Plastic, It’s What’s for Dinner: A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey
Abstract
:1. Introduction
2. Materials and Methods
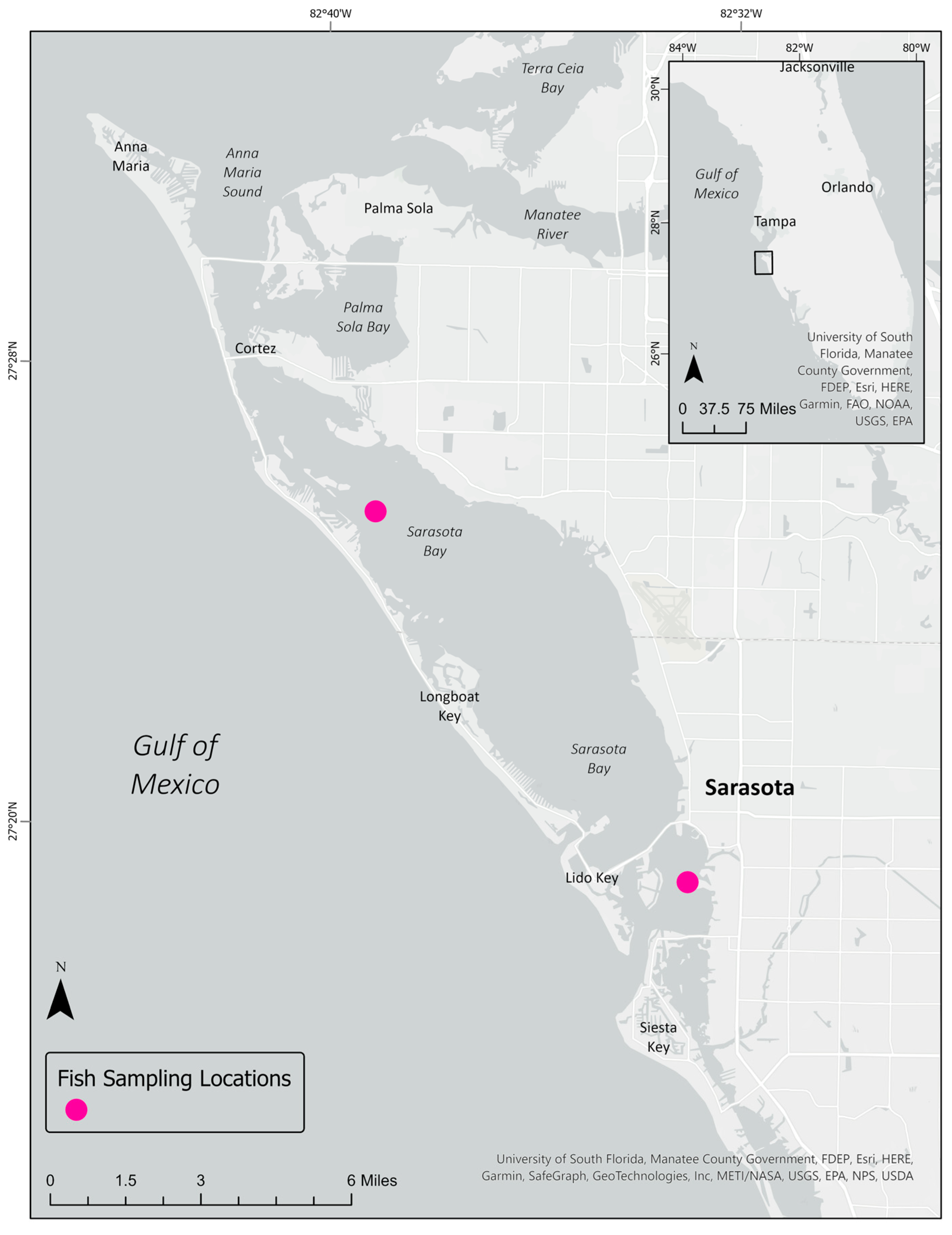
2.1. Fish Collection and Sampling
2.2. Dolphin Sampling
2.3. Microplastic Screening
2.4. Quality Assurance/Quality Control
2.5. Statistical Methods
3. Results
3.1. QA/QC Results
3.2. Suspected Plastics in Prey Fish Muscle Tissue
3.3. Suspected Plastics in Prey Fish Gastrointestinal Tracts
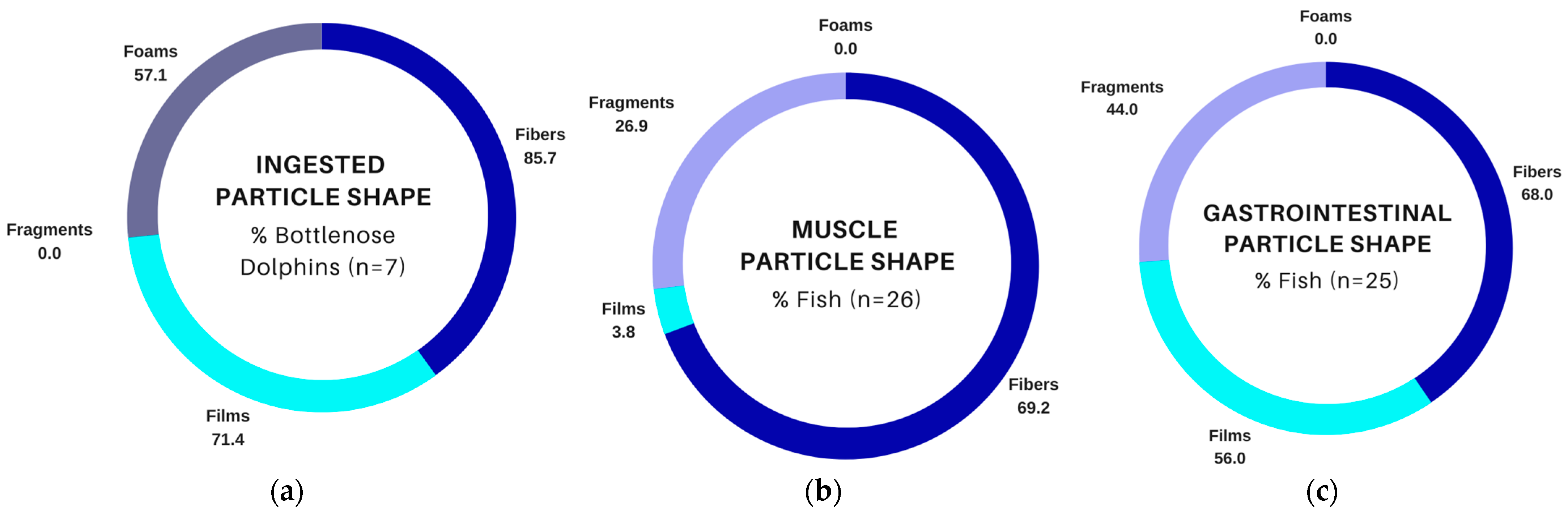
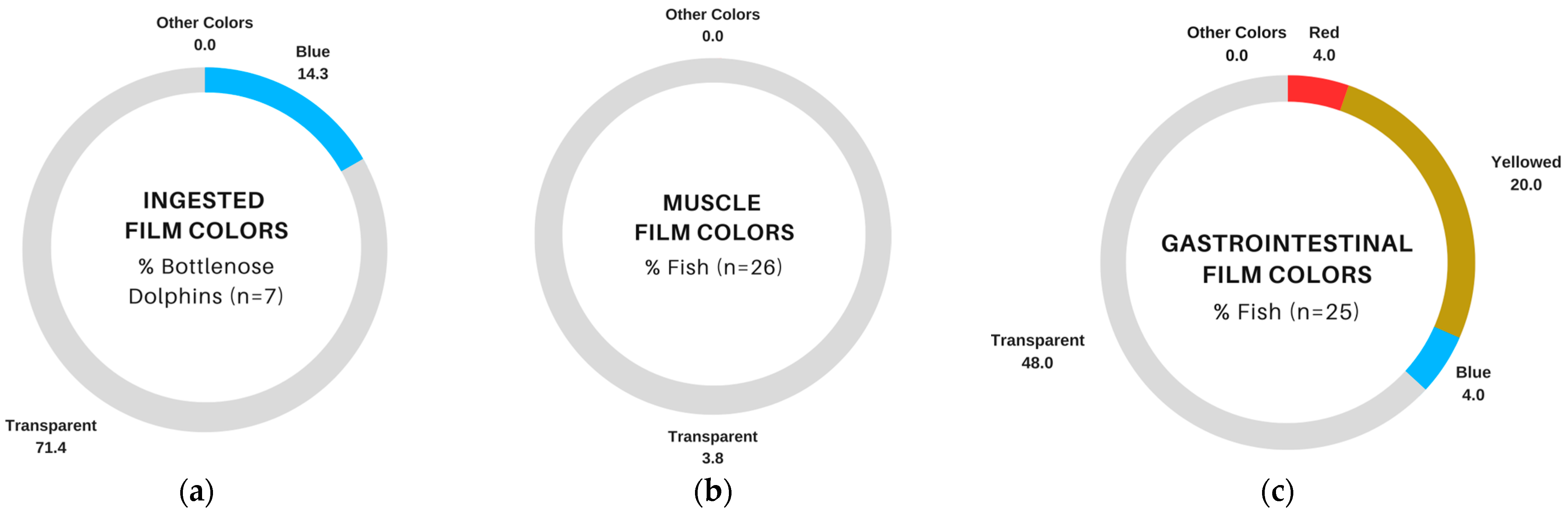
3.4. Comparison of Suspected Plastics in Fish and Bottlenose Dolphins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2023, 20, 789. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A Growing Plastic Smog, Now Estimated to Be over 170 Trillion Plastic Particles Afloat in the World’s Oceans—Urgent Solutions Required. PLoS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; van Franeker, J.A. Quantitative Overview of Marine Debris Ingested by Marine Megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in Marine Biota: A Review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Campani, T.; Baini, M.; Giannetti, M.; Cancelli, F.; Mancusi, C.; Serena, F.; Marsili, L.; Casini, S.; Fossi, M.C. Presence of plastic debris in loggerhead turtle stranded along the Tuscany coasts of the Pelagos Sanctuary for Mediterranean Marine Mammals (Italy). Mar. Pollut. Bull. 2013, 74, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Foekema, E.M.; Van Franeker, J.A.; Leopold, M.F.; Kühn, S.; Bravo Rebolledo, E.L.; Heße, E.; Mielke, L.; IJzer, J.; Kamminga, P.; et al. Microplastic in a Macro Filter Feeder: Humpback Whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015, 95, 248–252. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and Macroplastic Ingestion by a Deep Diving, Oceanic Cetacean: The True’s Beaked Whale Mesoplodon Mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Denuncio, P.; Bastida, R.; Dassis, M.; Giardino, G.; Gerpe, M.; Rodríguez, D. Plastic Ingestion in Franciscana Dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar. Pollut. Bull. 2011, 62, 1836–1841. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The Occurrence of Microplastic in Specific Organs in Commercially Caught Fishes from Coast and Estuary Area of East China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake Routes of Microplastics in Fishes: Practical and Theoretical Approaches to Test Existing Theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in Wild Fish from North East Atlantic Ocean and Its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chèvre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, Tissue Distribution and Toxicological Effects of Environmental Microplastics in Early Juvenile Fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef]
- Chen, K.-J.; Chen, M.-C.; Chen, T.-H. Plastic Ingestion by Fish in the Coastal Waters of the Hengchun Peninsula, Taiwan: Associated with Human Activity but No Evidence of Biomagnification. Ecotoxicol. Environ. Saf. 2021, 213, 112056. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef]
- Hart, L.B.; Dziobak, M.; Wells, R.S.; Ertel, B.; Weinstein, J. Microplastics in Gastric Samples from Common Bottlenose Dolphins (Tursiops truncatus) Residing in Sarasota Bay FL (USA). Front. Mar. Sci. 2022, 9, 947124. [Google Scholar] [CrossRef]
- Hart, L.B.; Beckingham, B.; Wells, R.S.; Alten Flagg, M.; Wischusen, K.; Moors, A.; Kucklick, J.; Pisarski, E.; Wirth, E. Urinary Phthalate Metabolites in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, FL, USA. GeoHealth 2018, 2, 313–326. [Google Scholar] [CrossRef]
- Dziobak, M.K.; Wells, R.S.; Pisarski, E.C.; Wirth, E.F.; Hart, L.B. Demographic Assessment of Mono(2-Ethylhexyl) Phthalate (MEHP) and Monoethyl Phthalate (MEP) Concentrations in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, FL, USA. GeoHealth 2021, 5, e2020GH000348. [Google Scholar] [CrossRef]
- Hart, L.B.; Dziobak, M.K.; Pisarski, E.C.; Wirth, E.F.; Wells, R.S. Sentinels of Synthetics—A Comparison of Phthalate Exposure between Common Bottlenose Dolphins (Tursiops truncatus) and Human Reference Populations. PLoS ONE 2020, 15, e0240506. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Bolton, B.; Litz, J.A.; Trevillian, J.; Kieszak, S.; Kucklick, J. Environmental Contaminants in Coastal Populations: Comparisons with the National Health and Nutrition Examination Survey (NHANES) and Resident Dolphins. Sci. Total Environ. 2019, 696, 134041. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.B.; Wells, R.S. Prey and Feeding Patterns of Resident Bottlenose Dolphins (Tursiops truncatus) in Sarasota Bay, Florida. J. Mammal. 1998, 79, 1045–1059. [Google Scholar] [CrossRef]
- Berens McCabe, E.J.; Gannon, D.P.; Barros, N.B.; Wells, R.S. Prey Selection by Resident Common Bottlenose Dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar. Biol. 2010, 157, 931–942. [Google Scholar] [CrossRef]
- Wells, R.; McHugh, K.; Douglas, D.; Shippee, S.; Berens McCabe, E.; Barros, N.; Phillips, G. Evaluation of Potential Protective Factors Against Metabolic Syndrome in Bottlenose Dolphins: Feeding and Activity Patterns of Dolphins in Sarasota Bay, Florida. Front. Endocrinol. 2013, 4, 139. [Google Scholar] [CrossRef]
- Gannon, D.P.; McCabe, E.J.B.; Camilleri, S.A.; Gannon, J.G.; Brueggen, M.K.; Barleycorn, A.A.; Palubok, V.I.; Kirkpatrick, G.J.; Wells, R.S. Effects of Karenia brevis harmful algal blooms on nearshore fish communities in southwest Florida. Mar. Ecol. Prog. Ser. 2009, 378, 171–186. [Google Scholar] [CrossRef]
- Wells, R.S.; Rhinehart, H.L.; Hansen, L.J.; Sweeney, J.C.; Townsend, F.I.; Stone, R.; Casper, D.R.; Scott, M.D.; Hohn, A.A.; Rowles, T.K. Bottlenose Dolphins as Marine Ecosystem Sentinels: Developing a Health Monitoring System. EcoHealth 2004, 1, 246–254. [Google Scholar] [CrossRef]
- Wells, R.S. Learning from Nature: Bottlenose Dolphin Care and Husbandry. Zoo Biol. 2009, 28, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Barratclough, A.; Wells, R.S.; Schwacke, L.H.; Rowles, T.K.; Gomez, F.M.; Fauquier, D.A.; Sweeney, J.C.; Townsend, F.I.; Hansen, L.J.; Zolman, E.S.; et al. Health Assessments of Common Bottlenose Dolphins (Tursiops truncatus): Past, Present, and Potential Conservation Applications. Front. Vet. Sci. 2019, 6, 444. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Anal. Methods 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Leads, R.R.; Weinstein, J.E. Occurrence of Tire Wear Particles and Other Microplastics within the Tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Manikanda Bharath, K.; Muthulakshmi, A.L.; Natesan, U. Microplastic Contamination around the Landfills: Distribution, Characterization and Threats: A Review. Curr. Opin. Environ. Sci. Health 2023, 31, 100422. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, K.F.R.; Higgs, A.; Walter, B.; Cullen-Unsworth, L.C.; Inman, I.; Jones, B.L. Canopy accumulation: Are seagrass meadows a sink of microplastics? Oceans 2021, 2, 162–178. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality Assessment of the Blue Mussel (Mytilus Edulis): Comparison between Commercial and Wild Types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic Contamination in Brown Shrimp (Crangon crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Corcoran, P.L. Degradation of microplastics in the environment. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 531–542. [Google Scholar]
- Sarasota Bay Comprehensive Conservation and Management Plan Update & State of the Bay Report 2014. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/ccmp.stateofthebay-for-website-august2014.pdf (accessed on 14 August 2023).
- USF 2020. Sarasota Bay Watershed: Geography and Land Use—Sarasota.WaterAtlas.Org. Available online: https://www.sarasota.wateratlas.usf.edu/watershed/geography.asp?wshedid=5&wbodyatlas=watershed (accessed on 14 August 2023).
- Aswathy, J.; Sharma, S.R.K.; Mini, K.G. Microplastic contamination in commercially important bivalves from the southwest coast of India. Environ. Pollut. 2022, 305, 119250. [Google Scholar]
- Rojas-Jimenez, K.; Villalobos-Rojas, F.; Gatgens-García, J.; Rodríguez-Arias, M.; Hernández-Montero, N.; Wehrtmann, I.S. Presence of Microplastics in Six Bivalve Species (Mollusca, Bivalvia) Commercially Exploited at the Pacific Coast of Costa Rica, Central America. Mar. Pollut. Bull. 2022, 183, 114040. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; González, G.P.; Martínez, R.M.G.; Rojas, D.L.S.; Hernando, P.F.; Deudero, S. Assessing microplastic ingestion and occurrence of bisphenols and phthalates in bivalves, fish and holothurians from a Mediterranean marine protected area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Patricio Ojeda, F.; Duarte, C.; Galbán-Malagón, C. Is the Feeding Type Related with the Content of Microplastics in Intertidal Fish Gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, N.; Gong, S.; Gao, S. The Patterns of Trophic Transfer of Microplastic Ingestion by Fish in the Artificial Reef Area and Adjacent Waters of Haizhou Bay. Mar. Pollut. Bull. 2022, 177, 113565. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.L.; da Costa, I.D.; da Silva Oliveira, A.; Zalmon, I.R. Microplastic Ecology: Testing the Influence of Ecological Traits and Urbanization in Microplastic Ingestion by Sandy Beach Fauna. Estuar. Coast. Shelf Sci. 2023, 290, 108406. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.P.; Li, H.; Lin, L.; Xu, X.; Yuan, X.; Koongolla, J.B.; Li, H. Microplastic contamination in coral reef fishes and its potential risks in the remote Xisha areas of the South China Sea. Mar. Pollut. Bull. 2023, 186, 114399. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating Microplastic Trophic Transfer in Marine Top Predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Donohue, M.J.; Masura, J.; Gelatt, T.; Ream, R.; Baker, J.D.; Faulhaber, K.; Lerner, D.T. Evaluating Exposure of Northern Fur Seals, Callorhinus Ursinus, to Microplastic Pollution through Fecal Analysis. Mar. Pollut. Bull. 2019, 138, 213–221. [Google Scholar] [CrossRef]
- McIvor, A.J.; Pires, R.; Lopes, C.; Raimundo, J.; Campos, P.F.; Pais, M.P.; Canning-Clode, J.; Dinis, A. Assessing Microplastic Exposure of the Critically Endangered Mediterranean Monk Seal (Monachus monachus) on a Remote Oceanic Island. Sci. Total Environ. 2023, 856, 159077. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are Baleen Whales Exposed to the Threat of Microplastics? A Case Study of the Mediterranean Fin Whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Zantis, L.J.; Bosker, T.; Lawler, F.; Nelms, S.E.; O’Rorke, R.; Constantine, R.; Sewell, M.; Carroll, E.L. Assessing Microplastic Exposure of Large Marine Filter-Feeders. Sci. Total Environ. 2022, 818, 151815. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, A.; Saavedra, C.; Gago, J.; Covelo, P.; Santos, M.B.; Pierce, G.J. Microplastics in the Stomach Contents of Common Dolphin (Delphinus delphis) Stranded on the Galician Coasts (NW Spain, 2005–2010). Mar. Pollut. Bull. 2018, 137, 526–532. [Google Scholar] [CrossRef]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in Beluga Whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, D.; Yu, R.-Q.; Xie, Z.; He, L.; Wu, Y. Microplastics in the Endangered Indo-Pacific Humpback Dolphins (Sousa chinensis) from the Pearl River Estuary, China. Environ. Pollut. 2021, 270, 116057. [Google Scholar] [CrossRef] [PubMed]
- Merga, L.B.; Redondo-Hasselerharm, P.E.; Van den Brink, P.J.; Koelmans, A.A. Distribution of Microplastic and Small Macroplastic Particles across Four Fish Species and Sediment in an African Lake. Sci. Total Environ. 2020, 741, 140527. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, C.M.; Corcoran, P.L.; Neff, B.D. Factors Influencing the Variation of Microplastic Uptake in Demersal Fishes from the Upper Thames River Ontario. Environ. Pollut. 2022, 313, 120095. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Kang, J.-H.; Hong, S.H.; Shim, W.J. Spatial Distribution of Microplastic in the Surface Waters along the Coast of Korea. Mar. Pollut. Bull. 2020, 155, 110729. [Google Scholar] [CrossRef]
- Peters, C.A.; Bratton, S.P. Urbanization Is a Major Influence on Microplastic Ingestion by Sunfish in the Brazos River Basin, Central Texas, USA. Environ. Pollut. 2016, 210, 380–387. [Google Scholar] [CrossRef]
- Garcia, T.D.; Cardozo, A.L.P.; Quirino, B.A.; Yofukuji, K.Y.; Ganassin, M.J.M.; dos Santos, N.C.L.; Fugi, R. Ingestion of Microplastic by Fish of Different Feeding Habits in Urbanized and Non-Urbanized Streams in Southern Brazil. Water Air Soil Pollut. 2020, 231, 434. [Google Scholar] [CrossRef]
- Lato, K.A.; Thorne, L.H.; Fuirst, M.; Brownawell, B.J. Microplastic Abundance in Gull Nests in Relation to Urbanization. Mar. Pollut. Bull. 2021, 164, 112058. [Google Scholar] [CrossRef]
- Peters, C.; Thomas, P.; Rieper, K.; Bratton, S. Foraging Preferences Influence Microplastic Ingestion by Six Marine Fish Species from the Texas Gulf Coast. Mar. Pollut. Bull. 2017, 124, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hastuti, A.R.; Lumbanbatu, D.T.; Wardiatno, Y. The Presence of Microplastics in the Digestive Tract of Commercial Fishes off Pantai Indah Kapuk Coast, Jakarta, Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 1233–1242. [Google Scholar] [CrossRef]
- de Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic Ingestion by Fish: Body Size, Condition Factor and Gut Fullness Are Not Related to the Amount of Plastics Consumed. Mar. Pollut. Bull. 2020, 151, 110827. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.W.; Beckingham, B.A.; Ingram, B.C.; Ballenger, J.C.; Weinstein, J.E.; Sancho, G. Microplastic and Tire Wear Particle Occurrence in Fishes from an Urban Estuary: Influence of Feeding Characteristics on Exposure Risk. Mar. Pollut. Bull. 2020, 160, 111539. [Google Scholar] [CrossRef] [PubMed]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe Scad Decapterus Muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wu, J.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Accumulation of Microplastics in Fish Guts and Gills from a Large Natural Lake: Selective or Non-Selective? Environ. Pollut. 2022, 309, 119785. [Google Scholar] [CrossRef] [PubMed]
- Bowen, L.W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish ingest microplastics unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in Different Tissues of Fish and Prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating Microplastics Bioaccumulation and Biomagnification in Seafood from the Persian Gulf: A Threat to Human Health? Food Addit. Contam. Part A 2019, 36, 1696–1708. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Microplastics in the Edible and Inedible Tissues of Pelagic Fishes Sold for Human Consumption in Kerala, India. Environ. Pollut. 2020, 266, 115365. [Google Scholar] [CrossRef]
- Di Giacinto, F.; Di Renzo, L.; Mascilongo, G.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Bogdanović, T.; Petričević, S.; Listeš, E.; Brkljača, M.; et al. Detection of Microplastics, Polymers and Additives in Edible Muscle of Swordfish (Xiphias Gladius) and Bluefin Tuna (Thunnus thynnus) Caught in the Mediterranean Sea. J. Sea Res. 2023, 192, 102359. [Google Scholar] [CrossRef]
- Zeytin, S.; Wagner, G.; Mackay-Roberts, N.; Gerdts, G.; Schuirmann, E.; Klockmann, S.; Slater, M. Quantifying Microplastic Translocation from Feed to the Fillet in European Sea Bass Dicentrarchus labrax. Mar. Pollut. Bull. 2020, 156, 111210. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakaoka, M. Trophic transfer of microplastics from mysids to fish greatly exceeds direct ingestion from the water column. Environ. Pollut. 2021, 273, 116468. [Google Scholar] [CrossRef] [PubMed]
- Sucharitakul, P.; Pitt, K.A.; Welsh, D.T. Trophic transfer of microbeads to jellyfish and the importance of aging microbeads for microplastic experiments. Mar. Pollut. Bull. 2021, 172, 112867. [Google Scholar] [CrossRef]
- Xiong, X.; Tu, Y.; Chen, X.; Jiang, X.; Shi, H.; Wu, C.; Elser, J.J. Ingestion and Egestion of Polyethylene Microplastics by Goldfish (Carassius auratus): Influence of Color and Morphological Features. Heliyon 2019, 5, e03063. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and Amount of Microplastic Ingested by Fishes in Watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Baalkhuyur, F.M.; Bin Dohaish, E.-J.A.; Elhalwagy, M.E.A.; Alikunhi, N.M.; AlSuwailem, A.M.; Røstad, A.; Coker, D.J.; Berumen, M.L.; Duarte, C.M. Microplastic in the Gastrointestinal Tract of Fishes along the Saudi Arabian Red Sea Coast. Mar. Pollut. Bull. 2018, 131, 407–415. [Google Scholar] [CrossRef]
- Adika, S.A.; Mahu, E.; Crane, R.; Marchant, R.; Montford, J.; Folorunsho, R.; Gordon, C. Microplastic Ingestion by Pelagic and Demersal Fish Species from the Eastern Central Atlantic Ocean, off the Coast of Ghana. Mar. Pollut. Bull. 2020, 153, 110998. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Mejía-Esquivia, K.A.; Sierra-Labastidas, T.; Patiño, A.; Blandón, L.M.; Espinosa Díaz, L.F. Prevalence of Microplastic Contamination in the Digestive Tract of Fishes from Mangrove Ecosystem in Cispata, Colombian Caribbean. Mar. Pollut. Bull. 2020, 154, 111085. [Google Scholar] [CrossRef]
- Wootton, N.; Ferreira, M.; Reis-Santos, P.; Gillanders, B.M. A Comparison of Microplastic in Fish from Australia and Fiji. Front. Mar. Sci. 2021, 8, 690991. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Z.; Yu, R.; Yan, C.; Zhou, S.; Xing, B. Tire Wear Particles: Trends from Bibliometric Analysis, Environmental Distribution with Meta-Analysis, and Implications. Environ. Pollut. 2023, 322, 121150. [Google Scholar] [CrossRef]
- Werbowski, L.M.; Gilbreath, A.N.; Munno, K.; Zhu, X.; Grbic, J.; Wu, T.; Sutton, R.; Sedlak, M.D.; Deshpande, A.D.; Rochman, C.M. Urban Stormwater Runoff: A Major Pathway for Anthropogenic Particles, Black Rubbery Fragments, and Other Types of Microplastics to Urban Receiving Waters. ACS EST Water 2021, 1, 1420–1428. [Google Scholar] [CrossRef]
- Unice, K.M.; Kreider, M.L.; Panko, J.M. Comparison of Tire and Road Wear Particle Concentrations in Sediment for Watersheds in France, Japan, and the United States by Quantitative Pyrolysis GC/MS Analysis. Environ. Sci. Technol. 2013, 47, 8138–8147. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, H.; Krause, T.; Koelling, S.; Lautenschläger, I.; Frey, A. The Gut Wall Provides an Effective Barrier against Nanoparticle Uptake. Beilstein J. Nanotechnol. 2014, 5, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Kuczaj, S.A.; Yeater, D.B. Observations of rough-toothed dolphins (Steno bredanensis) off the coast of Utila, Honduras. J. Mar. Biol. Assoc. UK 2007, 87, 141–148. [Google Scholar] [CrossRef]
- Martin, A.; da Silva, V.; Rothery, P. Object Carrying as Socio-Sexual Display in an Aquatic Mammal. Biol. Lett. 2008, 4, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in north sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Berna, F. Fourier transform infrared spectroscopy (FTIR). In Encyclopedia of Earth Sciences Series; Gilbert, A.S., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 285–286. [Google Scholar]
- Xie, Q.; Ning, X.; He, X.; Deng, L.; Wu, Z.; Huang, B.; Gui, D.; Wu, Y. First Evaluation of Quantitative Fatty Acid Signature Analysis (QFASA) in Dolphins. Reg. Stud. Mar. Sci. 2022, 50, 102141. [Google Scholar] [CrossRef]
- Martínez, L.M.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]

| Species | Sample Size | Muscle Mass (g) Mean (s.d.) | GIT Mass (g) Mean (s.d.) |
|---|---|---|---|
| Hardhead catfish (Ariopsis felis) | 2 | 16.3–23.2 1 | 12.8 1 |
| Pigfish (Orthopristis chrysoptera) | 12 | 2.8 (0.8) | 6.4 (2.1) |
| Pinfish (Lagodon rhomboides) | 10 | 3.8 (1.3) | 12.0 (7.0) |
| Gulf toadfish (Opsanus beta) | 5 | 4.1 (3.0) | 4.8 (1.9) |
| Blank Type | n | Single Fibers | Fiber Bundles | Films | Fragments (TWP) | Fragments (Non-TWP) | Foams |
|---|---|---|---|---|---|---|---|
| Dissection | 6 | 115 | 0 | 2 | 0 | 1 | 0 |
| Laboratory | 11 | 60 | 0 | 1 | 0 | 1 | 0 |
| Species | Sample Size | Total Particles | Particle Load (#/g Tissue) | Single Fibers | Fiber Bundles | Films | Non-TWP Fragments | TWP Fragments |
|---|---|---|---|---|---|---|---|---|
| Hardhead catfish (Ariopsis felis) | 2 | 6 | 0.2 | 2 | 0 | 0 | 0 | 4 |
| Pigfish (Orthopristis chrysoptera) | 11 | 88 | 2.9 | 86 | 0 | 0 | 1 | 1 |
| Pinfish (Lagodon rhomboides) | 8 | 50 | 1.6 | 49 | 0 | 0 | 0 | 1 |
| Gulf toadfish (Opsanus beta) | 5 | 28 | 1.4 | 18 | 0 | 1 | 0 | 9 |
| Total | 26 | 172 | - | 155 | 0 | 1 | 1 | 15 |
| Species | Sample Size | Total Particles | Particle Load (#/g Tissue) | Single Fibers | Fiber Bundles | Films | Non-TWP Fragments | TWP Fragments |
|---|---|---|---|---|---|---|---|---|
| Hardhead catfish (Ariopsis felis) | 1 | 9 | 0.7 | 1 | 0 | 3 | 0 | 0 |
| Pigfish (Orthopristis chrysoptera) | 10 | 161 | 2.5 | 32 | 44 | 77 | 3 | 5 |
| Pinfish (Lagodon rhomboides) | 9 | 97 | 0.9 | 36 | 34 | 23 | 2 | 2 |
| Gulf toadfish (Opsanus beta) | 5 | 76 | 3.2 | 22 | 28 | 22 | 1 | 3 |
| Total | 25 | 343 | 97 | 100 | 125 | 6 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, L.B.; Dziobak, M.; Wells, R.S.; Berens McCabe, E.; Conger, E.; Curtin, T.; Knight, M.; Weinstein, J. Plastic, It’s What’s for Dinner: A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey. Oceans 2023, 4, 409-422. https://doi.org/10.3390/oceans4040028
Hart LB, Dziobak M, Wells RS, Berens McCabe E, Conger E, Curtin T, Knight M, Weinstein J. Plastic, It’s What’s for Dinner: A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey. Oceans. 2023; 4(4):409-422. https://doi.org/10.3390/oceans4040028
Chicago/Turabian StyleHart, Leslie B., Miranda Dziobak, Randall S. Wells, Elizabeth Berens McCabe, Eric Conger, Tita Curtin, Maggie Knight, and John Weinstein. 2023. "Plastic, It’s What’s for Dinner: A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey" Oceans 4, no. 4: 409-422. https://doi.org/10.3390/oceans4040028
APA StyleHart, L. B., Dziobak, M., Wells, R. S., Berens McCabe, E., Conger, E., Curtin, T., Knight, M., & Weinstein, J. (2023). Plastic, It’s What’s for Dinner: A Preliminary Comparison of Ingested Particles in Bottlenose Dolphins and Their Prey. Oceans, 4(4), 409-422. https://doi.org/10.3390/oceans4040028








