Ocean Acidification Does Not Affect Fish Ectoparasite Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parasite Culture and Gnathiid Collection
2.2. Seawater CO2 Manipulation and Aquatic Systems
2.3. Gnathiid Survival and Stage Determination
2.4. Statistical Analysis
2.5. Ethics
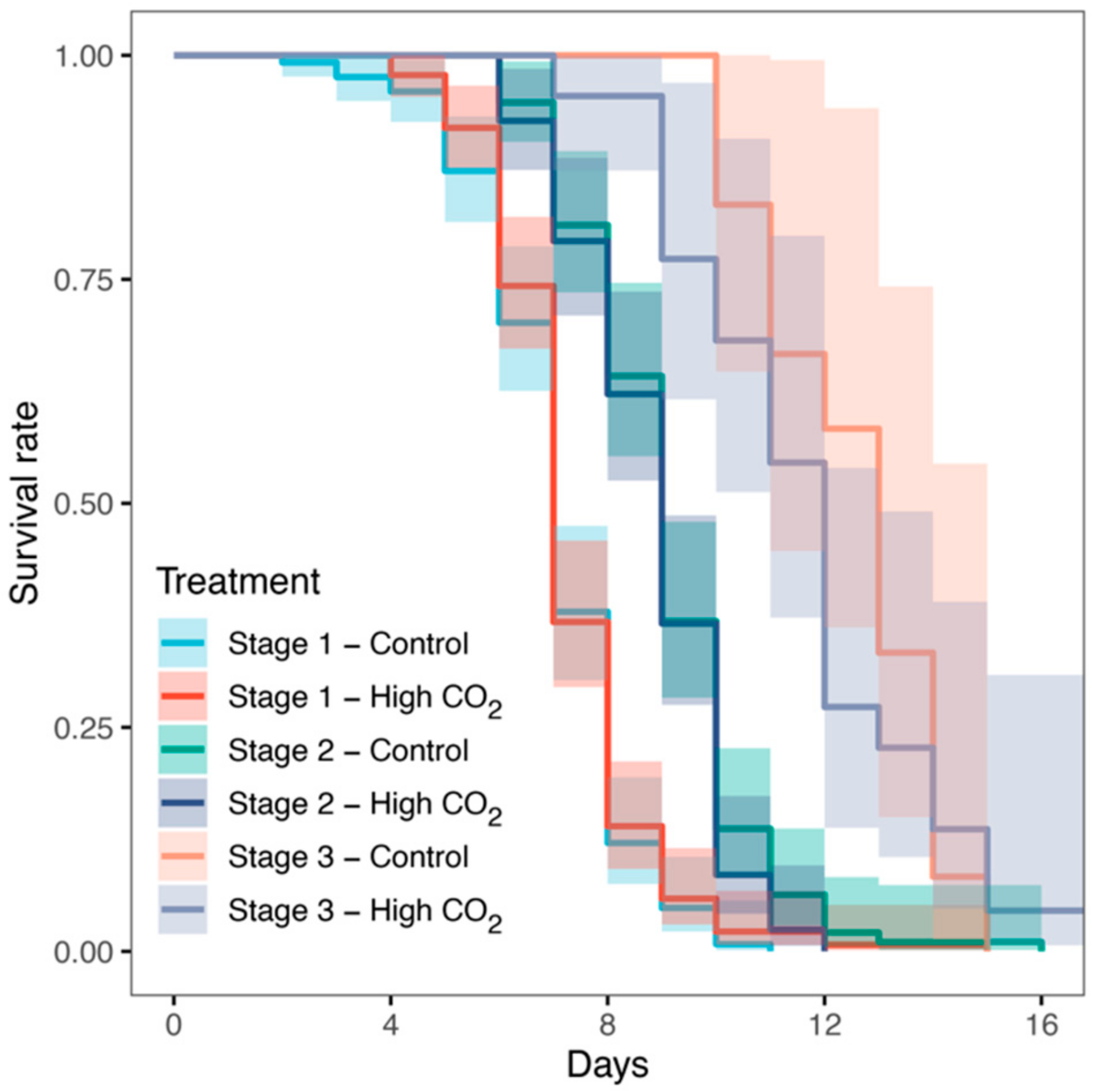
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- IPCC Climate Change 2013: The physical science basis contribution of working group i to the fifth assessment report of the Intergovernmental Panel on Climate Change; Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Dupont, S.; Pörtner, H. Get ready for ocean acidification. Nature 2013, 489, 429. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.N. Coral reefs under climate change and ocean acidification: challenges and opportunities for management and policy. Annu. Rev. Environ. Resour. 2016, 41, 59–81. [Google Scholar] [CrossRef]
- Kuffner, I.B.; Andersson, A.J.; Jokiel, P.L.; Rodgers, K.S.; MacKenzie, F.T. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 2008, 1, 114–117. [Google Scholar] [CrossRef]
- Smith, J.N.; De’Ath, G.; Richter, C.; Cornils, A.; Hall-Spencer, J.M.; Fabricius, K.E. Ocean acidification reduces demersal zooplankton that reside in tropical coral reefs. Nat. Clim. Chang. 2016, 6, 1124–1129. [Google Scholar] [CrossRef] [Green Version]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef] [Green Version]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Munday, P.L.; Cheal, A.J.; Dixson, D.L.; Rummer, J.L.; Fabricius, K.E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Chang. 2014, 4, 487–492. [Google Scholar] [CrossRef]
- Clark, T.D.; Raby, G.D.; Roche, D.G.; Binning, S.A.; Speers-roesch, B.; Jutfelt, F.; Sundin, J. Ocean acidification does not impair the behaviour of coral reef fishes. Nature 2020, 577, 370–375. [Google Scholar] [CrossRef]
- Raby, G.D.; Sundin, J.; Jutfelt, F.; Cooke, S.J.; Clark, T.D. Exposure to elevated carbon dioxide does not impair short-term swimming behaviour or shelter-seeking in a predatory coral-reef fish. J. Fish Biol. 2018, 93, 138–142. [Google Scholar] [CrossRef]
- Sundin, J.; Amcoff, M.; Mateos-González, F.; Raby, G.D.; Jutfelt, F.; Clark, T.D. Long-term exposure to elevated carbon dioxide does not alter activity levels of a coral reef fish in response to predator chemical cues. Behav. Ecol. Sociobiol. 2017, 71, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, D.B.; Grutter, A.S.; Costello, M.J.; Hutson, K.S. Cleaner fishes and shrimp diversity and a re-evaluation of cleaning symbioses. Fish Fish. 2017, 18, 698–716. [Google Scholar] [CrossRef]
- Paula, J.R.; Repolho, T.; Pegado, M.R.; Thörnqvist, P.O.; Bispo, R.; Winberg, S.; Munday, P.L.; Rosa, R. Neurobiological and behavioural responses of cleaning mutualisms to ocean warming and acidification. Sci. Rep. 2019, 9, 12728. [Google Scholar] [CrossRef] [Green Version]
- Quimbayo, J.P.; Cantor, M.; Dias, M.S.; Grutter, A.S.; Gingins, S.; Becker, J.H.A.; Floeter, S.R. The global structure of marine cleaning mutualistic networks. Glob. Ecol. Biogeogr. 2018, 27, 1238–1250. [Google Scholar] [CrossRef]
- Grutter, A.; Poulin, R. Intraspecific and interspecific relationships between host size and the abundance of parasitic larval gnathiid isopods on coral reef fishes. Mar. Ecol. Prog. Ser. 1998, 164, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Sikkel, P.C.; Welicky, R.L. The ecological significance of parasitic crustaceans. In Parasitic Crustacea, Zoological Monographs; Smit, N., Bruce, N., Hadfield, K., Eds.; Springer: Cham, Switzerland, 2019; Volume 3, pp. 421–477. [Google Scholar]
- Grutter, A.S.; Blomberg, S.P.; Fargher, B.; Kuris, A.M.; McCormick, M.I.; Warner, R.R. Size-related mortality due to gnathiid isopod micropredation correlates with settlement size in coral reef fishes. Coral Reefs 2017, 36, 549–559. [Google Scholar] [CrossRef]
- Grutter, A.S.; Crean, A.J.; Curtis, L.M.; Kuris, A.M.; Warner, R.R.; Mccormick, M.I. Indirect effects of an ectoparasite reduce successful establishment of a damselfish at settlement. Funct. Ecol. 2011, 25, 586–594. [Google Scholar] [CrossRef]
- Grutter, A.S. Cleaner fish really do clean. Nature 1999, 398, 672–673. [Google Scholar] [CrossRef]
- Grutter, A.S.; Blomberg, S.P.; Box, S.; Bshary, R.; Ho, O.; Madin, E.M.P.; McClure, E.C.; Meekan, M.G.; Murphy, J.M.; Richardson, M.A.; et al. Changes in local free-living parasite populations in response to cleaner manipulation over 12 years. Oecologia 2019, 190, 783–797. [Google Scholar] [CrossRef]
- Tanaka, K. Life history of gnathiid isopods - current knowledge and future directions. Plankt. Benthos Res. 2007, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hutson, K.S.; Cable, J.; Grutter, A.S.; Paziewska-Harris, A.; Barber, I. Aquatic Parasite Cultures and Their Applications. Trends Parasitol. 2018, 34, 1082–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grutter, A.S. Parasite infection rather than tactile stimulation is the proximate cause of cleaning behaviour in reef fish. Proc. Biol. Sci. 2001, 268, 1361–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grutter, A.S. Feeding ecology of the fish ectoparasite Gnathia sp. (Crustacea: Isopoda) from the Great Barrier Reef, and its implications for fish cleaning behaviour. Mar. Ecol. Prog. Ser. 2003, 259, 295–302. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Fox, J.; Weisberg, S. An r Companion to Applied Regression, 2nd ed.; SAGE Publications, Inc: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Kassambra, A.; Kosinski, M. Survminer: drawing survival curves using “ggplot2” 2018. Available online: https://cran.r-project.org/web/packages/survminer/survminer.pdf (accessed on 25 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2018. Available online: https://www.R-project.org/ (accessed on 25 December 2019).
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Melzner, F.; Mark, F.C.; Seibel, B.A.; Tomanek, L. Ocean acidification and coastal marine invertebrates: tracking CO2 effects from seawater to the cell. Ann. Rev. Mar. Sci. 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Kurihara, H.; Ishimatsu, A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar. Pollut. Bull. 2008, 56, 1086–1090. [Google Scholar] [CrossRef] [Green Version]
- Artim, J.M.; Sikkel, P.C. Live coral repels a common reef fish ectoparasite. Coral Reefs 2012, 32, 487–494. [Google Scholar] [CrossRef]
- Stumpp, M.; Hu, M.; Casties, I.; Saborowski, R.; Bleich, M.; Melzner, F.; Dupont, S. Digestion in sea urchin larvae impaired under ocean acidification. Nat. Clim. Chang. 2013, 3, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Harland, H.; MacLeod, C.D.; Poulin, R. Non-linear effects of ocean acidification on the transmission of a marine intertidal parasite. Mar. Ecol. Prog. Ser. 2015, 536, 55–64. [Google Scholar] [CrossRef]
- Keppel, A.G.; Breitburg, D.L.; Wikfors, G.H.; Burrell, R.B.; Clark, V.M. Effects of co-varying diel-cycling hypoxia and pH on disease susceptibility in the eastern oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 2015, 538, 169–183. [Google Scholar] [CrossRef]
- MacLeod, C.D.; Poulin, R. Differential tolerances to ocean acidification by parasites that share the same host. Int. J. Parasitol. 2015, 45, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Triki, Z.; Wismer, S.; Levorato, E.; Bshary, R. A decrease in the abundance and strategic sophistication of cleaner fish after environmental perturbations. Glob. Chang. Biol. 2018, 24, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Grutter, A.S.; De Brauwer, M.; Bshary, R.; Cheney, K.L.; Cribb, T.H.; Madin, E.M.P.; McClure, E.C.; Meekan, M.G.; Sun, D.; Warner, R.R.; et al. Parasite infestation increases on coral reefs without cleaner fish. Coral Reefs 2018, 37, 15–24. [Google Scholar] [CrossRef]
| D.f. | χ2 | p | |
|---|---|---|---|
| CO2 treatment | 1 | 3.29 | 0.070 |
| Larval stage | 2 | 6.35 | 0.042 |
| Headwidth | 1 | 27.35 | <0.001 |
| CO2 treatment × Headwidth | 1 | 2.10 | 0.147 |
| CO2 treatment × Larval stage | 2 | 1.18 | 0.555 |
| Stage × Headwidth | 2 | 11.63 | 0.003 |
| CO2 treatment × Stage × Headwidth | 2 | 0.80 | 0.680 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, J.R.; Otjacques, E.; Hildebrandt, C.; Grutter, A.S.; Rosa, R. Ocean Acidification Does Not Affect Fish Ectoparasite Survival. Oceans 2020, 1, 27-33. https://doi.org/10.3390/oceans1010003
Paula JR, Otjacques E, Hildebrandt C, Grutter AS, Rosa R. Ocean Acidification Does Not Affect Fish Ectoparasite Survival. Oceans. 2020; 1(1):27-33. https://doi.org/10.3390/oceans1010003
Chicago/Turabian StylePaula, José Ricardo, Eve Otjacques, Courtney Hildebrandt, Alexandra S. Grutter, and Rui Rosa. 2020. "Ocean Acidification Does Not Affect Fish Ectoparasite Survival" Oceans 1, no. 1: 27-33. https://doi.org/10.3390/oceans1010003
APA StylePaula, J. R., Otjacques, E., Hildebrandt, C., Grutter, A. S., & Rosa, R. (2020). Ocean Acidification Does Not Affect Fish Ectoparasite Survival. Oceans, 1(1), 27-33. https://doi.org/10.3390/oceans1010003