How to Survive Intensive Harvesting: The High Recruitment Rates of the Precious Mediterranean Red Coral (Corallium rubrum L. 1758)
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment Density and Colony Size/Age Distribution
2.2. Recruits, Juveniles, and Adult Colonies Spatial Distribution
2.3. C. rubrum Recruitment Data from the Literature
3. Results
3.1. Recruit, Juvenile, and Adult Densities
3.2. Spatial Distribution Pattern
3.3. C. rubrum Recruitment in Different Populations
4. Discussion
5. Conclusions
- (1)
- The recruitment rates of Corallium rubrum are characterized by large fluctuations; however, all but one population analyzed in the present study regularly recruited each year.
- (2)
- All but one of the data points on red coral recruitment were at least two orders of magnitude higher than those of most of the octocoral species reported here.
- (3)
- The only octocoral showing recruitment rates comparable to those of the red coral is another octocoral brooder (Heliopora coerulea) belonging to the order Scleralcyonacea.
- (4)
- Red coral tended to form monospecific patches, and at the smaller spatial scale examined (1 dm2), patchiness occurred more frequently on natural substrates than on settlement tiles. This suggests that the availability of a limited suitable surface for larval settlement on the natural substrate, rather than the settlement of small clouds of planulae in the same microarea, may have determined the observed patchiness.
- (5)
- Overall, the data collected in this study highlighted that red coral, in the shallower portion of its bathymetric distribution, is a highly recruiting species. High recruitment rates, together with an early age at first reproduction and a wide geographic and bathymetric distribution, are likely key factors allowing the shallow populations of this slow-growing, long-lived octocoral to survive a long-lasting, intensive exploitation to which some drastic mortality events linked to the GCC have recently been added.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caley, M.J.; Carr, M.H.; Hixon, M.A.; Hughes, T.P.; Jones, G.P.; Menge, B.A. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 1996, 27, 477–500. [Google Scholar] [CrossRef]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Ruzicka, R.R.; Colella, M.A.; Porter, J.W.; Morrison, J.M.; Kidney, J.A.; Brinkhuis; Lunz, K.S.; Macaulay, K.A.; Bartlett, L.A.; Meyers, M.K. Colee Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar. Ecol. Prog. Ser. 2013, 489, 125–141. [Google Scholar] [CrossRef]
- Lenz, E.A.; Bramanti, L.; Lasker, H.R.; Edmunds, P.J. Long-term variation of octocoral populations in St. John, US Virgin Islands. Coral Reefs 2015, 34, 1099–1109. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Lasker, H.R. Cryptic regime shift in benthic community structure on shallow reefs in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2016, 559, 1–12. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Gómez-Corrales, M.; Gutierrez-Cala, L.; Vergara, D.C.; Roa, P.; González-Zapata, F.L.; Gnecco, M.; Puerto, N.; Neira, L.; Sarmiento, A. Steady decline of corals and other benthic organisms in the SeaFlower Biosphere Reserve (Southwestern Caribbean). Front. Mar. Sci. 2019, 6, 73. [Google Scholar] [CrossRef]
- Cupido, R.; Cocito, S.; Barsanti, M.; Sgorbini, S.; Peirano, A.; Santangelo, G. Unexpected long-term population dynamics in a canopy-forming gorgonian coral following mass mortality. Mar. Ecol. Prog. Ser. 2009, 394, 195–200. [Google Scholar] [CrossRef]
- Lasker, H.R.; Martínez-Quintana, Á.; Bramanti, L.; Edmunds, P.J. Resilience of octocoral forests to catastrophic storms. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Ruffaldi Santori, S.; Benedetti, M.C.; Cocito, S.; Peirano, A.; Cupido, R.; Erra, F.; Santangelo, G. After the Fall: The Demographic Destiny of a Gorgonian Population Stricken by Catastrophic Mortality. Oceans 2021, 2, 337–350. [Google Scholar] [CrossRef]
- Bartlett, L.A.; Brinkhuis, V.I.; Ruzicka, R.R.; Colella, M.A.; Lunz, K.S.; Leone, E.H.; Hallock, P. Dynamics of stony coral and octocoral juvenile assemblages following disturbance on patch reefs of the Florida reef tract. In Corals in a Changing World; IntechOpen: London, UK, 2018; pp. 99–120. [Google Scholar]
- Rossi, S. The destruction of the ‘animal forests’ in the oceans: Towards an oversimplification of the benthic ecosystems. Ocean. Coast. Manag. 2013, 84, 77–85. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. Animal Forests of the World: An Overview. In The Marine Animal Forests; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–26. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. 422 Boil. 2006, 48, 123–195. [Google Scholar]
- Mundy, C.N. An appraisal of methods used in coral recruitment studies. Coral Reefs 2000, 19, 124–131. [Google Scholar] [CrossRef]
- Adjeroud, M.; Fernandez, J.M.; Carroll, A.G.; Harrison, P.L.; Penin, L. Spatial patterns and recruitment processes of coral assemblages among contrasting environmental conditions in the southwestern lagoon of New Caledonia. Mar. Pollut. Bull. 2010, 61, 375–386. [Google Scholar] [CrossRef]
- Vermeij, M.J.A. Substrate composition and adult distribution determine recruitment patterns in a Caribbean brooding coral. Mar. Ecol. Prog. Ser. 2005, 295, 123–133. [Google Scholar] [CrossRef]
- Tsounis, G.; Rossi, S.; Grigg, R.; Santangelo, G.; Bramanti, L.; Gili, J.M. The Exploitation and Conservation of Precious Corals; CRC Press: Boca Raton, FL, USA, 2010; pp. 161–212. [Google Scholar]
- Cattaneo-Vietti, R.; Bo, M.; Cannas, R.; Cau, A.; Follesa, C.; Meliadò, E.; Russo, G.F.; Sandulli, R.; Santangelo, G.; Bavestrello, G. An overexploited Italian treasure: Past and present distribution and exploitation of the precious red coral Corallium rubrum (L.; 1758) (Cnidaria: Anthozoa). Ital. J. Zool. 2016, 83, 443–455. [Google Scholar] [CrossRef]
- Santangelo, G.; Carletti, E.; Maggi, E.; Bramanti, L. Reproduction and population sexual structure of the overexploited Mediterranean red coral Corallium rubrum. Mar. Ecol. Prog. Ser. 2003, 248, 99–108. [Google Scholar] [CrossRef]
- Gallmetzer, I.; Haselmai, A.; Velimirov, B. Slow growth and early sexual maturity: Bane and boon for the red coral Corallium rubrum. Estuar. Coast. Shelf Sci. 2010, 90, 1–10. [Google Scholar] [CrossRef]
- Tsounis, G.; Rossi, S.; Bramanti, L.; Santangelo, G. Management hurdles for sustainable harvesting of Corallium rubrum. Mar. Policy 2013, 39, 361–364. [Google Scholar] [CrossRef]
- Guizien, K.; Viladrich, N.; Martínez-Quintana, Á.; Bramanti, L. Survive or swim: Different relationships between migration potential and larval size in three sympatric Mediterranean octocorals. Sci. Rep. 2020, 10, 18096. [Google Scholar] [CrossRef]
- Zelli, E.; Quéré, G.; Lago, N.; Di Franco, G.; Costantini, F.; Rossi, S.; Bramanti, L. Settlement dynamics and recruitment responses of Mediterranean gorgonians larvae to different crustose coralline algae species. J. Exp. Mar. Biol. Ecol. 2020, 530, 151427. [Google Scholar] [CrossRef]
- Costantini, F.; Taviani, M.; Remia, A.; Pintus, E.; Schembri, P.J.; Abbiati, M. Deep-water Corallium rubrum (L.; 1758) from the Mediterranean Sea: Preliminary genetic characterization. Mar. Ecol. 2010, 31, 261–269. [Google Scholar] [CrossRef]
- Bramanti, L.; Magagnini, G.; De Maio, L.; Santangelo, G. Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L 1758), a 4-year study. J. Exp. Mar. Biol. Ecol. 2005, 314, 69–78. [Google Scholar] [CrossRef]
- Bramanti, L.; Rossi, S.; Tsoounis, G.; Gili, J.M.; Santangelo, G. Settlement and early survival of red coral on artificial substrates in different geographic areas: Some clues for demography and restoration. Hydrobiologia 2007, 580, 219–224. [Google Scholar] [CrossRef]
- Santangelo, G.; Bramanti, L.; Rossi, S.; Tsounis, G.; Vielmini, I.; Lott, C.; Gili, J.M. Patterns of variation in recruitment and post-recruitment processes of the Mediterranean precious gorgonian coral Corallium rubrum. J. Exp. Mar. Biol. Ecol. 2012, 411, 7–13. [Google Scholar] [CrossRef]
- Angiolillo, M.; Gori, A.; Canese, S.; Bo, M.; Priori, C.; Bavestrello, G.; Salvati, E.; Erra, F.; Greenacre, M.; Santangelo, G. Distribution and population structure of a deep-dwelling red coral in W Mediterranean. Mar. Ecol. 2015, 1, 1–17. [Google Scholar] [CrossRef]
- GFCM (General Fisheries Commission for the Mediterranean Scientific Advisory Committee SAC). In Proceedings of the Report of the Transversal Workshop on Red Coral Ajaccio (Corsica), Ajaccio, France, 5–7 October 2011.
- Bramanti, L.; Vielmini, I.; Rossi, S.; Tsounis, G.; Iannelli, M.; Cattaneo-Vietti, R.; Priori, C.; Santangelo, G. Demographic parameters of two populations of red coral (Corallium rubrum L. 1758) in the North Western Mediterranean. Mar. Biol. 2014, 161, 1015–1026. [Google Scholar] [CrossRef]
- Bramanti, L.; Magagnini, G.; Santangelo, G. Settlement and recruitment: The first stages in the life cycle of two epibenthic suspension feeders (Corallium rubrum and Anomia ephippium). Ital. J. Zool. 2003, 70, 175–178. [Google Scholar] [CrossRef]
- Teixidó, N.; Garrabou, J.; Arntz, W.E. Spatial pattern quantification of Antarctic benthic communities using landscape indices. Mar. Ecol. Prog. Ser. 2002, 242, 1–14. [Google Scholar] [CrossRef]
- Pielou, E.C. Segregation and symmetry in two-species populations as studied by nearest-neighbour relationships. J. Ecol. 1961, 255–269. [Google Scholar] [CrossRef]
- Clark, P.J.; Evans, F.C. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 1954, 35, 445–453. [Google Scholar] [CrossRef]
- Upton, G.; Fingleton, B. Spatial Data Analysis by Example. Volume 1: Point Pattern and Quantitative Data; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1985. [Google Scholar]
- Lison, L. Statistica Applicate Alla Biologia Sperimentale; CEA: Ambrosiana, Italy, 1961.
- Cerrano, C.; Bavestrello, G.; Cicogna, F.; Cattaneo-Vietti, R. New experiences on transplantation and red coral harvesting effects in the Ligurian Sea. In Red Coral and Other Mediterranean Octocorals, Biology and Protection; Ministero Risorse Agricole: Roma, Italy, 1999; pp. 62–67. [Google Scholar]
- Garrabou, J.; Harmelin, J.G. A 20-year study on life-history traits of a harvested long-lived temperate coral in the NW Mediterranean: Insights into conservation and management needs. J. Anim. Ecol. 2002, 71, 966–978. [Google Scholar] [CrossRef]
- Renieri, D. Studio della Distribuzione Spaziale di Una Popolazione di Corallium rubrum (L. 1758). Master’s Thesis, University of Pisa, Pisa, Italy, 2005. [Google Scholar]
- Benedetti, A.; Bramanti, L.; Tsounis, G.; Faimali, M.; Pavanello, G.; Rossi, S.; Gili, J.M.; Santangelo, G. Applying cathodically polarised substrata to the restoration of a high value coral. Biofouling 2011, 27, 799–809. [Google Scholar] [CrossRef]
- Costantini, F.; Rugiu, L.; Cerrano, C.; Abbiati, M. Living upside down: Patterns of red coral settlement in a cave. PeerJ 2018, 6, e4649. [Google Scholar] [CrossRef]
- Villechanoux, J.; Bierwirth, J.; Mantas, T.P.; Cerrano, C. Testing transplantation techniques for the red coral Corallium rubrum. Water 2022, 14, 1071. [Google Scholar] [CrossRef]
- Benedetti, M.C. Demography and Growth of the Long-Lived Octocoral Corallium rubrum. Ph.D. Thesis, University of Pisa, Pisa, Italy, 2018. [Google Scholar]
- Santangelo, G.; Bramanti, L.; Iannelli, M. Population dynamics and conservation biology of the over-exploited Mediterranean red coral. J. Theor. Biol. 2007, 244, 416–423. [Google Scholar] [CrossRef]
- Priori, C.; Mastascusa, V.; Erra, F.; Angiolillo, M.; Canese, S.; Santangelo, G. Demography of deep-dwelling red coral populations: Age and reproductive structure of a highly valued marine species. Estuar. Coast. Shelf Sci. 2013, 118, 43–49. [Google Scholar] [CrossRef]
- Giannini, F.; Gili, J.M.; Santangelo, G. Relationships between the spatial distribution of red coral Corallium rubrum and coexisting suspension feeders at Medas Islands Marine Protected Area (Spain). Ital. J. Zool. 2003, 70, 233–239. [Google Scholar] [CrossRef]
- Santangelo, G.; Abbiati, M. Red coral: Conservation and management of an over-exploited Mediterranean species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 253–259. [Google Scholar] [CrossRef]
- Theodor, J. Contribution á l’ètude des Gorgones (VII): Ecologie et comportement de la planula. Vie Milieu 1967, 291–302. [Google Scholar]
- Gómez-Gras, D.; Linares, C.; López-Sanz, A.; Amate, R.; Ledoux, J.B.; Bensoussan, N.; Drap, P.; Bianchimani, O.; Marschal, C.; Torrents, O.; et al. Population collapse of habitat-forming species in the Mediterranean: A long-term study of gorgonian populations affected by recurrent marine heatwaves. Proc. R. Soc. B 2021, 288, 20212384. [Google Scholar] [CrossRef] [PubMed]
- Tsounis, G.; Rossi, S.; Gili, J.M.; Arntz, W.E. Red coral fishery at the Costa Brava (NW Mediterranean): Case study of an overharvested precious coral. Ecosystems 2007, 10, 975–986. [Google Scholar] [CrossRef]
- Linares, C.; Bianchimani, O.; Torrents, O.; Marschal, C.; Drap, P.; Garrabou, J. Marine Protected Areas and the conservation of long-lived marine invertebrates: The Mediterranean red coral. Mar. Ecol. Prog. Ser. 2010, 402, 69–79. [Google Scholar] [CrossRef]
- Santangelo, G. Conservation of long-lived marine species: Some hints for adopting a population approach. Arq. Ciên. Mar. 2022, 55, 231–259, Especial Labomar-60 anos. [Google Scholar] [CrossRef]
- Caswell, H. Matrix Population Models: Construction, Analysis and Interpretation, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Cau, A.; Bramanti, L.; Cannas, R.; Follesa, M.C.; Angiolillo, M.; Canese, S.; Bo, M.; Cuccu, D.; Guizien, K. Habitat constraints and self-thinning shape Mediterranean red coral deep population structure: Implications for conservation practice. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Lasker, H.R.; Boller, M.L.; Castanaro, J.; Sanchez, J.A. Determinate growth and modularity in a gorgonian octocoral. Biol. Bull. 2003, 205, 319–330. [Google Scholar] [CrossRef]
- Bramanti, L.; Edmunds, P.J. Density-associated recruitment mediates coral population dynamics on a coral reef. Coral Reefs 2016, 35, 543–553. [Google Scholar] [CrossRef]
- Szmant, A.M. Reproductive ecology of Caribbean reef corals. Coral Reefs 1986, 5, 43–53. [Google Scholar] [CrossRef]
- Grigg, R.W. Population dynamics of two gorgonian corals. Ecology 1977, 58, 278–290. [Google Scholar] [CrossRef]
- Gotelli, N.J. Determinants of recruitment, juvenile growth, and spatial distribution of a shallow-water gorgonian. Ecology 1988, 69, 157–166. [Google Scholar] [CrossRef]
- Yoshioka, P.M. Variable recruitment and its effects on the population and community structure of shallow-water gorgonians. Bull. Mar. Sci. 1996, 59, 433–443. [Google Scholar]
- Karlson, R.H.; Hughes, T.P.; Karlson, S.R. Density-dependent dynamics of soft coral aggregations: The significance of clonal growth and form. Ecology 1996, 77, 1592–1599. [Google Scholar] [CrossRef]
- Lasker, H.R.; Porto-Hannes, I. Species level identification of Antillogorgia spp. recruits identifies multiple pathways of octocoral success on Caribbean reefs. Coral Reefs 2021, 40, 41–51. [Google Scholar] [CrossRef]
- Harii, S.; Kayanne, H. Larval dispersal, recruitment, and adult distribution of the brooding stony octocoral Heliopora coerulea on Ishigaki Island, southwest Japan. Coral Reefs 2003, 22, 188–196. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Azzini, F.; Calcinai, B.; Castellano, L.; Muti, C.; Valisano, L.; Zega, G.; Bavestrello, G. Gorgonian population recovery after a mass mortality event. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 147–157. [Google Scholar] [CrossRef]
- Cupido, R.; Cocito, S.; Manno, V.; Ferrando, S.; Peirano, A.; Iannelli, M.; Bramanti, L.; Santangelo, G. Sexual structure of a highly reproductive, recovering gorgonian population: Quantifying reproductive output. Mar. Ecol. Prog. Ser. 2012, 469, 25–36. [Google Scholar] [CrossRef][Green Version]
- Lasker, H.R.; Kim, K.; Coffroth, M.A. Production, settlement, and survival of plexaurid gorgonian recruits. Mar. Ecol. Prog. Ser. 1998, 162, 111–123. [Google Scholar] [CrossRef]
- Toma, M.; Bo, M.; Giudice, D.; Canese, S.; Cau, A.; Andaloro, F.; Angiolillo, M.; Greco, S.; Bavestrello, G. Structure and status of the Italian red coral forests: What can a large-scale study tell? Front. Mar. Sci. 2022, 2577. [Google Scholar] [CrossRef]
- Carugati, L.; Moccia, D.; Bramanti, L.; Cannas, R.; Follesa, M.C.; Salvadori, S.; Cau, A. Deep-Dwelling Populations of Mediterranean Corallium rubrum and Eunicella cavolini: Distribution, Demography, and Co-Occurrence. Biology 2022, 11, 333. [Google Scholar] [CrossRef]
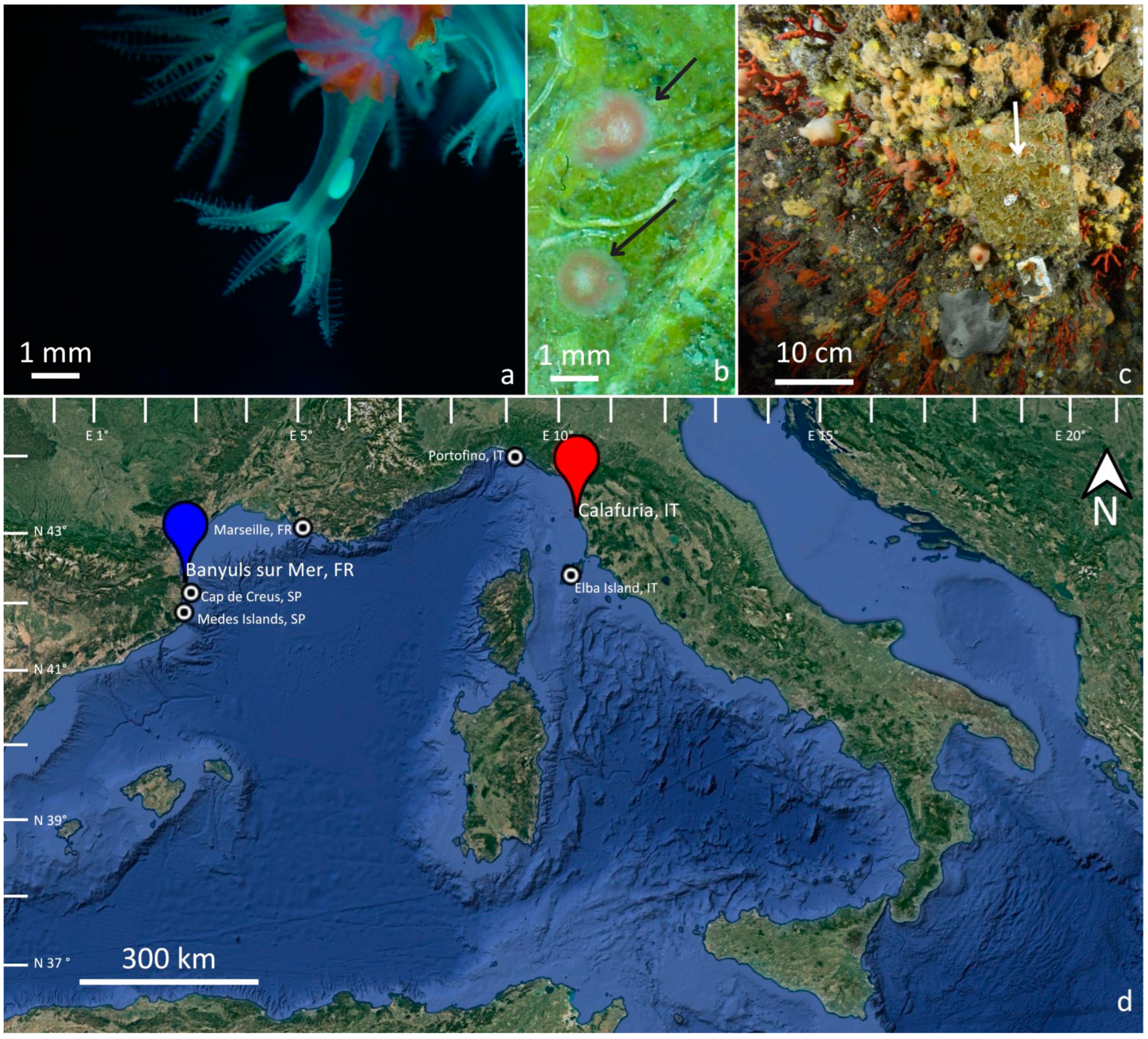

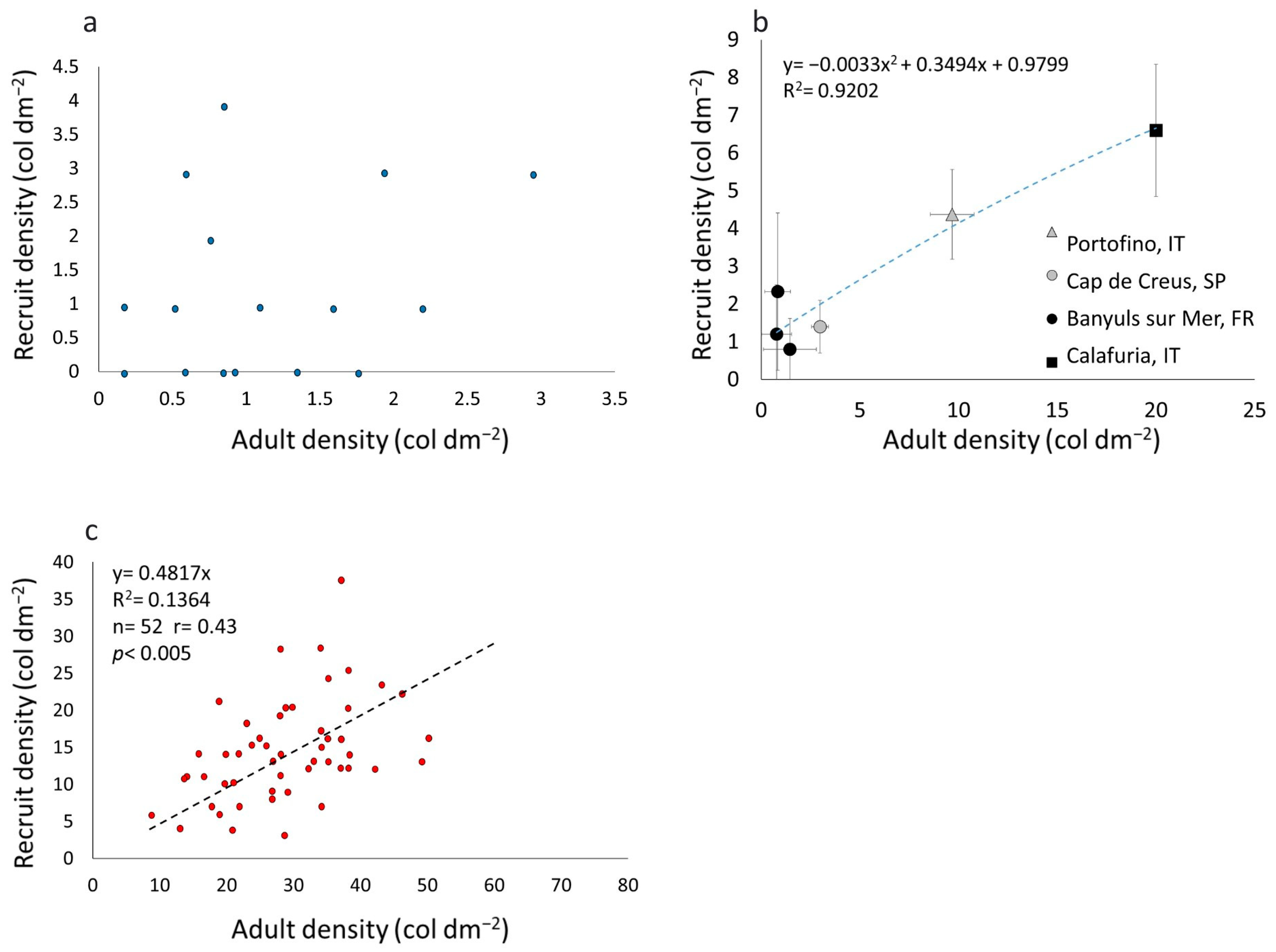
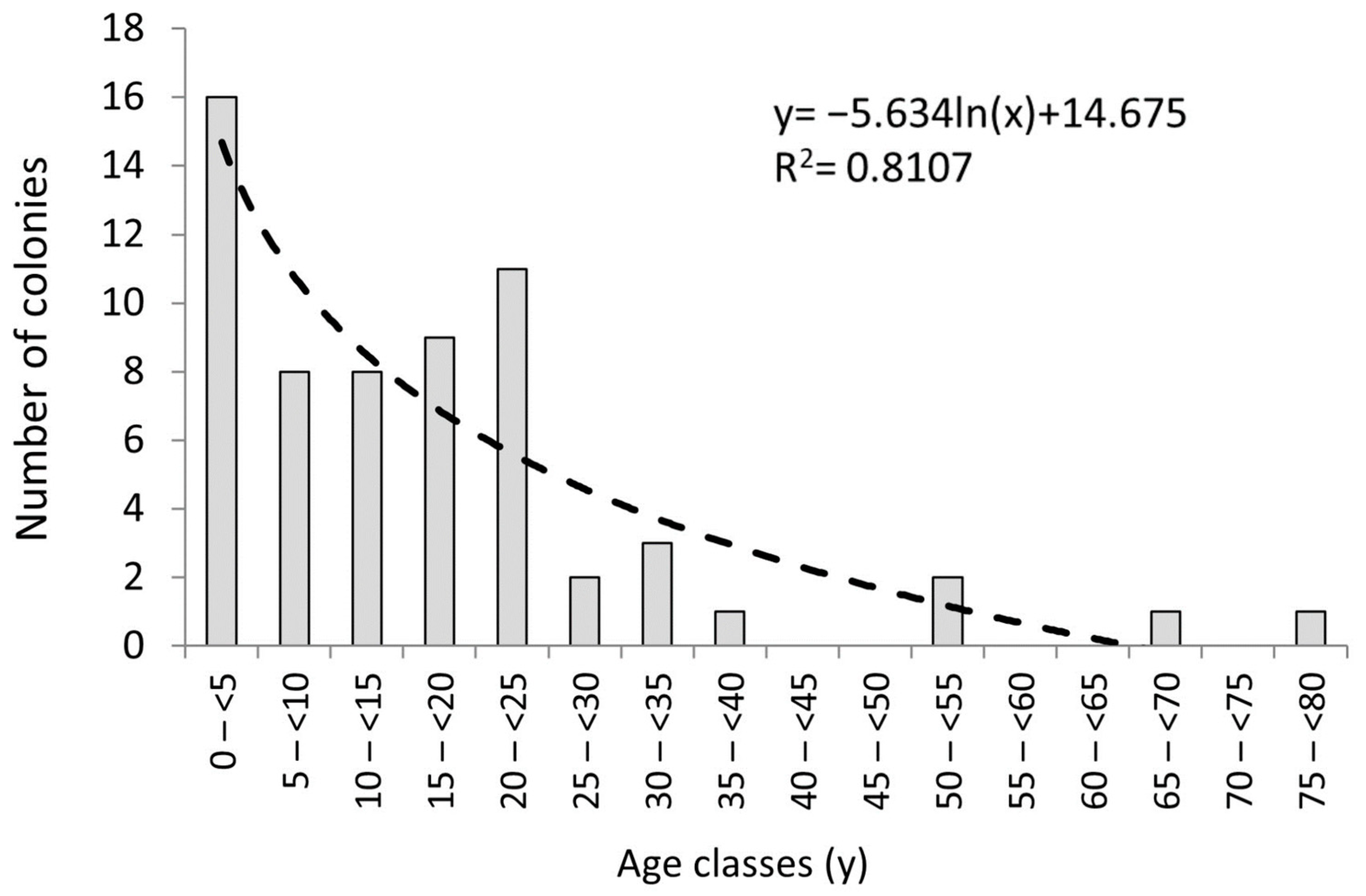
| Location | Depth (Meters) | Substrate | Recruit Density (col dm−2) | Adult Density (col dm−2) | Recruit Density/Adult Density | References |
|---|---|---|---|---|---|---|
| Portofino (It.) * | 34 | Natural | 2.77 (11.11 over 4 years) | 4.0 | 0.69 | Cerrano et al., 1999 [37] |
| Portofino (It.) * | 40–50 | Fiber cement | 1.6–11.7 | ND | Cerrano et al., 1999 [37] | |
| Marseille (Fr.) | 27 (cave walls) | Local limestone | 0.43 | 0.65 ± 0.3 | 0.66 | Garrabou and Harmelin, 2002 [38] |
| Calafuria (It.) | 25–35 | Marble | 12.3 ± 6.1–2.7 ± 2.4 (5.4 average over 4 years) | 20 | 0.61–0.13 | Bramanti et al., 2005 [25] |
| Calafuria (It.) | 25–35 | Natural | 13.19 ± 4.7 | 29 | 0.48 | Renieri, 2005 [39] |
| Calafuria (It.) | 35 | Marble—CaCO3 § | 3 ± 2.5–2.7 ± 1.1 | 20 | 0.15–0.13 | Benedetti et al., 2011 [40] |
| Calafuria (It.) | 35 ± 1 | Marble | 6.06 ± 1.75 | 20 | 0.33 | Santangelo et al., 2012 [27] |
| Elba Island (It.) | 35 ± 1 | Marble | 4.6 ± 1.01 | ND | Santangelo et al., 2012 [27] | |
| Medes Is. MPA (Sp.) * | 35 ± 1 | Marble | 0.56 ± 0.21 | ND | Santangelo et al., 2012 [27] | |
| Cap de Creus (Sp.) | 35 | Natural | 1.4 ± 0.7 | 3 ± 1.7 | 0.45 | Bramanti et al., 2014 [30] |
| Portofino (It.) * | 35 | Natural | 4.4 ± 1.20 | 9.9 ± 4.6 | 0.44 | Bramanti et al., 2014 [30] |
| Portofino (It.) * | 34–37 (cave ceiling) | PVC | 0.92 | 3.5 ± 2.1 | 2.48 | Costantini et al., 2018 [41] |
| Portofino (It.) * | 34–37 (cave ceiling) | PVC | 8.7 ± 5.96 | 3.5 ± 2.1 | 1.53 | Costantini et al., 2018 [41] |
| Portofino (It.) * | 34–70 | PVC | 1.87 ± 1.94–1.63 ± 0.5 | 2.5–3 | 0.75–0.54 | Villechanoux, 2022 [42] |
| Banyuls sur Mer (Fr) | 24–29 | Marble | 1.17 ± 1.0 | 1.11 ± 0.11 | 1.05 | Benedetti MC PhD Thesis, 2018 [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, M.C.; Bramanti, L.; Santangelo, G. How to Survive Intensive Harvesting: The High Recruitment Rates of the Precious Mediterranean Red Coral (Corallium rubrum L. 1758). Oceans 2023, 4, 301-314. https://doi.org/10.3390/oceans4030021
Benedetti MC, Bramanti L, Santangelo G. How to Survive Intensive Harvesting: The High Recruitment Rates of the Precious Mediterranean Red Coral (Corallium rubrum L. 1758). Oceans. 2023; 4(3):301-314. https://doi.org/10.3390/oceans4030021
Chicago/Turabian StyleBenedetti, Maria Carla, Lorenzo Bramanti, and Giovanni Santangelo. 2023. "How to Survive Intensive Harvesting: The High Recruitment Rates of the Precious Mediterranean Red Coral (Corallium rubrum L. 1758)" Oceans 4, no. 3: 301-314. https://doi.org/10.3390/oceans4030021
APA StyleBenedetti, M. C., Bramanti, L., & Santangelo, G. (2023). How to Survive Intensive Harvesting: The High Recruitment Rates of the Precious Mediterranean Red Coral (Corallium rubrum L. 1758). Oceans, 4(3), 301-314. https://doi.org/10.3390/oceans4030021







