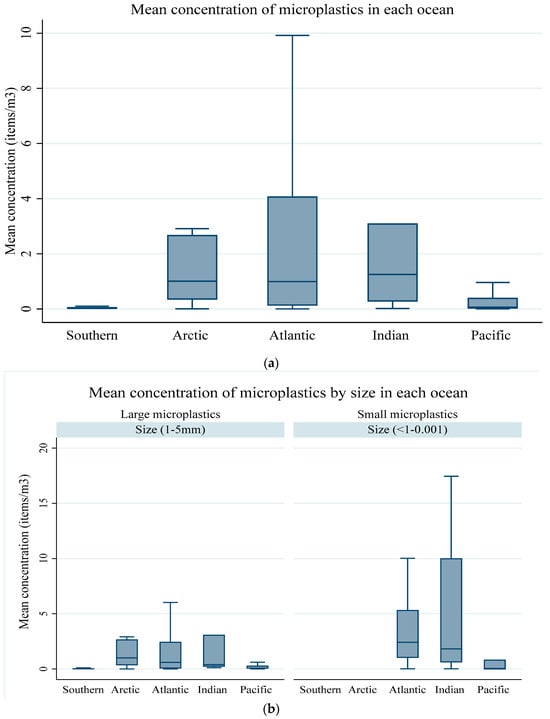
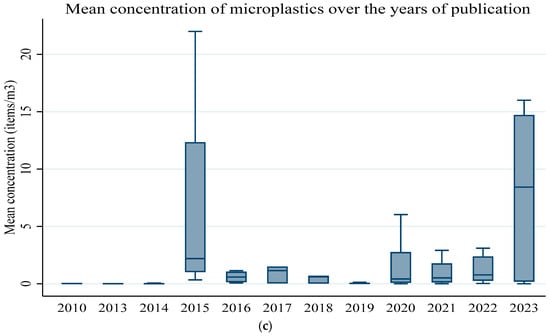
Figure 8.
(a) Mean concentrations of microplastics in each ocean. (b) Mean concentrations of microplastics in each ocean by size. (c) Mean concentrations of microplastics over the years of publication.
Figure 8b illustrates the mean concentration of microplastics in each ocean by size, small (1–5 mm) and large microplastics (<1–0.001 mm). When the two classes of microplastics were compared to the overall mean concentrations and sizes, a two-sample Kolmogorov–Smirnov test failed to reject the null hypothesis of normal distribution at 5% (p-value = 0.068) and 1% (p-value = 0.328). A non-parametric Spearman test between the mean concentration and size showed an insignificant negative correlation (r = −0.138; p-value = 0.160). The negative association was in line with research by [113], who alluded that the concentration of microplastics increases with a decrease in size, though our results were insignificant. The distribution of the microplastic abundance across the five oceans over time by year of publication is shown in Figure 8c. The mean concentration of microplastics varied over the years, with 2015 and 2023 exhibiting the highest values. A Kruskal–Wallis H test failed to reject the null hypothesis of no significant differences at alpha = 5% (Chi (11) = 35.64; p-value = 0.002) for the mean concentrations.
The variation in microplastic concentrations among the five oceans can be attributed to a complex interplay of diverse environmental factors, anthropogenic activities, and oceanographic dynamics. For instance, the Atlantic Ocean exhibited the highest mean concentration of microplastics (4.98 items/m3), which could potentially be explained by the extensive subtropical gyres, maritime traffic, high coastal population density, and inappropriate plastic waste disposal practices in surrounding regions [42,114]. The Indian Ocean followed closely, with a mean concentration of 3.170 items/m3, likely influenced by shipping routes, coastal urbanization, and regional waste management practices [115]. The Pacific Ocean, with a mean concentration of 1.49 items/m3, may indicate the impact of ocean currents, especially the subtropical gyres as well as proximity to major plastic pollution sources, such as the Great Pacific Garbage Patch [64]. The Arctic Ocean, characterized by its unique circulation patterns and cold-water conditions, exhibited a moderate mean concentration of 1.35 items/m3, influenced by both local and long-range transport of microplastics [116]. The Southern Ocean, with the lowest mean concentration of 0.04 items/m3, reflects its remoteness, limited human activities, and potential isolation from major plastic sources [117]. These variations highlight the multifaceted nature of microplastic distribution, which is influenced by both local and global factors. A map illustration of the distribution of the mean sizes of microplastics across the oceans is shown in Figure 9a and the distribution of the mean concentrations of microplastics across the oceans is depicted in Figure 9b.
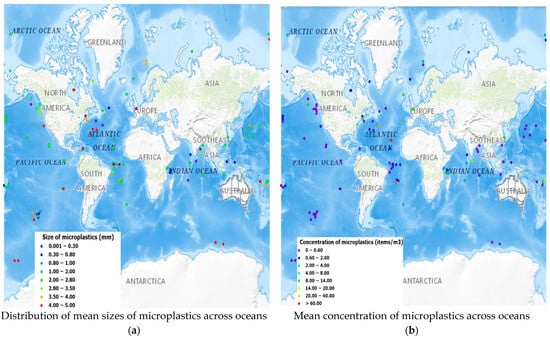
Figure 9.
Distribution of mean sizes and mean concentrations of microplastics across the ocean.
To summarise our results, we highlight other researchers estimating the abundance of microplastics across oceans in different scopes. A study by [54] estimated the accumulated number of microplastic particles in 2014, ranging from 15 to 51 trillion particles across the subtropical gyres in the North Atlantic and Pacific Oceans, weighing between 93,000 and 236,000 metric tonnes, with more than 90% of observations collected using a manta or neuston net with 333 mm mesh. Ref. [22] estimated the total number of plastic particles floating in the world’s five sub-tropical gyres between 2007 and 2013, using surface net tows and visual survey transects at a minimum of 5.25 trillion particles, weighing 268,940 tonnes. Ref. [73] estimated the average global microplastic abundance on upper surface oceans and the Laurentian Great Lakes at 24.4 trillion pieces using pelagic samples, with the sampling mesh size varying between 0.1 and 0.505 mm. A review by [118] highlighted that over 80% of field studies only sampled microplastics larger than 300 µm; therefore, microplastics smaller than this size, including 95% of cosmetic microbeads, synthetic microfibres, and secondary microplastics with diameters less than 300 µm, are absent from microplastic datasets. In the current study, the overall mean microplastic concentration in surface waters across the world’s five oceans was 2.76 items/m3, with a standard deviation of 7.48 items/m3 for microplastics ranging between 0.15 and 5 mm, using sampling mesh sizes between 25 and 500 µm. Therefore, standardisation and inter-calibration protocols for sampling microplastics in surface waters are essential if greater comparability between studies is to be achieved. In addition, most studies collected a one-time sample from the ocean waters, which may not be sufficient to draw important information about microplastics [37].
Forest Plot
We plotted a cumulative forest plot to help visualize the variation in the mean concentration of the microplastics for the reviewed studies at a 95% Confidence Interval (CI) (Figure 10). Figure 10 suggests that the precision of estimates increased as the CI narrowed. The largest CI was observed in 2010, which could be explained by the commencement of the standardisation of microplastic measurements [119], then it decreased between the years 2013 and 2020, and further decreased between 2020 and 2023. Ref. [120] posited that microplastic contamination in marine environments became a global environmental and public health concern between 2010 and 2020.
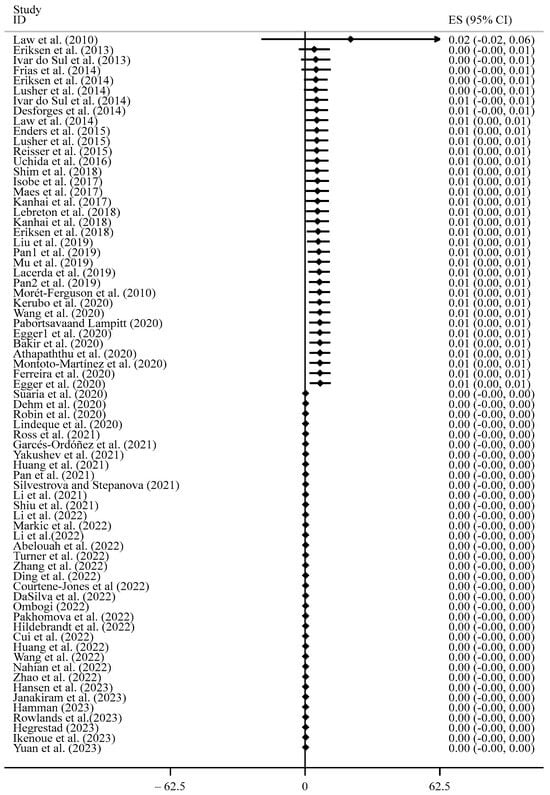
Figure 10.
Cumulative forest plot of the mean concentrations of microplastics [12,14,20,22,40,42,43,49,58,64,74,89,93,106,109,111,114,116,117,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171].
4. Discussion and Conclusions
Microplastics are ubiquitous in marine environments, and their adverse effects have been documented across all biota and humans; as a result, there has been an expansion in research to quantify the microplastics occurring in all ocean matrices. This has led to diverse methods and non-standardised approaches to reporting the sample collection, extraction, identification, and consequently, data analysis. This systematic review summarised the concentrations of microplastics in open surface waters of the world’s five oceans.
The overall concentration of microplastics in all oceans ranged between 0.002 and 62.50 items/m3, with a mean abundance of 2.76 items/m3, a median of 0.28 items/m3, and the first quartile (Q1) and third quartiles (Q3) were between 0.03 and 1.77 items/m3. The overall mean size for microplastics in all oceans ranged between 0.15 and 5 mm, with a mean and median of 2.50 mm and 2.63 mm, respectively, a first quartile (Q1) of 1.5 mm, and a third quartile (Q3) of 3.25 mm. The dominant colours, shapes, and polymers of microplastics were as follows: black, blue, and transparent; fragments, fibres, and films; polystyrene (PS), polyethylene terephthalate (PET), and polyphthalamide (PPA). This information can be used to inform interventions and evaluate their effectiveness in reducing marine plastic pollution in marine environments.
While it is possible to compare microplastic abundances in the world’s oceans across studies, one must be cognisant of the disparities in sampling, processing, and analytical methodologies for microplastics’ identification. In addition, it should be noted that microplastic concentrations are influenced by a plethora of variables, some of which include the location, atmospheric parameters, and oceanographic conditions. Even though we may not directly compare the results of this study to other studies due to the context and scope, assumptions of sampling depth, and mesh sizes employed to construct the pooled data, we can highlight a few studies that attempted to assess the global abundance of microplastics across surface waters of oceans or seas.
However, there is a need to specifically address the knowledge gaps that have been identified in this study. Unique techniques and insufficient reporting of data render the rapidly increasing microplastic studies relevant only in a limited scope, rather than useful and comparable in a more universal regional or global scope. Therefore, the ensuing findings are unsupportive of the common research efforts to standardise the microplastic protocols and methods. Despite the challenges encountered in estimations of microplastic abundance across ocean surface waters, these studies provide unequivocal baseline evidence that marine plastic debris and the subsequent microplastics are a persistent, transboundary problem, and thus a potential threat to the social, economic, and ecological well-being of marine environments. Comprehensively addressing the global issue of microplastic pollution requires a thorough understanding of their physical properties and abundance in marine environments. The distribution of microplastics in the world’s oceans is influenced by a multitude of interconnected factors, reflecting the complexity of their origin, human activities, transport pathways, and environmental interactions.
We recommend that studies provide detailed technique descriptions, including sampling device dimensions in width, depth, and height, as well as the device’s submersion depth to facilitate unit conversion or modelling. In addition, studies should routinely report both the count and weight data when using different trawl models or other sampling techniques to aid in the characterisation of variability. Sampling coordinates at each sampled location could also be reported to aid in mapping the concentrations across the globe. Estimating the concentration of microplastics necessitates a long-term effort; therefore, repeated samples and measurements should be collected over time, as intermittent site visits will not produce the trajectory required to evaluate trends and the efficacy of management interventions.
Furthermore, survey identification protocols for exceedingly small microplastics, particularly nanoplastics, require further development and optimisation of spectroscopy methods. The utilisation of the most common and tested procedures to standardise microplastic datasets, protocols, and methods would aid in global understanding of the problem. This will allow for data comparability, which will facilitate reproducible data for future meta-analyses. This study may contribute to a worldwide monitoring system and provide a current synthesis, which could aid policymakers in developing the appropriate mitigation strategies to curb marine plastic pollution in marine environments.
For future microplastic research, we recommend at a minimum documenting all the essential information for translating units and metrics, as standardisation is critical for compiling datasets. At the moment, the inconsistency in approaches has made meta-analysis challenging for microplastics due to the diversity of methods employed and insufficient study details being reported. The utilisation of the most prevalent and tested procedures and the reporting of exhaustive data will yield informative and universally comparable datasets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oceans5030024/s1, PRISMA 2020 Checklist [172].
Author Contributions
Conceptualization, J.M. and D.H.M.; methodology, J.M. and D.H.M.; validation, J.M.; formal analysis, J.M.; data curation, J.M.; writing—original draft preparation, J.M.; writing—review and editing, J.M., D.H.M., M.Y. and M.T.; visualization, J.M.; supervision, D.H.M., M.Y. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available at https://data.mendeley.com/datasets/hb3ty366bg/2.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Summary of the studies included in the review.
Table A1.
Summary of the studies included in the review.
| Authors | Observations | Year of Publication |
|---|---|---|
| Abelouah et al. (2022) [156] | 1 | 2022 |
| Athapaththu et al. (2020) [121] | 1 | 2020 |
| Bakir et al. (2020) [122] | 1 | 2020 |
| Courtene-Jones et al. (2022) [157] | 1 | 2022 |
| Cui et al. (2022) [111] | 1 | 2022 |
| da Silva et al. (2022) [158] | 1 | 2022 |
| Dehm et al. (2020) [123] | 1 | 2020 |
| Desforges et al. (2014) [106] | 1 | 2014 |
| Ding et al. (2022) [159] | 1 | 2022 |
| Egger et al. (2020) [124] | 2 | 2020 |
| Egger et al. (2020) [125] | 6 | 2020 |
| Enders et al. (2015) [126] | 1 | 2015 |
| Eriksen et al. (2014) [22] | 6 | 2014 |
| Eriksen et al. (2018) [127] | 1 | 2018 |
| Eriksen et al. (2013) [128] | 3 | 2013 |
| Ferreira et al. (2020) [129] | 1 | 2020 |
| Frias et al. (2014) [43] | 1 | 2014 |
| Garcés-Ordóñez et al. (2021) [130] | 1 | 2021 |
| Hamman (2023) [160] | 1 | 2023 |
| Hansen et al. (2023) [161] | 1 | 2023 |
| Hegrestad (2023) [162] | 3 | 2023 |
| Hildebrandt et al. (2022) [163] | 1 | 2022 |
| Huang et al. (2021) [131] | 1 | 2021 |
| Huang et al. (2022) [164] | 1 | 2022 |
| Ikenoue et al. (2023) [165] | 1 | 2023 |
| Isobe et al. (2017) [132] | 1 | 2017 |
| Ivar do Sul et al. (2014) [133] | 1 | 2014 |
| Ivar do Sul et al. (2013) [134] | 1 | 2013 |
| Janakiram et al. (2023) [89] | 1 | 2023 |
| Kanhai et al. (2018) [116] | 1 | 2018 |
| Kanhai et al. (2017) [42] | 1 | 2017 |
| Kerubo et al. (2020) [135] | 4 | 2020 |
| Lacerda et al. (2019) [136] | 2 | 2019 |
| Law et al. (2010) [137] | 1 | 2010 |
| Law et al. (2014) [138] | 1 | 2014 |
| Lebreton et al. (2018) [139] | 1 | 2018 |
| Li et al. (2021) [58] | 1 | 2021 |
| Li et al. (2022) [109] | 1 | 2022 |
| Lindeque et al. (2020) [40] | 6 | 2020 |
| Lindeque et al. (2020) [40] | 6 | 2020 |
| Liu et al. (2019) [140] | 1 | 2019 |
| Lusher et al. (2014) [93] | 1 | 2014 |
| Lusher et al. (2015) [141] | 2 | 2015 |
| Maes et al. (2017) [142] | 1 | 2017 |
| Markic et al. (2022) [143] | 1 | 2022 |
| Montoto-Martínez et al. (2020) [144] | 1 | 2020 |
| Morét-Ferguson et al. (2010) [145] | 1 | 2010 |
| Mu et al. (2019) [146] | 1 | 2019 |
| Nahian et al. (2022) [166] | 1 | 2022 |
| Ombongi (2022) [167] | 1 | 2022 |
| Pabortsava and Lampitt (2020) [147] | 3 | 2020 |
| Pakhomova et al. (2022) [49] | 1 | 2022 |
| Pan et al. (2019) [148] | 1 | 2019 |
| Pan et al. (2021) [149] | 1 | 2021 |
| Pan et al. (2019) [150] | 1 | 2019 |
| Reisser et al. (2015) [12] | 1 | 2015 |
| Robin et al. (2020) [151] | 1 | 2020 |
| Ross et al. (2021) [152] | 3 | 2021 |
| Rowlands et al. (2023) [168] | 1 | 2023 |
| Shim et al. (2018) [20] | 3 | 2018 |
| Shiu et al. (2021) [74] | 1 | 2021 |
| Silvestrova and Stepanova (2021) [114] | 2 | 2021 |
| Suaria et al. (2020) [153] | 1 | 2020 |
| Turner et al. (2022) [169] | 1 | 2022 |
| Uchida et al. (2016) [154] | 1 | 2016 |
| Wang et al. (2020) [64] | 1 | 2020 |
| Wang et al. (2022) [170] | 1 | 2022 |
| Yakushev et al. (2021) [155] | 1 | 2021 |
| Yuan et al. (2023) [171] | 1 | 2023 |
| Zhang et al. (2022) [117] | 1 | 2022 |
| Zhang et al. (2017) [85] | 1 | 2017 |
| Zhang et al. (2023) [21] | 1 | 2023 |
| Zhao et al. (2022) [14] | 1 | 2022 |
| Total number of observations | 105 |
References
- Harris, P.T.; Maes, T.; Raubenheimer, K.; Walsh, J.P. A marine plastic cloud-Global mass balance assessment of oceanic plastic pollution. Cont. Shelf Res. 2023, 255, 104947. [Google Scholar] [CrossRef]
- UNEP. Marine Plastic Debris and Microplastics-Global Lessons and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- Wang, Y.; Zhong, Z.; Chen, X.; Sokolova, I.; Ma, L.; Yang, Q.; Qiu, K.; Khan, F.U.; Tu, Z.; Guo, B.; et al. Microplastic pollution and ecological risk assessment of Yueqing Bay affected by intensive human activities. J. Hazard. Mater. 2024, 461, 132603. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Gaga, E.O.; Gedik, K. How can contamination be prevented during laboratory analysis of atmospheric samples for microplastics? Environ. Monit. Assess. 2024, 196, 159. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.D.; Kupchik, M.J.; Benfield, M.C. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut. 2017, 230, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, extraction of microplastics from edible salts and its removal from contaminated seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Nyadjro, E.S.; Webster, J.A.; Boyer, T.P.; Cebrian, J.; Collazo, L.; Kaltenberger, G.; Larsen, K.; Lau, Y.H.; Mickle, P.; Toft, T.; et al. The NOAA NCEI marine microplastics database. Sci. Data 2023, 10, 726. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Reisser, J.; Slat, B.; Noble, K.; Du Plessis, K.; Epp, M.; Proietti, M.; De Sonneville, J.; Becker, T.; Pattiaratchi, C. The vertical distribution of buoyant plastics at sea: An observational study in the North Atlantic Gyre. Biogeosciences 2015, 12, 1249–1256. [Google Scholar] [CrossRef]
- Sim, B.R.; Kim, H.C.; Kang, S.; Lee, D.I.; Hong, S.; Lee, S.H.; Kim, Y. Geochemical indicators for the recovery of sediment quality after the abandonment of oyster Crassostrea gigas farming in South Korea. Korean J. Fish. Aquat. Sci. 2020, 53, 773–783. [Google Scholar] [CrossRef]
- Zhao, S.; Zettler, E.R.; Bos, R.P.; Lin, P.; Amaral-Zettler, L.A.; Mincer, T.J. Large quantities of small microplastics permeate the surface ocean to abyssal depths in the South Atlantic Gyre. Glob. Chang. Biol. 2022, 28, 2991–3006. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sources, fate and effects of microplastics in the marine environment: A global assessment. In IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; Kershaw, P.J., Ed.; GESAMP: London, UK, 2015. [Google Scholar]
- Biswas, T.; Pal, S.C. Emerging threats of microplastics on marine environment: A critical review of toxicity measurement, policy practice gap and future research direction. J. Clean. Prod. 2023, 434, 139941. [Google Scholar] [CrossRef]
- Du, J.; Gao, J.; Liu, G.; Song, Y.; Yang, A.; Wang, H.; Ding, Y.; Wang, Q. Bibliometric profile of global microplastics research from 2004 to 2019. Int. J. Environ. Res. Public Health 2020, 17, 5639. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.C.; Pham, M.H. Ecotoxicological effects of microplastics on aquatic organisms: A review. Environ. Sci. Pollut. Res. 2021, 28, 44716–44725. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S. Marine microplastics: Abundance, distribution, and composition. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Yan, P.; Wang, J.; Yan, S.; Liu, X.; Aurangzeib, M. Microplastic migration and distribution in the terrestrial and aquatic environments: A threat to biotic safety. J. Environ. Manag. 2023, 333, 117412. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; King, A.E.; Ramirez, J.M. Plastic debris: An overview of composition, sources, environmental occurrence, transport, and fate. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–31. [Google Scholar]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- UNIDO. Addressing the Challenge of Marine Plastic Litter Using Circular Economy Methods: Relevant Considerations. United Nations Working Paper. 2019. Available online: https://www.unido.org/sites/default/files/files/2019-06/UNIDO_Addressing_the_challenge_of_Marine_Plastic_Litter_Using_Circular_Economy_0.pdf (accessed on 28 May 2024).
- Thacharodi, A.; Meenatchi, R.; Hassan, S.; Hussain, N.; Bhat, M.A.; Arockiaraj, J.; Ngo, H.H.; Le, Q.H.; Pugazhendhi, A. Microplastics in the environment: A critical overview on its fate, toxicity, implications, management, and bioremediation strategies. J. Environ. Manag. 2024, 349, 119433. [Google Scholar] [CrossRef]
- Thompson, R.C. Microplastics in the marine environment: Sources, consequences and solutions. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 185–200. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Duarte, A.C. Introduction to the analytical methodologies for the analysis of microplastics. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of microplastics in commercial seafood under the perspective of the human food chain. A review. J. Agric. Food Chem. 2020, 68, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, Z.; Van Berkel, R. Unlocking circular economy for prevention of marine plastic pollution: An exploration of G20 policy and initiatives. J. Environ. Manag. 2021, 277, 111457. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, F.; Quan, S.; Chen, L.; Shen, A.; Jiao, A.; Qi, H.; Yu, G. Microplastics in the Bronchoalveolar lavage fluid of Chinese children: Associations with age, City development, and disease features. Environ. Sci. Technol. 2023, 57, 12594–12601. [Google Scholar] [CrossRef] [PubMed]
- Johannes, H.P.; Kojima, M.; Iwasaki, F.; Edita, E.P. Applying the extended producer responsibility towards plastic waste in Asian developing countries for reducing marine plastic debris. Waste Manag. Res. 2021, 39, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef]
- Russell, M.; Webster, L. Microplastics in sea surface waters around Scotland. Mar. Pollut. Bull. 2021, 166, 112210. [Google Scholar] [CrossRef] [PubMed]
- Fok, L.; Lam, T.W.L.; Li, H.X.; Xu, X.R. A meta-analysis of methodologies adopted by microplastic studies in China. Sci. Total Environ. 2020, 718, 135371. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Thomposon, R.C. Microplastics in the ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 265–268. [Google Scholar] [CrossRef]
- Cowger, W.; Booth, A.M.; Hamilton, B.M.; Thaysen, C.; Primpke, S.; Munno, K.; Lusher, A.L.; Dehaut, A.; Vaz, V.P.; Liboiron, M.; et al. Reporting guidelines to increase the reproducibility and comparability of research on microplastics. Appl. Spectrosc. 2020, 74, 1066–1077. [Google Scholar] [CrossRef]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 250. [Google Scholar] [CrossRef]
- Kanhai, L.D.K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Report and Studies No. 99. In Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean; GESAMP: London, UK, 2019; Available online: http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean (accessed on 28 May 2024).
- Liu, S.; Li, Y.; Wang, F.; Gu, X.; Li, Y.; Liu, Q.; Li, L.; Bai, F. Temporal and spatial variation of microplastics in the urban rivers of Harbin. Sci. Total Environ. 2024, 910, 168373. [Google Scholar] [CrossRef]
- Lu, H.C.; Ziajahromi, S.; Neale, P.A.; Leusch, F.D. A systematic review of freshwater microplastics in water and sediments: Recommendations for harmonization to enhance future study comparisons. Sci. Total Environ. 2021, 781, 146693. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Gimenez, B.C.G. Microplastics in the marine environment: Current trends and future perspectives. Mar. Pollut. Bull. 2015, 97, 5–12. [Google Scholar] [CrossRef]
- Pakhomova, S.; Berezina, A.; Lusher, A.L.; Zhdanov, I.; Silvestrova, K.; Zavialov, P.; van Bavel, B.; Yakushev, E. Microplastic variability in subsurface water from the Arctic to Antarctica. Environ. Pollut. 2022, 298, 118808. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Kreczak, H.; Willmott, A.J.; Baggaley, A.W. Subsurface dynamics of buoyant microplastics subject to algal biofouling. Limnol. Oceanogr. 2021, 66, 3287–3299. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Ballent, A.; Pando, S.; Purser, A.; Juliano, M.F.; Thomsen, L. Modelled transport of benthic marine microplastic pollution in the Nazaré Canyon. Biogeosciences 2013, 10, 7957–7970. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef]
- Kukulka, T.; Proskurowski, G.; Morét-Ferguson, S.; Meyer, D.W.; Law, K.L. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 2012, 39, 7. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic microplastics in surface water of the Eastern Indian Ocean during monsoon transition period: Abundance, distribution, and characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Huang, J.; Ho, Y.W.; Fang, J.K.H.; Lam, E.Y. High-throughput microplastic assessment using polarization holographic imaging. Sci. Rep. 2024, 14, 2355. [Google Scholar] [CrossRef] [PubMed]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sadri, S.S.; Thompson, R.C. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014, 81, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, L.D.K.; Asmath, H.; Gobin, J.F. The status of marine debris/litter and plastic pollution in the Caribbean Large Marine Ecosystem (CLME): 1980–2020. Environ. Pollut. 2022, 300, 118919. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Filgueiras, A.; Pedrotti, M.L.; Caetano, M.; Frias, J. Standardised Protocol for Monitoring Microplastics in Seawater. Deliverable 4.1; JPI Oceans: Brussels, Belgium, 2019. [Google Scholar] [CrossRef]
- Brander, S.M.; Renick, V.C.; Foley, M.M.; Steele, C.; Woo, M.; Lusher, A.; Carr, S.; Helm, P.; Box, C.; Cherniak, S.; et al. Sampling and quality assurance and quality control: A guide for scientists investigating the occurrence of microplastics across matrices. Appl. Spectrosc. 2020, 74, 1099–1125. [Google Scholar] [CrossRef]
- Michida, Y.; Chavanich, S.; Chiba, S.; Cordova, M.R.; Cozsar Cabanas, A.; Glagani, F.; Hagmann, P.; Hinata, H.; Isobe, A.; Kershaw, P.; et al. Guidelines for Harmonizing Ocean Surface Microplastic Monitoring Methods, Version 1.1; Ministry of the Environment, Japan: Tokyo, Japan, 2019. [Google Scholar] [CrossRef]
- Provencher, J.F.; Liboiron, M.; Borrelle, S.B.; Bond, A.L.; Rochman, C.; Lavers, J.L.; Avery-Gomm, S.; Yamashita, R.; Ryan, P.G.; Lusher, A.L.; et al. A Horizon Scan of research priorities to inform policies aimed at reducing the harm of plastic pollution to biota. Sci. Total Environ. 2020, 733, 139381. [Google Scholar] [CrossRef]
- Mofokeng, R.P.; Faltynkova, A.; Alfonso, M.B.; Boujmil, I.; Carvalho, I.R.B.; Lunzalu, K.; Zanuri, N.M.; Nyadjro, E.S.; Puskic, P.S.; Lindsay, D.J.; et al. The future of ocean plastics: Designing diverse collaboration frameworks. ICES J. Mar. Sci. 2024, 81, 43–54. [Google Scholar] [CrossRef]
- Uhrin, A.V.; Hong, S.; Burgess, H.K.; Lim, S.; Dettloff, K. Towards a North Pacific long-term monitoring program for ocean plastic pollution: A systematic review and recommendations for shorelines. Environ. Pollut. 2022, 310, 119862. [Google Scholar] [CrossRef]
- United Nation Environmental Programme (UNEP). From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; United Nation Environmental Programme (UNEP): Nairobi, Kenya, 2021; Available online: https://malaysia.un.org/en/171922-pollution-solution-global-assessment-marine-litter-and-plastic-pollution (accessed on 28 May 2024).
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Azuma, T.; Cordova, M.R.; Cózar, A.; Galgani, F.; Hagita, R.; Kanhai, L.D.; Imai, K.; Iwasaki, S.; Kako, S.I.; et al. A multilevel dataset of microplastic abundance in the world’s upper ocean and the Laurentian Great Lakes. Microplast. Nanoplast. 2021, 1, 16. [Google Scholar] [CrossRef]
- Shiu, R.F.; Gong, G.C.; Fang, M.D.; Chow, C.H.; Chin, W.C. Marine microplastics in the surface waters of “pristine” Kuroshio. Mar. Pollut. Bull. 2021, 172, 112808. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and nanoplastics analysis: Options, imaging, advancements and challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]
- Zeng, G.; Ma, Y.; Du, M.; Chen, T.; Lin, L.; Dai, M.; Luo, H.; Hu, L.; Zhou, Q.; Pan, X. Deep convolutional neural networks for aged microplastics identification by Fourier transform infrared spectra classification. Sci. Total Environ. 2024, 913, 169623. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Schüttrumpf, H. Effects of particle properties on the settling and rise velocities of microplastics in freshwater under laboratory conditions. Environ. Sci. Technol. 2019, 53, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, J.S.; Kim, S.Y.; Song, N.S.; La, H.S.; Yang, E.J. Arctic Ocean sediments as important current and future sinks for marine microplastics missing in the global microplastic budget. Sci. Adv. 2023, 9, eadd2348. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is it or isn’t it: The importance of visual classification in microplastic characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Galgani, F.; Pham, C.K.; Reisser, J. Plastic pollution. Front. Mar. Sci. 2017, 4, 307. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The colors of the ocean plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. Available online: https://pubs.acs.org/doi/pdf/10.1021/acs.est.9b06400 (accessed on 28 May 2024). [CrossRef] [PubMed]
- Wang, Z.M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.A.P.; Da Costa, J.; Duarte, A.C.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Duarte, A.C.; da Costa, J.P. Staining methodologies for microplastics screening. TrAC Trends Anal. Chem. 2024, 172, 117555. [Google Scholar] [CrossRef]
- Fox, S.; Stefánsson, H.; Ásbjörnsson, E.J.; Peternell, M.; Wanner, P.; Sturkell, E.; Konrad-Schmolke, M.; Zlotskiy, E. Physical characteristics of microplastic particles and potential for global atmospheric transport: A meta-analysis. Environ. Pollut. 2023, 342, 122938. [Google Scholar] [CrossRef]
- Janakiram, R.; Keerthivasan, R.; Janani, R.; Ramasundaram, S.; Martin, M.V.; Venkatesan, R.; Murthy, M.R.; Sudhakar, T. Seasonal distribution of microplastics in surface waters of the Northern Indian Ocean. Mar. Pollut. Bull. 2023, 190, 114838. [Google Scholar] [CrossRef]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Hui, J.; Irvine, J.T.; Lawton, L.A. Characterisation of microplastics is key for reliable data interpretation. Chemosphere 2023, 331, 138691. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Bergmann, M.; Tekman, M.B.; Gutow, L. Sea change for plastic pollution. Nature 2017, 544, 297. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Lee, D.Y.; Park, J.; Zhou, J.L.; Yoon, M.H. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, X.; Niu, X.; Zeng, J.; Zhou, Y.; Suona, Z.; Yuan, Y.; Chen, X. Overview of analytical methods for the determination of microplastics: Current status and trends. TrAC Trends Anal. Chem. 2023, 167, 117261. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Strafella, P.; López Correa, M.; Pyko, I.; Teichert, S.; Gomiero, A. Distribution of microplastics in the marine environment. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 813–847. [Google Scholar]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Li, H.X.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef]
- So, W.K.; Chan, K.; Not, C. Abundance of plastic microbeads in Hong Kong coastal water. Mar. Pollut. Bull. 2018, 133, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A complete guide to extraction methods of microplastics from complex environmental matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Chae, B.; Oh, S. Is 5 mm still a good upper size boundary for microplastics in aquatic environments? Perspectives on size distribution and toxicological effects. Mar. Pollut. Bull. 2023, 196, 115591. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Mar. Anthropog. Litter; 2015; pp. 229–244. Available online: https://library.oapen.org/bitstream/handle/20.500.12657/28030/1001966.pdf? (accessed on 28 May 2024).
- Galloway, T.S.; Lewis, C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA 2016, 113, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, L.; Wang, X.; Liu, K.; Li, D. Cross-oceanic distribution and origin of microplastics in the subsurface water of the South China Sea and Eastern Indian Ocean. Sci. Total Environ. 2022, 805, 150243. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Furue, R. A model for the size distribution of marine microplastics: A statistical mechanics approach. PLoS ONE 2021, 16, e0259781. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Gao, P.; Zhang, M.; Wang, L.; Sun, H.; Liu, C. Adverse effects of microplastics on earthworms: A critical review. Sci. Total Environ. 2022, 850, 158041. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef]
- Silvestrova, K.; Stepanova, N. The distribution of microplastics in the surface layer of the Atlantic Ocean from the subtropics to the equator according to visual analysis. Mar. Pollut. Bull. 2021, 162, 111836. [Google Scholar] [CrossRef] [PubMed]
- Pattiaratchi, C.; van der Mheen, M.; Schlundt, C.; Narayanaswamy, B.E.; Sura, A.; Hajbane, S.; White, R.; Kumar, N.; Fernandes, M.; Wijeratne, S. Plastics in the Indian Ocean–sources, fate, distribution and impacts. Ocean Sci. Discuss. 2021, 2021, 1–40. [Google Scholar]
- Kanhai, L.D.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Ju, M.; Qu, L.; Chu, X.; Huo, C.; Wang, J. Distribution characteristics of microplastics in surface and subsurface Antarctic seawater. Sci. Total Environ. 2022, 838, 156051. [Google Scholar] [CrossRef] [PubMed]
- Conke, L.S.; Nascimento, E.P.D. Selective waste collection in Brazil: Comparing reports and research methodologies. Urbe. Rev. Bras. Gestão Urbana 2018, 10, 199–212. [Google Scholar] [CrossRef]
- Oslo and Paris Convention (OSPAR). Guideline for Monitoring Marine Litter on the Beaches in the OSPAR Maritime Area; OSPAR Commission: London, UK, 2010. [Google Scholar] [CrossRef]
- Perumal, K.; Muthuramalingam, S. Global sources, abundance, size, and distribution of microplastics in marine sediments-A critical review. Estuar. Coast. Shelf Sci. 2022, 264, 107702. [Google Scholar] [CrossRef]
- Athapaththu, A.M.A.I.K.; Thushari, G.G.N.; Dias, P.C.B.; Abeygunawardena, A.P.; Egodauyana, K.P.U.T.; Liyanage, N.P.P.; Pitawala, H.M.J.C.; Senevirathna, J.D.M. Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): Distribution and characterization by FTIR. Mar. Pollut. Bull. 2020, 161, 111750. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Desender, M.; Wilkinson, T.; Van Hoytema, N.; Amos, R.; Airahui, S.; Graham, J.; Maes, T. Occurrence and abundance of meso and microplastics in sediment, surface waters, and marine biota from the South Pacific region. Mar. Pollut. Bull. 2020, 160, 111572. [Google Scholar] [CrossRef] [PubMed]
- Dehm, J.; Singh, S.; Ferreira, M.; Piovano, S. Microplastics in subsurface coastal waters along the southern coast of Viti Levu in Fiji, South Pacific. Mar. Pollut. Bull. 2020, 156, 111239. [Google Scholar] [CrossRef]
- Egger, M.; Nijhof, R.; Quiros, L.; Leone, G.; Royer, S.J.; McWhirter, A.C.; Kantakov, G.A.; Radchenko, V.I.; Pakhomov, E.A.; Hunt, B.P.; et al. A spatially variable scarcity of floating microplastics in the eastern North Pacific Ocean. Environ. Res. Lett. 2020, 15, 114056. [Google Scholar] [CrossRef]
- Egger, M.; Sulu-Gambari, F.; Lebreton, L. First evidence of plastic fallout from the North Pacific Garbage Patch. Sci. Rep. 2020, 10, 7495. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Liboiron, M.; Kiessling, T.; Charron, L.; Alling, A.; Lebreton, L.; Richards, H.; Roth, B.; Ory, N.C.; Hidalgo-Ruz, V.; et al. Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 2018, 232, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Maximenko, N.; Thiel, M.; Cummins, A.; Lattin, G.; Wilson, S.; Hafner, J.; Zellers, A.; Rifman, S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013, 68, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Thompson, J.; Paris, A.; Rohindra, D.; Rico, C. Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar. Pollut. Bull. 2020, 153, 110991. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Espinosa, L.F.; Costa Muniz, M.; Salles Pereira, L.B.; Meigikos dos Anjos, R. Abundance, distribution, and characteristics of microplastics in coastal surface waters of the Colombian Caribbean and Pacific. Environ. Sci. Pollut. Res. 2021, 28, 43431–43442. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Song, B.; Deng, J.; Shen, M.; Chen, Q.; Zeng, G.; Liang, J. Microplastics in the coral reefs and their potential impacts on corals: A mini-review. Sci. Total Environ. 2021, 762, 143112. [Google Scholar] [CrossRef]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F.; Fillmann, G. Microplastics in the pelagic environment around oceanic islands of the Western Tropical Atlantic Ocean. Water Air Soil Pollut. 2014, 225, 2004. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Costa, M.F.; Barletta, M.; Cysneiros, F.J.A. Pelagic microplastics around an archipelago of the Equatorial Atlantic. Mar. Pollut. Bull. 2013, 75, 305–309. [Google Scholar] [CrossRef]
- Kerubo, J.O.; Muthumbi, A.W.; Onyari, J.M.; Kimani, E.N.; Robertson-Andersson, D. Microplastic pollution in the surface waters of creeks along the Kenyan coast, Western Indian Ocean (WIO). West. Indian Ocean J. Mar. Sci. 2020, 19, 75–88. [Google Scholar] [CrossRef]
- Lacerda, A.L.D.F.; Rodrigues, L.D.S.; Van Sebille, E.; Rodrigues, F.L.; Ribeiro, L.; Secchi, E.R.; Kessler, F.; Proietti, M.C. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Morét-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic accumulation in the North Atlantic subtropical gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; DeForce, E.; Kukulka, T.; Proskurowski, G. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, F.; Song, Z.; Zong, C.; Wei, N.; Li, D. A novel method enabling the accurate quantification of microplastics in the water column of deep ocean. Mar. Pollut. Bull. 2019, 146, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef]
- Maes, T.; Van der Meulen, M.D.; Devriese, L.I.; Leslie, H.A.; Huvet, A.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastics baseline surveys at the water surface and in sediments of the North-East Atlantic. Front. Mar. Sci. 2017, 4, 135. [Google Scholar] [CrossRef]
- Markic, A.; Bridson, J.H.; Morton, P.; Hersey, L.; Maes, T.; Bowen, M. Microplastic pollution in the surface waters of Vava’u, Tonga. Mar. Pollut. Bull. 2022, 185, 114243. [Google Scholar] [CrossRef]
- Montoto-Martinez, T.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Pump-underway ship intake: An unexploited opportunity for Marine Strategy Framework Directive (MSFD) microplastic monitoring needs on coastal and oceanic waters. PLoS ONE 2020, 15, e0232744. [Google Scholar] [CrossRef]
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhang, S.; Qu, L.; Jin, F.; Fang, C.; Ma, X.; Zhang, W.; Wang, J. Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea. Mar. Pollut. Bull. 2019, 143, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guo, H.; Chen, H.; Wang, S.; Sun, X.; Zou, Q.; Zhang, Y.; Lin, H.; Cai, S.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Liu, Q.; Sun, X.; Li, W.; Zou, Q.; Cai, S.; Lin, H. Widespread occurrence of microplastic pollution in open sea surface waters: Evidence from the mid-North Pacific Ocean. Gondwana Res. 2021, 108, 31–40. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Sun, Y.; Sun, X.; Lin, H. Environmental implications of microplastic pollution in the Northwestern Pacific Ocean. Mar. Pollut. Bull. 2019, 146, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Robin, R.S.; Karthik, R.; Purvaja, R.; Ganguly, D.; Anandavelu, I.; Mugilarasan, M.; Ramesh, R. Holistic assessment of microplastics in various coastal environmental matrices, southwest coast of India. Sci. Total Environ. 2020, 703, 134947. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.A.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive distribution of polyester fibres in the Arctic Ocean is driven by Atlantic inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro-and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Uchida, K.; Hagita, R.; Hayashi, T.; Tokai, T. Distribution of small plastic fragments floating in the western Pacific Ocean from 2000 to 2001. Fish. Sci. 2016, 82, 969–974. [Google Scholar] [CrossRef]
- Yakushev, E.; Gebruk, A.; Osadchiev, A.; Pakhomova, S.; Lusher, A.; Berezina, A.; van Bavel, B.; Vorozheikina, E.; Chernykh, D.; Kolbasova, G.; et al. Microplastics distribution in the Eurasian Arctic is affected by Atlantic waters and Siberian rivers. Commun. Earth Environ. 2021, 2, 23. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Ben-Haddad, M.; Hajji, S.; De-la-Torre, G.E.; Aziz, T.; Abou Oualid, J.; Banni, M.; Alla, A.A. Floating microplastics pollution in the Central Atlantic Ocean of Morocco: Insights into the occurrence, characterization, and fate. Mar. Pollut. Bull. 2022, 182, 113969. [Google Scholar] [CrossRef] [PubMed]
- Courtene-Jones, W.; Liu, K.; Li, D. Estimating Microplastics in Deep Water. Plast. Ocean. Orig. Charact. Fate Impacts 2022, 131–149. [Google Scholar] [CrossRef]
- Da Silva, E.F.; do Carmo, D.D.F.; Muniz, M.C.; Dos Santos, C.A.; Cardozo, B.B.I.; de Oliveira Costa, D.M.; Dos Anjos, R.M.; Vezzone, M. Evaluation of microplastic and marine debris on the beaches of Niterói Oceanic Region, Rio De Janeiro, Brazil. Mar. Pollut. Bull. 2022, 175, 113161. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Zheng, L.; Dai, D.; Li, F. Atmospheric microplastics in the Northwestern Pacific Ocean: Distribution, source, and deposition. Sci. Total Environ. 2022, 829, 154337. [Google Scholar] [CrossRef] [PubMed]
- Hamman, M. Microplastic Pollution in The Ocean and Coral Reefs of the Western Indian Ocean. Ph.D. Dissertation, North-West University, Potchefstroom, South Africa, 2023. Available online: https://repository.nwu.ac.za/bitstream/handle/10394/42127/Hamman_M.pdf?sequence=1 (accessed on 28 May 2024).
- Hansen, J.; Hildebrandt, L.; Zimmermann, T.; El Gareb, F.; Fischer, E.K.; Pröfrock, D. Quantification and characterization of microplastics in surface water samples from the Northeast Atlantic Ocean using laser direct infrared imaging. Mar. Pollut. Bull. 2023, 190, 114880. [Google Scholar] [CrossRef] [PubMed]
- Hegrestad, M. Microplastic in Subsurface Water in the South Pacific Ocean (Master’s Thesis, ). Master's Thesis, The University of Bergen, Bergen, Norway, 2023. Available online: https://bora.uib.no/bora-xmlui/bitstream/handle/11250/3086744/Microplastic-in-subsurface-water-in-the-South-Pacific-Ocean.pdf?sequence=1 (accessed on 28 May 2024).
- Hildebrandt, L.; El Gareb, F.; Zimmermann, T.; Klein, O.; Kerstan, A.; Emeis, K.C.; Pröfrock, D. Spatial distribution of microplastics in the tropical Indian Ocean based on laser direct infrared imaging and microwave-assisted matrix digestion. Environ. Pollut. 2022, 307, 119547. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Zhang, S.; Jin, F.; Fang, C.; Ma, X.; Wang, J.; Mu, J. Systematical insights into distribution and characteristics of microplastics in near-surface waters from the East Asian Seas to the Arctic Central Basin. Sci. Total Environ. 2022, 814, 151923. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Nakajima, R.; Fujiwara, A.; Onodera, J.; Itoh, M.; Toyoshima, J.; Watanabe, E.; Murata, A.; Nishino, S.; Kikuchi, T. Horizontal distribution of surface microplastic concentrations and water-column microplastic inventories in the Chukchi Sea, western Arctic Ocean. Sci. Total Environ. 2023, 855, 159564. [Google Scholar] [CrossRef]
- Nahian, S.; Rakib, M.R.J.; Haider, S.M.B.; Kumar, R.; Mohsen, M.; Sharma, P.; Khandaker, M.U. Occurrence, spatial distribution, and risk assessment of microplastics in surface water and sediments of Saint Martin Island in the Bay of Bengal. Mar. Pollut. Bull. 2022, 179, 113720. [Google Scholar] [CrossRef]
- Ombongi, J.K. Microplastic Pollution along the Kenya Coast in the Western Indian Ocean (Wio), Region. Ph.D. Dissertation, University of Nairobi, Nairobi, Kenya, 2022. Available online: http://erepository.uonbi.ac.ke/handle/11295/161672 (accessed on 28 May 2024).
- Rowlands, E.; Galloway, T.; Cole, M.; Peck, V.L.; Posacka, A.; Thorpe, S.; Manno, C. Vertical flux of microplastic, a case study in the Southern Ocean, South Georgia. Mar. Pollut. Bull. 2023, 193, 115117. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. PBDEs in the marine environment: Sources, pathways and the role of microplastics. Environ. Pollut. 2022, 301, 118943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, N.; Liu, K.; Zhu, L.; Li, C.; Zong, C.; Li, D. Exponential decrease of airborne microplastics: From megacity to open ocean. Sci. Total Environ. 2022, 849, 157702. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Corvianawatie, C.; Cordova, M.R.; Surinati, D.; Li, Y.; Wang, Z.; Li, X.; Li, R.; Wang, J.; He, L.; et al. Microplastics in the tropical Northwestern Pacific Ocean and the Indonesian seas. J. Sea Res. 2023, 194, 102406. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).