How Is Artificial Intelligence Transforming the Intersection of Pediatric and Special Care Dentistry? A Scoping Review of Current Applications and Ethical Considerations
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- Focused on pediatric patients, including children with intellectual, developmental, or physical disabilities.
- Described the application of AI technologies in any domain of pediatric dentistry.
- Addressed AI use in clinical or behavioral settings, including diagnostics, treatment planning, preventive care, patient monitoring, behavior management, or teledentistry.
- Included original research articles, narrative reviews, systematic reviews, scoping reviews, or technology-focused overviews.
2.3. Exclusion Criteria
- Focused exclusively on adult populations.
- Mentioned AI superficially without describing practical applications in pediatric dental contexts.
- Were non-peer-reviewed formats (editorials, letters, conference abstracts).
- Were published in languages other than English.
2.4. Study Selection and Data Extraction
3. Results
3.1. Study Selection
3.2. Overview of the Evidence Base
3.3. Diagnostic Imaging and Caries Detection
3.4. Three-Dimensional Imaging
3.5. Interceptive and Preventive Orthodontics
3.6. Chatbots and Teledentistry
3.7. Decision Support, Patient Engagement, and Predictive Analytics
3.8. Pain Assessment and Discomfort Monitoring
3.9. Behavior Management
3.10. Behavior Modeling
3.11. Ethical and Environmental Considerations and Challenges
3.11.1. Rigorous Validation of AI
3.11.2. Ensuring the Complementary Role of AI
3.11.3. AI-Induced Overtreatment
3.11.4. Carbon Cost of Cloud AI Inference
4. Discussion
4.1. Implications for Practice
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ML | Machine Learning |
| DL | Deep Learning |
| NLP | Natural Language Processing |
| CBCT | Cone Beam Computed Tomography |
| VR | Virtual Reality |
| ECC | Early Childhood Caries |
| ASD | Autism Spectrum Disorder |
References
- Mintz, Y.; Brodie, R. Introduction to artificial intelligence in medicine. Minim. Invasive Ther. Allied Technol. MITAT 2019, 28, 73–81. [Google Scholar] [CrossRef]
- Rasteau, S.; Ernenwein, D.; Savoldelli, C.; Bouletreau, P. Artificial intelligence for oral and maxillo-facial surgery: A narrative review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 276–282. [Google Scholar] [CrossRef]
- Zeitoun, J.-D.; Ravaud, P. Artificial intelligence in health care: Value for whom? Lancet Digit. Health 2020, 2, 338–339. [Google Scholar] [CrossRef]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metab. Clin. Exp. 2017, 69, 36–40. [Google Scholar] [CrossRef]
- Sharma, V.; Pattnaik, S.; Dutta, B.; Shukla, P.; Mahakul, B.; Dhull, K.S. Preventing the Risk of Dental Problems in Children with Special Health Care Needs: A Case Series. Cureus 2025, 17, e86320. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, J.; Zhao, W.; Matinlinna, J.P.; Burrow, M.F.; Tsoi, J.K.H. Artificial intelligence in dentistry—A review. Front. Dent. Med. 2023, 4, 1085251. [Google Scholar] [CrossRef]
- Howard, J. Artificial intelligence: Implications for the future of work. Am. J. Ind. Med. 2019, 62, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Alessa, N. Application of Artificial Intelligence in Pediatric Dentistry: A Literature Review. J. Pharm. Bioallied Sci. 2024, 16, 1938–1940. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, J.; Singla, M.; Nath, A.; Arya, A. Revolutionizing the diagnosis of dental caries using artificial intelligence-based methods. J. Conserv. Dent. Endod. 2025, 28, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Porwal, P.; Porwal, A. Transforming Dental Caries Diagnosis Through Artificial Intelligence-Based Techniques. Cureus 2023, 15, e41694. [Google Scholar] [CrossRef]
- Alharbi, N.; Alharbi, A.S. AI-Driven Innovations in Pediatric Dentistry: Enhancing Care and Improving Outcome. Cureus 2024, 16, e69250. [Google Scholar] [CrossRef]
- Cohen, S.; Levenson, R.; Pantanowitz, L. Artificial Intelligence in Pathology. Am. J. Pathol. 2021, 191, 1670–1672. [Google Scholar] [CrossRef]
- Suresh, A.; Naidu, S.N.; Inginshetty, V. ENDO AI: A Novel Artificial Intelligence Framework for Predicting Treatment Outcomes in Endodontic Therapy. J. Med. Dent. Sci. Res. 2025, 12, 12–19. [Google Scholar] [CrossRef]
- Ammar, N.; Kühnisch, J. Diagnostic performance of artificial intelligence-aided caries detection on bitewing radiographs: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2024, 60, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.M.; Darvish, S.; Salman, B.N.; Luchian, I. Artificial Intelligence in Adult and Pediatric Dentistry: A Narrative Review. Bioengineering 2024, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, D.G. Artificial Intelligence in Oral and Maxillofacial Surgery Education. Oral Maxillofac. Surg. Clin. North Am. 2022, 34, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Akitomo, T.; Hamada, M.; Asao, Y.; Iwamoto, Y.; Tachikake, M.; Mitsuhata, C.; Nomura, R. Usefulness of Generative Artificial Intelligence (AI) Tools in Pediatric Dentistry. Diagnostics 2024, 14, 2818. [Google Scholar] [CrossRef]
- Rokhshad, R.; Zhang, P.; Mohammad-Rahimi, H.; Shobeiri, P.; Schwendicke, F. Current Applications of Artificial Intelligence for Pediatric Dentistry: A Systematic Review and Meta-Analysis. Pediatr. Dent. 2024, 46, 27–35. [Google Scholar] [PubMed]
- Vishwanathaiah, S.; Fageeh, H.N.; Khanagar, S.B.; Maganur, P.C. Artificial Intelligence Its Uses and Application in Pediatric Dentistry: A Review. Biomedicines 2023, 11, 788. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71–79. [Google Scholar] [CrossRef]
- AI, M.; Rehman, A.U.; Umar, S.; Zubair, M.; Sajjad, Z.; Ghaffar, A.; Jalil, M. Diagnostic accuracy of AI-based versus conventional radiographic caries detection in pediatric patients: A cross-sectional study. Insights-J. Health Rehabil. 2025, 3, 29–34. [Google Scholar] [CrossRef]
- Silva-Filho, J.E.; Silva, B.B.; Pascoal, S.C.D.; Filgueira, A.A.; Mendes, T.A.D.; Albuquerque, D.F. Sensitivity Evaluation of Deep Learning-Based Models for Dental Caries Detection in Bitewing Radiographs: A Systematic Review and Meta-analysis. J. Adv. Med. Med. Res. 2024, 36, 190–203. [Google Scholar] [CrossRef]
- Bayati, M.; Alizadeh Savareh, B.; Ahmadinejad, H.; Mosavat, F. Advanced AI-driven detection of interproximal caries in bitewing radiographs using YOLOv8. Sci. Rep. 2025, 15, 4641. [Google Scholar] [CrossRef]
- Dhanak, N.; Chougule, V.T.; Nalluri, K.; Kakkad, A.; Dhimole, A.; Parihar, A.S. Artificial intelligence enabled smart phone app for real-time caries detection on bitewing radiographs. Bioinformation 2024, 20, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Panyarak, W.; Wantanajittikul, K.; Charuakkra, A.; Prapayasatok, S.; Suttapak, W. Enhancing Caries Detection in Bitewing Radiographs Using YOLOv7. J. Digit. Imaging 2023, 36, 2635–2647. [Google Scholar] [CrossRef]
- Albano, D.; Galiano, V.; Basile, M.; Di Luca, F.; Gitto, S.; Messina, C.; Cagetti, M.G.; Del Fabbro, M.; Tartaglia, G.M.; Sconfienza, L.M. Artificial intelligence for radiographic imaging detection of caries lesions: A systematic review. BMC Oral Health 2024, 24, 274. [Google Scholar] [CrossRef]
- Fux-Noy, A.; Rohana, R.; Rettman, A.; Moskovitz, M.; Nadler, C. Panoramic errors in pediatric patients with special needs. Sci. Rep. 2023, 13, 11757. [Google Scholar] [CrossRef]
- Ameli, N.; Miri Moghaddam, M.; Lai, H.; Pacheco-Pereira, C. Automated quality evaluation of dental panoramic radiographs using deep learning. Imaging Sci. Dent. 2025, 55, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Eby, P.R.; Martis, L.M.; Paluch, J.T.; Pak, J.J.; Chan, A.H.L. Impact of Artificial Intelligence-driven Quality Improvement Software on Mammography Technical Repeat and Recall Rates. Radiol. Artif. Intell. 2023, 5, e230038. [Google Scholar] [CrossRef]
- Thummerer, A.; Schmidt, L.; Hofmaier, J.; Corradini, S.; Belka, C.; Landry, G.; Kurz, C. Deep learning based super-resolution for CBCT dose reduction in radiotherapy. Med. Phys. 2025, 52, 1629–1642. [Google Scholar] [CrossRef]
- Usui, K.; Kamiyama, S.; Arita, A.; Ogawa, K.; Sakamoto, H.; Sakano, Y.; Kyogoku, S.; Daida, H. Reducing image artifacts in sparse projection CT using conditional generative adversarial networks. Sci. Rep. 2024, 14, 3917. [Google Scholar] [CrossRef]
- Wajer, R.; Wajer, A.; Kazimierczak, N.; Wilamowska, J.; Serafin, Z. The Impact of AI on Metal Artifacts in CBCT Oral Cavity Imaging. Diagnostics 2024, 14, 1280. [Google Scholar] [CrossRef]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Lu, H.-P.; Hou, J.; Wang, Q.; Yu, F.-Y.; Zhong, C.; Huang, C.-Y.; Chen, S. Deep learning-based prediction of mandibular growth trend in children with anterior crossbite using cephalometric radiographs. BMC Oral Health 2023, 23, 28. [Google Scholar] [CrossRef]
- Yamada, S.; Yoon, H.J.; Huh, Y.; Yanagi, Y.; Park, J.H.; Tai, K.; Kim, N.; Baek, S. Accuracy of Artificial Intelligence in Predicting the Treatment Effects of Headgear and/or Functional Appliance on the Maxillo-Mandibular Growth in Preadolescent Patients with Skeletal Class II Malocclusion. Orthod. Craniofacial Res. 2025, 2, 189–194. [Google Scholar] [CrossRef]
- Volovic, J.; Badirli, S.; Ahmad, S.; Leavitt, L.; Mason, T.; Bhamidipalli, S.S.; Eckert, G.; Albright, D.; Turkkahraman, H. A Novel Machine Learning Model for Predicting Orthodontic Treatment Duration. Diagnostics 2023, 13, 2740. [Google Scholar] [CrossRef]
- Moeini, A.; Torabi, S. The Role of Artificial Intelligence in Dental Diagnosis and Treatment Planning. J. Oral Dent. Health Nexus 2025, 2, 14–26. [Google Scholar] [CrossRef]
- Kılıç, B.; İbrahim, A.H.; Aksoy, S.; Sakman, M.C.; Demircan, G.S.; Önal-Süzek, T. A family-centered orthodontic screening approach using a machine learning-based mobile application. J. Dent. Sci. 2024, 19, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Rapaka, R. AI-driven evolution in teledentistry: A comprehensive overview of technology and clinical applications. Dent. Rev. 2025, 5, 100154. [Google Scholar] [CrossRef]
- Bayraktar, N.C. Can ChatGPT be guide in pediatric dentistry? BMC Oral Health 2025, 25, 9. [Google Scholar] [CrossRef]
- Thorat, V.; Rao, P.; Joshi, N.; Talreja, P.; Shetty, A.R. Role of Artificial Intelligence (AI) in Patient Education and Communication in Dentistry. Cureus 2024, 16, 59799. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Knights, N.; Husbands, D.; Graham, C.A.; Llewellyn, C.D.; Buchanan, T.; Montgomery, I.; Ridge, D.; Silva, J.N.A. Achieving health equity through conversational AI: A roadmap for design and implementation of inclusive chatbots in healthcare. PLOS Digit. Health 2024, 3, e0000492. [Google Scholar] [CrossRef]
- Paschal, A.M.; Wilroy, J.D.; Hawley, S.R. Unmet needs for dental care in children with special health care needs. Prev. Med. Rep. 2016, 3, 62–67. [Google Scholar] [CrossRef]
- Alghamdi, S.A. Parent perceptions regarding virtual pediatric dental clinics during COVID-19 pandemic: A cross-sectional study. PeerJ 2023, 11, e15289. [Google Scholar] [CrossRef] [PubMed]
- Abaklı İnci, M.; Korkut, E.; Koç, M.; Tüzüner, T. Retrospective evaluation of the effectiveness of teledentistry approach during COVID-19 in pediatric dentistry: A parental perspective. Digit. Health 2022, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Lipsky, M.S.; Phuatrakoon, T.N.; Nguyen, M.; Licari, F.W.; Unni, E.J. Teledentistry Implementation During the COVID-19 Pandemic: Scoping Review. Interact. J. Med. Res. 2022, 11, e39955. [Google Scholar] [CrossRef] [PubMed]
- Sakr, L.; Abbas, H.; Thabet, N.; Abdelgawad, F. Reliability of teledentistry mobile photos versus conventional clinical examination for dental caries diagnosis on occlusal surfaces in a group of school children: A diagnostic accuracy study. BMC Oral Health 2025, 25, 545. [Google Scholar] [CrossRef]
- Hasan, F.; Tantawi MEl Haque, F.; Foláyan, M.O.; Virtanen, J.I. Early childhood caries risk prediction using machine learning approaches in Bangladesh. BMC Oral Health 2025, 25, 49. [Google Scholar] [CrossRef]
- Al-Namankany, A. Influence of Artificial Intelligence-Driven Diagnostic Tools on Treatment Decision-Making in Early Childhood Caries: A Systematic Review of Accuracy and Clinical Outcomes. Dent. J. 2023, 11, 214. [Google Scholar] [CrossRef]
- Pupong, K.; Hunsrisakhun, J.; Pithpornchaiyakul, S.; Naorungroj, S. Development of Chatbot-Based Oral Health Care for Young Children and Evaluation of its Effectiveness, Usability, and Acceptability: Mixed Methods Study. JMIR Pediatr. Parent. 2025, 8, e62738. [Google Scholar] [CrossRef]
- Hasei, J.; Hanzawa, M.; Nagano, A.; Maeda, N.; Yoshida, S.; Endo, M.; Yokoyama, N.; Ochi, M.; Ishida, H.; Katayama, H.; et al. Empowering pediatric, adolescent, and young adult patients with cancer utilizing generative AI chatbots to reduce psychological burden and enhance treatment engagement: A pilot study. Front. Digit. Health 2025, 7, 1543543. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Zadeh, S.-A.; Bagheri, M.; Saadat, M. Decoding children dental health risks: A machine learning approach to identifying key influencing factors. Front. Artif. Intell. 2024, 7, 1392597. [Google Scholar] [CrossRef] [PubMed]
- Lakhotia, S.; Godrej, H.; Kaur, A.; Nutakki, C.S.; Mun, M.; Eber, P.; Anthony Celi, L. Machine learning in dentistry: A scoping review. PLOS Digit. Health 2025, 4, e0000940. [Google Scholar] [CrossRef]
- Badrov, M.; Perkov, L.; Tadin, A. The Impact of Oral Health on the Quality of Life of Children with Autism Spectrum Disorder and Their Families: Parental Perspectives from an Online Cross-Sectional Study. Oral 2025, 5, 36. [Google Scholar] [CrossRef]
- Gomez-Rios, I.; Egea-Lopez, E.; Ortiz Ruiz, A.J. ORIENTATE: Automated machine learning classifiers for oral health prediction and research. BMC Oral Health 2023, 23, 408. [Google Scholar] [CrossRef]
- De Sario, G.D.; Haider, C.R.; Maita, K.C.; Torres-Guzman, R.A.; Emam, O.S.; Avila, F.R.; Garcia, J.P.; Borna, S.; McLeod, C.J.; Bruce, C.J.; et al. Using AI to Detect Pain through Facial Expressions: A Review. Bioengineering 2023, 10, 548. [Google Scholar] [CrossRef]
- Cascella, M.; Schiavo, D.; Cuomo, A.; Ottaiano, A.; Perri, F.; Patrone, R.; Migliarelli, S.; Bignami, E.G.; Vittori, A.; Cutugno, F.; et al. Artificial Intelligence for Automatic Pain Assessment: Research Methods and Perspectives. Pain Res. Manag. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Santana, M.D.R.; de Souza, A.C.A.; de Abreu, L.C.; Valenti, V.E. Association between oral variables and heart rate variability. Int. Arch. Med. 2013, 6, 49. [Google Scholar] [CrossRef]
- Gkikas, S.; Tachos, N.S.; Andreadis, S.; Pezoulas, V.C.; Zaridis, D.; Gkois, G.; Matonaki, A.; Stavropoulos, T.G.; Fotiadis, D.I. Multimodal automatic assessment of acute pain through facial videos and heart rate signals utilizing transformer-based architectures. Front. Pain Res. 2024, 5, 1372814. [Google Scholar] [CrossRef]
- Yue, J.-M.; Wang, Q.; Liu, B.; Zhou, L. Postoperative accurate pain assessment of children and artificial intelligence: A medical hypothesis and planned study. World J. Clin. Cases 2024, 12, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ramdhanie, G.G.; Wanda, D.; Agustini, N.; Abuzairi, T. Identification of technology-based models and efficacy of digital-based pain facial expression assessment tools among children: A systematic review. BMC Nurs. 2025, 24, 905. [Google Scholar] [CrossRef]
- Hughes, J.D.; Chivers, P.; Hoti, K. The Clinical Suitability of an Artificial Intelligence-Enabled Pain Assessment Tool for Use in Infants: Feasibility and Usability Evaluation Study. J. Med. Internet Res. 2023, 25, e41992. [Google Scholar] [CrossRef]
- Kasımoğlu, Y.; Kocaaydın, S.; Batu, Ş.; İnce, G.; Tuna-İnce, E.B. The Impact of a Humanoid Robot on Children’s Dental Anxiety, Behavior and Salivary Amylase Levels: A Randomized Clinical Trial. J. Pediatr. Res. 2023, 10, 132–141. [Google Scholar] [CrossRef]
- Suresh, L.R.; Shetty, V. Effect of Virtual Reality Distraction Method on the Level of Salivary Cortisol in Children with Autism Spectrum Disorder During Dental Treatment. J. Autism Dev. Disord. 2024, 3, 211–219. [Google Scholar] [CrossRef]
- Al Kheraif, A.A.; Adam, T.R.; Wasi, A.; Alhassoun, R.K.; Haddadi, R.M.; Alnamlah, M. Impact of Virtual Reality Intervention on Anxiety and Level of Cooperation in Children and Adolescents with Autism Spectrum Disorder during the Dental Examination. J. Clin. Med. 2024, 13, 6093. [Google Scholar] [CrossRef]
- Mehrotra, D.; Shetty, A.A.; Rai, K.; Kumara. Effect of audio and virtual reality distraction on the dental anxiety of children with mild intellectual disability. Spec. Care Dent. 2024, 44, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Shiraishi, T.; Sato, H.; Nihei, M.; Inoue, T.; Kuwabara, C. A concept for emotion recognition systems for children with profound intellectual and multiple disabilities based on artificial intelligence using physiological and motion signals. Disabil. Rehabil. Assist. Technol. 2024, 19, 1319–1326. [Google Scholar] [CrossRef]
- De Barros Padilha, D.X.; Veiga, N.J.; Mello-Moura, A.C.V.; Nunes Correia, P. Virtual reality and behaviour management in paediatric dentistry: A systematic review. BMC Oral Health 2023, 23, 995. [Google Scholar] [CrossRef]
- Nishat, F.; Hudson, S.; Panesar, P.; Ali, S.; Litwin, S.; Zeller, F.; Candelaria, P.; Foster, M.E.; Stinson, J. Exploring the needs of children and caregivers to inform design of an artificial intelligence-enhanced social robot in the pediatric emergency department. J. Clin. Transl. Sci. 2023, 7, 191–209. [Google Scholar] [CrossRef]
- Waldman, C.E.; Hermel, M.; Hermel, J.A.; Allinson, F.; Pintea, M.N.; Bransky, N.; Udoh, E.; Nicholson, L.; Robinson, A.; Gonzalez, J.; et al. Artificial intelligence in healthcare: A primer for medical education in radiomics. Pers. Med. 2022, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Quinzi, V.; Palazzo, G.; Ronsivalle, V.; Lo Giudice, A. The Implications of Artificial Intelligence in Pedodontics: A Scoping Review of Evidence-Based Literature. Healthcare 2024, 12, 1311. [Google Scholar] [CrossRef]
- Myllyaho, L.; Raatikainen, M.; Männistö, T.; Mikkonen, T.; Nurminen, J.K. Systematic literature review of validation methods for AI systems. J. Syst. Softw. 2021, 181, 111050. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y. Artificial Intelligence Decision-Making Transparency and Employees’ Trust: The Parallel Multiple Mediating Effect of Effectiveness and Discomfort. Behav. Sci. 2022, 12, 127. [Google Scholar] [CrossRef]
- Thurzo, A.; Thurzo, V. Embedding Fear in Medical AI: A Risk-Averse Framework for Safety and Ethics. AI 2025, 6, 101. [Google Scholar] [CrossRef]
- Sherif, I.A.; Nser, S.Y.; Bobo, A.; Afridi, A.; Hamed, A.; Dunbar, M.; Boutefnouchet, T. Can Ordinary AI-Powered Tools Replace a Clinician-Led Fracture Clinic Appointment? Cureus 2024, 4, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Alderman, J.E.; Palmer, J.; Ganapathi, S.; Laws, E.; McCradden, M.D.; Oakden-Rayner, L.; Pfohl, S.R.; Ghassemi, M.; McKay, F.; et al. The value of standards for health datasets in artificial intelligence-based applications. Nat. Med. 2023, 29, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A. Provable AI Ethics and Explainability in Medical and Educational AI Agents: Trustworthy Ethical Firewall. Electronics 2025, 14, 1294. [Google Scholar] [CrossRef]
- Labkoff, S.; Oladimeji, B.; Kannry, J.; Solomonides, A.; Leftwich, R.; Koski, E.; Joseph, A.L.; Lopez-Gonzalez, M.; A Fleisher, L.; Nolen, K.; et al. Toward a responsible future: Recommendations for AI-enabled clinical decision support. J. Am. Med. Inform. Assoc. 2024, 31, 2730–2739. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, L.; Xue, L. Overcoming medical overuse with AI assistance: An experimental investigation. J. Health Econ. 2025, 103, 103043. [Google Scholar] [CrossRef]
- Lefebvre, M.; Stenger, A. Short- & long-term effects of monetary and non-monetary incentives to cooperate in public good games: An experiment. PLoS ONE 2020, 15, e0227360. [Google Scholar] [CrossRef]
- Weiner, E.B.; Dankwa-Mullan, I.; Nelson, W.A.; Hassanpour, S. Ethical challenges and evolving strategies in the integration of artificial intelligence into clinical practice. PLOS Digit. Health 2025, 4, e0000810. [Google Scholar] [CrossRef]
- Smith, M.J.; Axler, R.; Bean, S.; Rudzicz, F.; Shaw, J. Four equity considerations for the use of artificial intelligence in public health. Bull. World Health Organ. 2020, 98, 290–292. [Google Scholar] [CrossRef]
- Bolón-Canedo, V.; Morán-Fernández, L.; Cancela, B.; Alonso-Betanzos, A. A review of green artificial intelligence: Towards a more sustainable future. Neurocomputing 2024, 599, 128096. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, J.; Song, Y.; Zheng, X.; He, G.; Chen, X.; Zhang, T.; Lee, W.-J.; Song, J. Tracking the carbon footprint of global generative artificial intelligence. Innovation 2025, 6, 100866. [Google Scholar] [CrossRef] [PubMed]
- Dauner, M.; Socher, G. Energy costs of communicating with AI. Front. Commun. 2025, 10, 119–126. [Google Scholar] [CrossRef]
- Aquino-Brítez, S.; García-Sánchez, P.; Ortiz, A.; Aquino-Brítez, D. Towards an Energy Consumption Index for Deep Learning Models: A Comparative Analysis of Architectures, GPUs, and Measurement Tools. Sensors 2025, 25, 846. [Google Scholar] [CrossRef]
- Carvalho, B.K.G.; Nolden, E.-L.; Schulze Wenning, A.; Kiss-Dala, S.; Agócs, G.; Róth, I.; Kerémi, B.; Géczi, Z.; Hegyi, P.; Kivovics, M. Diagnostic accuracy of artificial intelligence for approximal caries on bitewing radiographs: A systematic review and meta-analysis. J. Dent. 2024, 151, 105388. [Google Scholar] [CrossRef] [PubMed]
- Nordblom, N.F.; Büttner, M.; Schwendicke, F. Artificial Intelligence in Orthodontics: Critical Review. J. Dent. Res. 2024, 103, 577–584. [Google Scholar] [CrossRef]
- Larkin, A.; Kim, J.-S.; Kim, N.; Baek, S.; Yamada, S.; Park, K.; Tai, K.; Yanagi, Y.; Park, J.H. Accuracy of artificial intelligence-assisted growth prediction in skeletal Class I preadolescent patients using serial lateral cephalograms for a 2-year growth interval. Orthod. Craniofacial Res. 2024, 27, 535–543. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, M.; Dutta, B.; Bagchi, A. Knowledge and Awareness of Emotional Artificial Intelligence as Tool in Child’s Oral Health Care Assessed Among Dental Professionals of Eastern India. A Cross Sectional Study. J. Pharm. Bioallied Sci. 2024, 16, 2033–2035. [Google Scholar] [CrossRef]
- Ogawa, R.; Ogura, I. AI-based computer-aided diagnosis for panoramic radiographs: Quantitative analysis of mandibular cortical morphology in relation to age and gender. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 383–387. [Google Scholar] [CrossRef]
- Orhan, K.; Sanders, A.; Ünsal, G.; Ezhov, M.; Mısırlı, M.; Gusarev, M.; İçEn, M.; Shamshiev, M.; Keser, G.; Pekiner, F.N.; et al. Assessing the reliability of CBCT-based AI-generated STL files in diagnosing osseous changes of the mandibular condyle: A comparative study with ground truth diagnosis. Dentomaxillofacial Radiol. 2023, 52, 211–219. [Google Scholar] [CrossRef]
- Karakuş, R.; Öziç, M.Ü.; Tassoker, M. AI-Assisted Detection of Interproximal, Occlusal, and Secondary Caries on Bite-Wing Radiographs: A Single-Shot Deep Learning Approach. J. Imaging Inform. Med. 2024, 37, 3146–3159. [Google Scholar] [CrossRef]
- Kühnisch, J.; Meyer, O.; Hesenius, M.; Hickel, R.; Gruhn, V. Caries Detection on Intraoral Images Using Artificial Intelligence. J. Dent. Res. 2022, 101, 158–165. [Google Scholar] [CrossRef]
- Uribe, S.E.; Issa, J.; Sohrabniya, F.; Denny, A.; Kim, N.; Dayo, A.; Chaurasia, A.; Sofi-Mahmudi, A.; Büttner, M.; Schwendicke, F. Publicly Available Dental Image Datasets for Artificial Intelligence. J. Dent. Res. 2024, 103, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Nistelrooij, N.; Maier, E.; Bronkhorst, H.; Crins, L.; Xi, T.; Loomans, B.A.C.; Vinayahalingam, S. Automated monitoring of tooth wear progression using AI on intraoral scans. J. Dent. 2024, 150, 105323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Alqahtani, K.A.; Van den Bogaert, T.; Shujaat, S.; Jacobs, R.; Shaheen, E. Convolutional neural network for automated tooth segmentation on intraoral scans. BMC Oral Health 2024, 24, 804. [Google Scholar] [CrossRef] [PubMed]
- Freire-Maia, J.; Clementino, L.C.; Martins-Júnior, P.A.; Freire-Maia, F.B. Interest in oral health education through digital technologies: A cross-sectional study. Gen. Dent. 2021, 69, 13–17. [Google Scholar]
- Mendonça, T.S.; de Carvalho, S.T.; Aljafari, A.; Hosey, M.T.; Costa, L.R. Oral Health Education for Children: Development of a Serious Game with a User-Centered Design Approach. Games Health J. 2024, 13, 268–277. [Google Scholar] [CrossRef]
- Wallace, C.K.; Schofield, C.E.; Burbridge, L.A.L.; O’Donnell, K.L. Role of teledentistry in paediatric dentistry. Br. Dent. J. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Hammersmith, K.J.; Thiel, M.C.; Messina, M.J.; Casamassimo, P.S.; Townsend, J.A. Connecting Medical Personnel to Dentists via Teledentistry in a Children’s Hospital System: A Pilot Study. Front. Oral Health 2021, 2, 769988. [Google Scholar] [CrossRef] [PubMed]
- Surdu, S.; Langelier, M. Teledentistry: Increasing utilisation of oral-health services for children in rural areas. J. Telemed. Telecare 2023, 29, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.M.; Banja, J.D.; Meltzer, C.C. Ethical considerations in artificial intelligence. Eur. J. Radiol. 2020, 122, 108768. [Google Scholar] [CrossRef]
- Benzinger, L.; Ursin, F.; Balke, W.-T.; Kacprowski, T.; Salloch, S. Should Artificial Intelligence be used to support clinical ethical decision-making? A systematic review of reasons. BMC Med. Ethics 2023, 24, 48. [Google Scholar] [CrossRef]
- Semerci, Z.M.; Yardımcı, S. Empowering Modern Dentistry: The Impact of Artificial Intelligence on Patient Care and Clinical Decision Making. Diagnostics 2024, 14, 1260. [Google Scholar] [CrossRef] [PubMed]
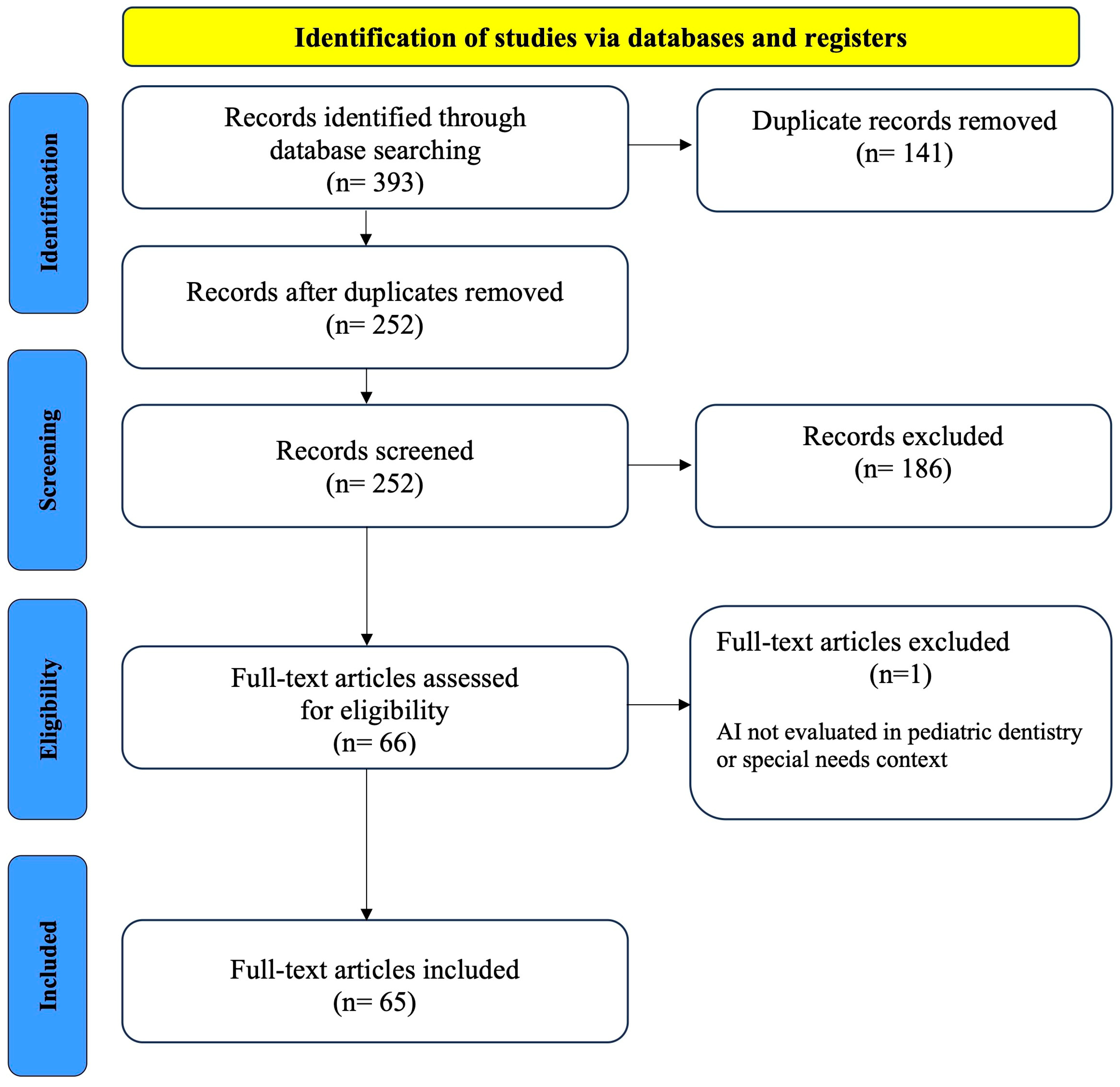
| Database | Applied Search Strategy |
|---|---|
| PubMed | (“Artificial Intelligence” [Mesh] OR “Machine Learning” [Mesh] OR “Neural Networks, Computer” [Mesh] OR “Natural Language Processing” [Mesh] OR “Robotics” [Mesh] OR “artificial intelligence” [tiab] OR “machine learning” [tiab] OR “deep learning” [tiab] OR “neural network*” [tiab] OR “natural language processing” [tiab] OR “large language model*” [tiab] OR “computer vision” [tiab] OR “predictive analytic*” [tiab] OR “teledentistry” [tiab]) AND (“Pediatric Dentistry” [Mesh] OR “Dentistry” [Mesh] OR “Orthodontics” [Mesh] OR “pediatric dent*” [tiab] OR “paediatric dent*” [tiab] OR “child dent*” [tiab] OR orthodontic* [tiab]) AND (“Child” [Mesh] OR “Adolescent” [Mesh] OR “Disabled Children” [Mesh] OR “Intellectual Disability” [Mesh] OR “Developmental Disabilities” [Mesh] OR “Autism Spectrum Disorder” [Mesh] OR child* [tiab] OR pediatric* [tiab] OR paediatric* [tiab] OR adolescent* [tiab] OR “special need*” [tiab] OR disability [tiab] OR disabilities [tiab] OR neurodivergen* [tiab]) AND (diagnos* [tiab] OR detection [tiab] OR screening [tiab] OR “treatment planning” [tiab] OR “decision support” [tiab] OR “behavior management” [tiab] OR “behaviour management” [tiab] OR triage [tiab] OR monitoring [tiab] OR “remote monitoring” [tiab] OR “cone beam” [tiab] OR CBCT [tiab] OR radiograph* [tiab] OR chatbot* [tiab] OR “virtual assistant*” [tiab] OR robotics [tiab] OR robot-assist* [tiab]) |
| Web of Science | TS = ((“artificial intelligence” OR “machine learning” OR “deep learning” OR “neural network*” OR “natural language processing” OR “large language model*” OR “computer vision” OR robotics OR “predictive analytic*” OR teledentistry) AND (“pediatric dent*” OR “paediatric dent*” OR “child dent*” OR orthodontic* OR dentistry) AND (child* OR pediatric* OR paediatric* OR adolescent* OR “special need*” OR disability OR disabilities OR neurodivergen*) AND (diagnos* OR detection OR screening OR “treatment planning” OR “decision support” OR “behavior management” OR “behaviour management” OR triage OR monitoring OR “remote monitoring” OR radiograph* OR CBCT OR “cone beam” OR chatbot* OR “virtual assistant*” OR robot*)) |
| Scopus | TITLE-ABS-KEY(“artificial intelligence” OR “machine learning” OR “deep learning” OR “neural network*” OR “natural language processing” OR “large language model*” OR “computer vision” OR robotics OR “predictive analytic*” OR teledentistry) AND TITLE-ABS-KEY(“pediatric dent*” OR “paediatric dent*” OR “child dent*” OR orthodontic* OR dentistry) AND TITLE-ABS-KEY(child* OR pediatric* OR paediatric* OR adolescent* OR “special need*” OR disability OR disabilities OR neurodivergen*) AND TITLE-ABS-KEY(diagnos* OR detection OR screening OR “treatment planning” OR “decision support” OR “behavior management” OR “behaviour management” OR triage OR monitoring OR “remote monitoring” OR radiograph* OR CBCT OR “cone beam” OR chatbot* OR “virtual assistant*” OR robot*) |
| Thematic Domain | Evidence Maturity | Children with Special Health Care Needs-Specific Evidence |
|---|---|---|
| Diagnostic imaging and caries detection | Established (systematic reviews, large annotated datasets) | Explored conceptually, but lacking SHCN-specific validation |
| Three-dimensional imaging | Emerging (feasibility and optimization studies) | No SHCN-focused validation; adult/mixed datasets dominate |
| Interceptive and preventive orthodontics | Emerging (growth prediction, treatment timelines) | No SHCN-focused validation; entirely general pediatric populations |
| Chatbots and teledentistry | Developing (pilot studies, scoping reviews) | Some early SHCN-tailored applications improved triage and follow-up, but limited overall |
| Decision support and predictive analytics | Emerging (ML-based risk prediction, systematic review) | Sparse |
| Pain assessment and discomfort monitoring | Emerging (diagnostic accuracy, multimodal feasibility) | Directly relevant to nonverbal/neurodivergent children, but validation remains limited |
| Behavior management | Emerging (small RCTs and intervention trials) | Trials in ASD and mild intellectual disability show reduced anxiety and better cooperation, but replication sparse |
| Behavior modeling | Early-stage (conceptual or prototype systems) | Entirely SHCN-focused (ASD, ADHD, profound disabilities), but unvalidated |
| Ethical and environmental issues | Conceptual (reviews, methodological perspectives) | No empirical SHCN-focused studies; implications inferred |
| Study | Study Type | Overview and Principal Findings |
|---|---|---|
| Diagnostic Imaging and Caries Detection | ||
| Malik et al., 2025 [21] | Cross-sectional study | Compared AI-based and conventional radiographic methods for caries detection in pediatric patients; AI achieved higher diagnostic accuracy, improving early lesion identification. |
| Silva-Filho et al., 2024 [22] | Systematic review and meta-analysis | Evaluated sensitivity of deep learning models for caries detection in bitewing radiographs; pooled results indicated consistently high sensitivity, supporting AI as a reliable adjunct to clinical diagnosis. |
| Bayati et al., 2025 [23] | Experimental study | Applied YOLOv8 deep learning model for interproximal caries detection; achieved rapid processing with high accuracy, outperforming earlier AI architectures. |
| Dhanak et al., 2024 [24] | Technical validation | Tested a smartphone app integrating AI for real-time caries detection on bitewing radiographs; showed strong diagnostic agreement with conventional readings, enabling point-of-care use. |
| Panyarak et al., 2023 [25] | Experimental study | Used YOLOv7 to enhance caries detection in bitewing radiographs; reported improved detection performance and processing efficiency over baseline models. |
| Albano et al., 2024 [26] | Systematic review | Synthesized evidence on AI for radiographic caries detection; concluded that AI achieves high diagnostic accuracy but stressed need for diverse, multi-center datasets. |
| Fux-Noy et al., 2023 [27] | Observational study | Documented high rates of panoramic imaging errors in pediatric patients with special needs, leading to increased repeat exposures; emphasized strategies to minimize repeats. |
| Ameli et al., 2025 [28] | Experimental study | Developed a deep learning system to automatically assess panoramic radiograph quality; successfully identified positioning/coverage issues, offering potential to reduce repeat imaging. |
| Eby et al., 2023 [29] | Observational study | Evaluated AI-driven quality improvement in mammography; demonstrated reduced technical repeat and recall rates, providing indirect evidence for similar benefits in dental radiography. |
| Three-Dimensional Imaging | ||
| Thummerer et al., 2025 [30] | Experimental study | Compared AI-based super-resolution CBCT reconstruction with standard protocols; AI maintained fine anatomical detail while reducing radiation dose by up to 50%, improving safety for pediatric and SHCN patients. |
| Usui et al., 2024 [31] | Experimental study | Compared AI-based sparse projection CBCT reconstruction with conventional methods; AI reduced streak artifacts and preserved diagnostic accuracy, enabling shorter scan times for pediatric and SHCN patients. |
| Wajer et al., 2024 [32] | Experimental study | Compared AI-based artifact reduction with unprocessed CBCT images; AI improved contrast-to-noise ratios and reduced metal artifacts, enhancing image quality in cases with orthodontic appliances or restorations. |
| Ezhov et al., 2021 [33] | Clinical evaluation | Compared AI-assisted CBCT interpretation with unaided clinician review; AI increased sensitivity for pathology detection from 76.7% to 85.4% and slightly improved specificity, benefiting diagnosis of pediatric trauma and infections. |
| Interceptive and Preventive Orthodontics | ||
| Zhang et al., 2023 [34] | Retrospective study | Compared deep learning-based mandibular growth prediction with orthodontist assessment in pediatric anterior crossbite; AI achieved 85% accuracy vs. 54.2% for junior orthodontists, supporting early interceptive care. |
| Yamada et al., 2025 [35] | Prospective clinical study | Evaluated CNN-based prediction of headgear/functional appliance effects on maxillo-mandibular growth in preadolescent Class II malocclusion; AI provided accurate growth forecasts to guide early intervention. |
| Volovic et al., 2023 [36] | Predictive modeling development and validation study | Developed and validated a machine learning model for orthodontic treatment duration; AI outperformed traditional regression models, improving scheduling and patient communication. |
| Moeini & Torabi, 2025 [37] | Narrative review | Summarized AI’s role in orthodontic diagnosis and treatment planning, highlighting applications for pediatric and special health care needs (SHCN) populations. |
| Kılıç et al., 2024 [38] | Cross-sectional study | Compared AI-based mobile orthodontic screening with in-clinic orthodontist assessment; AI accurately identified malocclusion risks in children, enabling early family-initiated referrals. |
| Chatbots and Teledentistry | ||
| Kaushik & Rapaka, 2025 [39] | Scoping review | Outlined AI’s role in teledentistry—enhancing remote diagnosis, treatment planning, and patient engagement, while noting gaps in bias assessment and clinical validation |
| Bayraktar, 2025 [40] | Diagnostic study | Assessed ChatGPT-3.5 (OpenAI, 2022) pediatric dental guidance; found high-quality responses for parent-focused queries (average score 4.3/5) but lower performance on academic questions, highlighting its potential and readability limitations. |
| Nadarzynski et al., 2024 [42] | Conceptual implementation paper | Proposed a framework for inclusive health care chatbots, emphasizing equity in conversational AI design to reduce access disparities, though not specific to dentistry. |
| Paschal et al., 2016 [43] | Cross-sectional study | Documented significant unmet dental needs among children with special health care needs, underscoring the opportunity for AI-facilitated solutions like teledentistry. |
| Alghamdi, 2023 [44] | Cross-sectional study | Found strong caregiver satisfaction (>80%) with virtual pediatric dental clinics during COVID-19, citing convenience and reduced access barriers. |
| Abakl et al., 2022 [45] | Retrospective study | Reported that teledentistry improved follow-up compliance and parental satisfaction during the pandemic in pediatric care, especially for those facing access challenges. |
| Hung et al., 2022 [46] | Scoping review | Explored global teledentistry implementations during COVID-19; AI-driven platforms supported continuity of pediatric dental services in resource-limited environments, though rigorous outcome studies remain scarce. |
| Sakr et al., 2025 [47] | Diagnostic accuracy study | Compared mobile phone photo-based teledentistry with conventional examination for occlusal caries in schoolchildren; found substantial agreement and high sensitivity, supporting its utility in school-based screenings. |
| Decision Support, Patient Engagement, and Predictive Analytics | ||
| Hasan et al., 2025 [48] | Diagnostic study | Developed and tested ML models to predict early childhood caries risk in Bangladeshi children; achieved high predictive accuracy, supporting early identification and prevention strategies. |
| Al-Namankany, 2023 [49] | Systematic review | Synthesized evidence on AI-driven diagnostic tools for ECC; found promising accuracy and potential to enhance treatment decision-making, though further validation is needed. |
| Pupong et al., 2025 [50] | Mixed methods study | Designed and evaluated a chatbot-based oral health care system for young children; reported high usability, acceptability, and positive caregiver engagement. |
| Hasei et al., 2025 [51] | Pilot study | Assessed generative AI chatbot use in pediatric, adolescent, and young adult cancer patients; reduced psychological burden and increased treatment engagement, with potential relevance to pediatric dentistry. |
| Sadegh-Zadeh et al., 2024 [52] | Machine learning study | Applied ML to identify key influencing factors in children’s oral health risk; highlighted the value of data-driven risk assessment for targeted prevention. |
| Lakhotia et al., 2025 [53] | Scoping review | Mapped ML applications across dentistry; identified growth areas, challenges in clinical translation, and gaps in pediatric-specific AI evidence. |
| Badrov et al., 2025 [54] | Cross-sectional study | Explored parental perspectives on oral health in children with autism spectrum disorder; poor oral health linked to lower quality of life, underscoring need for tailored AI-enhanced interventions. |
| Gomez-Rios et al., 2023 [55] | Development study | Introduced ORIENTATE, an AutoML platform for oral health prediction; demonstrated strong predictive performance for research and clinical use. |
| Pain Assessment and Discomfort Monitoring | ||
| De Sario et al., 2023 [56] | Narrative review | Reviewed AI-based facial expression analysis for pediatric pain detection; found convolutional neural networks effective in identifying subtle microexpressions, offering greater objectivity than conventional observation. |
| Cascella et al., 2023 [57] | Methodological review | Examined multimodal AI approaches integrating facial video analysis with physiological monitoring; highlighted potential to standardize pain scoring and reduce observer bias, though protocol harmonization is needed. |
| Santana et al., 2013 [58] | Observational study | Investigated heart rate variability as an indicator of discomfort in pediatric dental contexts; found correlations between autonomic changes and oral pain or anxiety. |
| Gkikas et al., 2024 [59] | Diagnostic accuracy study | Evaluated a transformer-based multimodal system combining facial video and heart rate data; achieved higher accuracy in acute pain detection than single-modality methods. |
| Yue et al., 2024 [60] | Medical hypothesis & planned study | Proposed development of an AI-powered facial expression recognition tool for perioperative pediatric pain assessment; aims to improve short- and long-term postoperative outcomes through automated scoring. |
| Ramdhanie et al., 2025 [61] | Systematic review | Synthesized evidence on technology-assisted facial expression recognition for pediatric pain; reported superior sensitivity, specificity, and inter-rater reliability compared to manual scoring, but noted lack of standardization. |
| Hughes et al., 2023 [62] | Feasibility study | Tested an AI-enabled rapid-assessment tool for infant pain detection; demonstrated ability to identify pain-related facial features within seconds, offering a fast, objective adjunct to caregiver-dependent scoring. |
| Behavior Management | ||
| Kasımoğlu et al., 2023 [63] | Randomized clinical trial | Tested a humanoid robot as a behavior management tool during pediatric dental treatment; significantly reduced dental anxiety, improved cooperation, and lowered salivary amylase compared to conventional methods. |
| Suresh & Shetty, 2024 [64] | Experimental study | Assessed VR distraction in children with ASD during dental procedures; significantly lowered salivary cortisol levels, indicating reduced stress. |
| Al Kheraif et al., 2024 [65] | Clinical intervention study | Used VR distraction in children/adolescents with ASD during dental exams; improved cooperation scores and reduced observed anxiety levels. |
| Mehrotra et al., 2024 [66] | Experimental study | Compared audio distraction and VR in children with mild intellectual disabilities; both methods reduced anxiety, with VR producing greater improvements in cooperation. |
| Behavior Modeling | ||
| Tanabe et al., 2024 [67] | Concept and development study | Proposed an AI-based emotion recognition system for children with profound intellectual and multiple disabilities using physiological and motion signals; aimed to facilitate adaptive behavior management. |
| Barros Padilha et al., 2023 [68] | Systematic review | Synthesized evidence on VR in pediatric dentistry; found consistent anxiety reduction and improved patient cooperation, but emphasized need for standardized protocols and long-term evaluation. |
| Nishat et al., 2023 [69] | Mixed-methods need assessment | Identified design requirements for an AI-enhanced social robot in pediatric emergency settings; highlighted potential for reducing distress and improving communication in children with special needs. |
| Ethical Issue | Concern | Potential Solution |
|---|---|---|
| Data Privacy | Sensitive patient data being processed by AI systems | Implementation of robust data protection protocols |
| Algorithmic Bias | AI models may be biased if trained on non-diverse data | Ensure diverse datasets and regular algorithm audits |
| Autonomy and Decision-Making | AI may influence decision-making, reducing clinician autonomy | AI as a support tool, with clinicians retaining final decision-making power |
| Overtreatment | Unnecessary diagnostics/interventions | Align AI incentives, retain clinician oversight |
| Carbon Cost | Environmental burden from cloud inference | Infrastructure optimization, carbon-aware computing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assiry, A.A.; Alrehaili, R.S.; Mahnashi, A.; Alkam, H.; Mahdi, R.; Hakami, R.; Alshammakhy, R.; Almallahi, W.; Alhawsah, Y.; Khalil, A.S. How Is Artificial Intelligence Transforming the Intersection of Pediatric and Special Care Dentistry? A Scoping Review of Current Applications and Ethical Considerations. Prosthesis 2025, 7, 119. https://doi.org/10.3390/prosthesis7050119
Assiry AA, Alrehaili RS, Mahnashi A, Alkam H, Mahdi R, Hakami R, Alshammakhy R, Almallahi W, Alhawsah Y, Khalil AS. How Is Artificial Intelligence Transforming the Intersection of Pediatric and Special Care Dentistry? A Scoping Review of Current Applications and Ethical Considerations. Prosthesis. 2025; 7(5):119. https://doi.org/10.3390/prosthesis7050119
Chicago/Turabian StyleAssiry, Ali A., Rawan S. Alrehaili, Abdulaziz Mahnashi, Hadia Alkam, Roaa Mahdi, Razan Hakami, Reem Alshammakhy, Walaa Almallahi, Yomna Alhawsah, and Ahmed S. Khalil. 2025. "How Is Artificial Intelligence Transforming the Intersection of Pediatric and Special Care Dentistry? A Scoping Review of Current Applications and Ethical Considerations" Prosthesis 7, no. 5: 119. https://doi.org/10.3390/prosthesis7050119
APA StyleAssiry, A. A., Alrehaili, R. S., Mahnashi, A., Alkam, H., Mahdi, R., Hakami, R., Alshammakhy, R., Almallahi, W., Alhawsah, Y., & Khalil, A. S. (2025). How Is Artificial Intelligence Transforming the Intersection of Pediatric and Special Care Dentistry? A Scoping Review of Current Applications and Ethical Considerations. Prosthesis, 7(5), 119. https://doi.org/10.3390/prosthesis7050119






