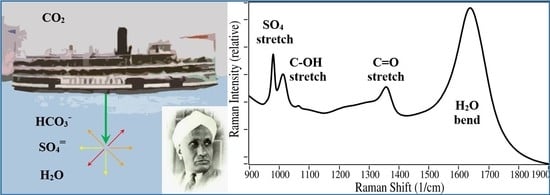
Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleypas, J.A.; Feely, R.A.; Fabry, V.J.; Langdon, C.; Sabine, C.L.; Robbins, L.L. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research. In Report of a Workshop Held; NOAA: St. Petersburg, FL, USA, 2005. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Mollica, N.R.; Guo, W.; Cohen, A.L.; Huang, K.-F.; Foster, G.L.; Donald, H.K.; Solow, A.R. Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. USA 2018, 115, 1754–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.R.A.; Gilleard, J.M.; Allen, M.C.; Deheyn, D.D. Effects of CO2-induced pH reduction on the exoskeleton structure and biophotonic properties of the shrimp Lysmata californica. Nat. Sci. Rep. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pearce, F. What Is the Carbon Limit? That Depends Who You Ask. Yale Envrironment360. 6 November 2014. Available online: https://e360.yale.edu/features/what_is_the_carbon_limit_that_depends_who_you_ask (accessed on 15 December 2020).
- Adams, E.E.; Caldeira, K. Ocean Storage of CO2. Elements 2008, 2, 319–324. [Google Scholar] [CrossRef]
- Friederici, P. Ocean Carbon Sequestration: The World’s Best Bad Idea. In Pacific Standard; The Social Justice Foundation: Santa Barbara, CA, USA, 2017. [Google Scholar]
- Witze, A. Southern Ocean—Climate Friend or Foe? Science News. Available online: https://www.sciencenewsdigital.org/sciencenews/june_8__2019/MobilePagedArticle.action?articleId=1491783#articleId1491783 (accessed on 15 December 2020).
- Brewer, P.G.; Peltzer, E.T.; Walz, P.; Aya, I.; Yamane, K.; Kojima, R.; Nakajima, Y.; Nakayama, N.; Haugan, P.; Johannessen, T. Deep ocean experiments with fossil fuel CO2: Creation and sensing of a controlled plume at 4 km depth. J. Mar. Res. 2005, 63, 9–33. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.R.; Oliver, B.G. A vibrational-spectroscopic study of the species present in the CO2-H2O system. J. Solut. Chem. 1972, 1, 329–339. [Google Scholar] [CrossRef]
- Dickson, A.G. Introduction to CO2 Chemistry in Sea Water. Available online: https://www.iaea.org/sites/default/files/18/07/oa-dickson-chemistry-1901015.pdf (accessed on 8 January 2020).
- Dunk, R.M.; Peltzer, E.T.; Walz, P.M.; Brewer, P.G. Seeing a deep ocean CO2 enrichment experiment in a new light: Laser Raman detection of dissolved CO2 in seawater. Environ. Sci. Technol. 2005, 39, 9630–9636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kirkwood, W.J.; Walz, P.M.; Peltzer, E.T.; Brewer, P.G. A review of advances in deep-ocean Raman spectroscopy. Appl. Spectrosc. 2012, 66, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Guo, J.; Liu, Q.; Luo, Z.; Yan, J.; Zheng, R. Highly sensitive Raman system for dissolved gas analysis in water. Appl. Opt. 2016, 55, 7744–7748. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, C.; Chetan Shende, C.; Farquharson, D.S.; Farquharson, S. A simple multi-pass optical system for Raman spectral measurements of ppm bicarbonate and carbonate in water. J. Raman Spectrosc. 2020, submitted. [Google Scholar]
- Farquharson, S.; Brouillette, C.; Shende, C.; Morrison, C. Measurement of CO2 in water from a UV oxidizer by Raman Spectroscopy”. Adv. Space Res. 2021. in preparation. [Google Scholar]
- Brewer, P.R. Ocean chemistry of the fossil fuel CO2 signal: The haline signal of “business as usual”. Geophys. Res. Lett. 1997, 24, 1367–1369. [Google Scholar] [CrossRef]
- Omand, M.M.; Govindarajan, R.; He, J.; Mahadevan, A. Sinking flux of particulate organic matter in the oceans: Sensitivity to particle characteristics. Sci. Rep. 2020, 10, 5582–5598. [Google Scholar] [CrossRef] [PubMed]
- Cross, P.C.; Burnham, J.; Leighton, P.A. The Raman Spectrum and the Structure of Water. JACS 1937, 59, 1134–1147. [Google Scholar] [CrossRef]
- Falcke, H.; Eberle, S.H. Raman Spectroscopic Identification of Carbonic Acid. Water Res. 1990, 24, 685. [Google Scholar] [CrossRef]
- Fung, K.H.; Tang, I.N. Relative Raman Scattering Cross-Section Measurements with Suspended Particles. Appl. Spectrosc. 1991, 45, 734–737. [Google Scholar] [CrossRef]
- Thor Labs Product Literature, at UV Fused Quartz Cuvettes. Available online: thorlabs.com (accessed on 15 December 2020).
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Raman, C.V. The Molecular Scattering of Light: The Colour of the Sea, Nobel Lecture December 11, 1930, Stockholm, Sweden. Available online: https://www.nobelprize.org/nobel_prizes/physics/laureates/1930/raman-lecture.pdf (accessed on 15 December 2020).



| Prepared Concentration (ppm) | 30 | 100 | 150 | 300 | 1000 | 3000 | 10,000 | Average |
| Corrected Concentration (ppm) | 22 | 71 | 140 | 299 | 1089 | 2955 | 10,006 | |
| 1360 cm−1 Peak Height | 23 | 49 | 85 | 168 | 580 | 1554 | 5235 | |
| Difference (ppm) | 8 | 29 | 10 | 1 | 89 | 45 | 6 | 26.9 |
| % Difference | 36.0% | 41.4% | 6.9% | 0.4% | 8.1% | 1.5% | 0.1% | 13.5% |
| Prepared Concentration (ppm) | 50 | 100 | 125 | 200 | 250 | 500 | 1000 | Average |
| Corrected Concentration (ppm) | 54 | 106 | 128 | 186 | 241 | 513 | 997 | |
| 1360 cm−1 Peak Height | 26 | 56 | 69 | 101 | 132 | 286 | 560 | |
| Difference (ppm) | 4 | 6 | 3 | 14 | 9 | 13 | 3 | 8.0 |
| % Difference | 6.6% | 5.5% | 2.7% | 7.6% | 3.8% | 2.5% | 0.3% | 3.7% |
| Prepared Concentration (ppm) | 50 | 100 | 125 | 200 | 250 | 500 | 1000 |
| Measurent 1 (peak height) | 29.1 | 52.2 | 68.9 | 101.5 | 128.9 | 286.7 | 562.9 |
| Measurent 2 (peak height) | 24.8 | 49.4 | 66.9 | 102.2 | 137.9 | 291.3 | 559.0 |
| Measurent 3 (peak height) | 24.6 | 57.9 | 69.3 | 98.4 | 134.1 | 284.0 | |
| Measurent 4 (peak height) | 24.5 | 54.2 | 72.0 | 107.6 | 127.4 | 284.1 | |
| Measurent 5 (peak height) | 28.1 | 58.6 | 66.4 | ||||
| Measurent 6 (peak height) | 25.5 | 63.2 | 74.3 | ||||
| Measurent 7 (peak height) | 27.4 | 51.2 | 64.3 | ||||
| Measurent 8 (peak height) | 27.5 | 59.4 | 76.8 | ||||
| Average | 26.4 | 55.8 | 69.8 | 102.4 | 132.1 | 286.5 | 561.0 |
| Standard Deviation | 1.8 | 4.7 | 4.2 | 3.8 | 4.8 | 3.4 | 2.8 |
| % Standard Deviation | 6.8% | 8.5% | 6.0% | 3.8% | 3.7% | 1.2% | 0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shende, C.; Farquharson, S.; Farquharson, D.; Brouillette, C. Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System. Oceans 2021, 2, 330-336. https://doi.org/10.3390/oceans2020019
Shende C, Farquharson S, Farquharson D, Brouillette C. Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System. Oceans. 2021; 2(2):330-336. https://doi.org/10.3390/oceans2020019
Chicago/Turabian StyleShende, Chetan, Stuart Farquharson, Duncan Farquharson, and Carl Brouillette. 2021. "Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System" Oceans 2, no. 2: 330-336. https://doi.org/10.3390/oceans2020019
APA StyleShende, C., Farquharson, S., Farquharson, D., & Brouillette, C. (2021). Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System. Oceans, 2(2), 330-336. https://doi.org/10.3390/oceans2020019