New-Onset Left Bundle Branch Block and Other Conduction Disturbances After TAVR: Incidence, Predictors, and Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
3. Comparison of TAVR and SAVR
4. New Conduction Disturbances After TAVR
4.1. Left Bundle Branch Block (LBBB)
4.2. Other Conduction Disturbances After TAVR
4.3. Consequences of Conduction Disturbances After TAVR
5. Pacemaker After TAVR
5.1. Predictors of Implantation PPM
| Category | Predictors |
|---|---|
| Clinical |
|
| Preprocedural ECG |
|
| Echocardiographic/CT |
|
| Valve Characteristics |
|
| Implantation Technique |
|
| Procedural Events |
|
| Postprocedural ECG |
|
5.1.1. Type of Valve Used
5.1.2. Conduction Disturbances Before TAVR
5.1.3. Other ECG Changes
5.1.4. Length of Membranous Septum
5.1.5. Position of the New Valve in Heart
5.1.6. Implantation Technique
5.1.7. Compression Ratio
5.1.8. Infra-Annular Extension
5.1.9. Other Risk Factors
5.2. Stratification of Risks
6. Management of Conduction Disturbances
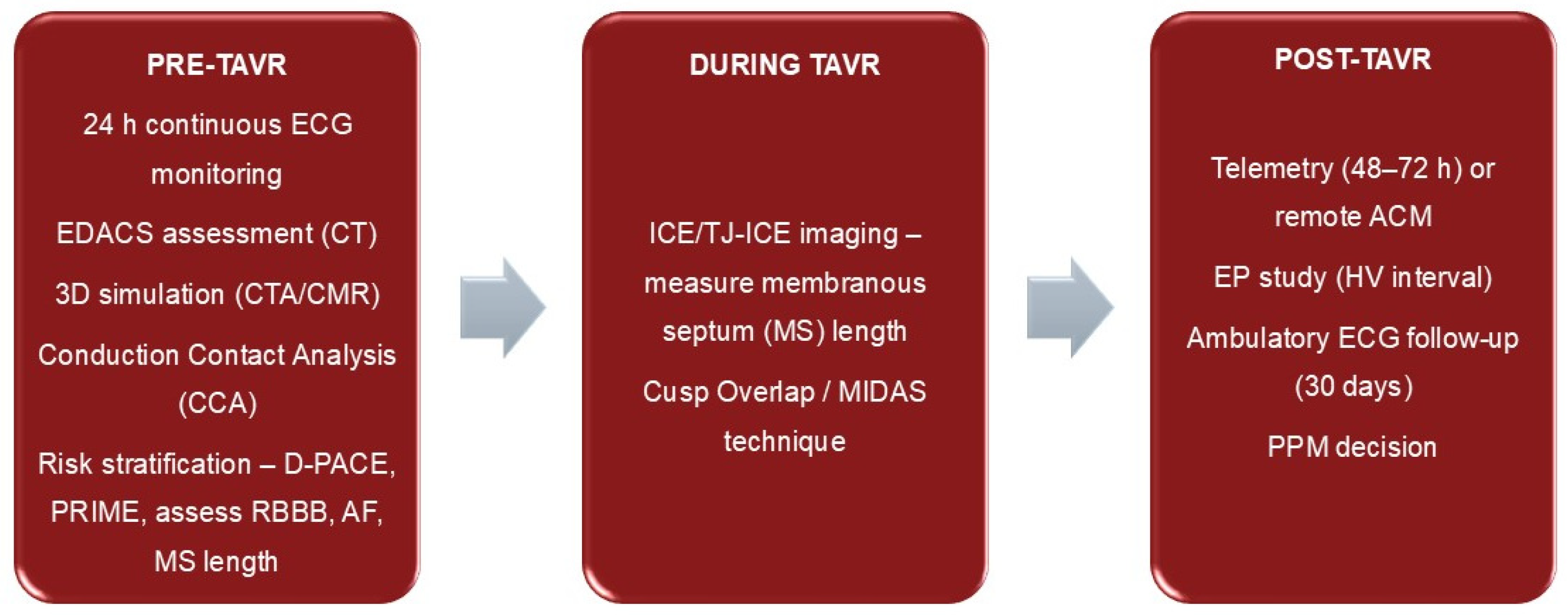
Follow-Up and Management Strategies After TAVR
7. Future Prospects and Scope of Research
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of Patients with Aortic Valve Stenosis. Mayo Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, F.S.; Wijtsma, I.; Amrane, H.; Vossenberg, T.N.E.; Haenen, J.; Porta, F.; Van Boven, A.J.; Hofma, S.H. Outcome of Transcatheter Aortic Valve Replacement in Patients over 85 Years of Age versus Patients Aged 85 and Younger. Neth. Heart J. 2022, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Unzue, L.; Antón, B.D.; Laderas, A.P.; Polo, L.M.; Vázquez, J.M.C.; Rodrigo, F.J.R.; Jimenez, F.J.P.; Fernández-Friera, L.; Mestre, R.T.; Fernández, E.G. Commissural Alignment in SAPIEN 3 Valves: Impact on Gradient and Mortality at Follow-Up. Catheter. Cardiovasc. Interv. 2025, 105, 1067–1076. [Google Scholar] [CrossRef]
- Ellison, M.B.; Goldstein, S.; Anjum, F.; Grose, B.W. Intraoperative Echocardiography. In StatPearls; National Library of Medicine: Bethesda, MD, USA, 2025. [Google Scholar]
- Leclercq, F.; Meunier, P.A.; Gandet, T.; Macia, J.-C.; Delseny, D.; Gaudard, P.; Mourad, M.; Schmutz, L.; Robert, P.; Roubille, F.; et al. Simplified TAVR Procedure: How Far Is It Possible to Go? J. Clin. Med. 2022, 11, 2793. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.E.; Hussein, M.A.; Naeim, H.; Abuelatta, R.; Alghamdy, S. A Comparative Study of TAVR versus SAVR in Moderate and High-Risk Surgical Patients: Hospital Outcome and Midterm Results. Heart Surg. Forum 2019, 22, E331–E339. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Lodo, V.; Italiano, E.G.; Zingarelli, E.; Pietropaolo, C.; Pidello, S.; Buono, G.; Centofanti, P. Transcatheter Aortic Valve Implantation versus Surgery: 4-Year Survival According to Life Expectancy. J. Geriatr. Cardiol. 2024, 21, 846–854. [Google Scholar] [CrossRef]
- Hahn, R.T.; Ternacle, J.; Silva, I.; Giuliani, C.; Zanuttini, A.; Théron, A.; Cristell, N.; Bernier, M.; Skaf, S.; Beaudoin, J.; et al. 5-Year Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. JACC Cardiovasc. Imaging 2025, 18, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. Self-Expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Crouch, J.; Merhi, W.; Wai Sang, S.L.; et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2025, 85, 1523–1532. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or Surgical Aortic Valve Implantation: 10-Year Outcomes of the NOTION Trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef]
- Poels, T.T.; Houthuizen, P.; Van Garsse, L.A.F.M.; Soliman Hamad, M.A.; Maessen, J.G.; Prinzen, F.W.; Van Straten, A.H.M. Frequency and Prognosis of New Bundle Branch Block Induced by Surgical Aortic Valve Replacement. Eur. J. Cardiothorac. Surg. 2015, 47, 47–53. [Google Scholar] [CrossRef]
- Dokollari, A.; Torregrossa, G.; Bisleri, G.; Hassanabad, A.F.; Sa, M.P.; Sicouri, S.; Veshti, A.; Prifti, E.; Bacchi, B.; Cabrucci, F.; et al. Early and Long-Term Clinical and Echocardiographic Outcomes of Sutureless vs. Sutured Bioprosthesis for Aortic Valve Replacement. J. Cardiovasc. Dev. Dis. 2023, 10, 224. [Google Scholar] [CrossRef]
- Regeer, M.V.; Merkestein, L.R.; de Weger, A.; Kamperidis, V.; van der Kley, F.; van Rosendael, P.J.; Marsan, N.A.; Klautz, R.J.M.; Schalij, M.J.; Bax, J.J.; et al. Left Bundle Branch Block after Sutureless, Transcatheter, and Stented Biological Aortic Valve Replacement for Aortic Stenosis. EuroIntervention 2017, 12, 1660–1666. [Google Scholar] [CrossRef]
- Vilalta, V.; Cediel, G.; Mohammadi, S.; López, H.; Kalavrouziotis, D.; Resta, H.; Dumont, E.; Voisine, P.; Philippon, F.; Escabia, C.; et al. Incidence, Predictors and Prognostic Value of Permanent Pacemaker Implantation Following Sutureless Valve Implantation in Low-Risk Aortic Stenosis Patients. Eur. J. Cardiothorac. Surg. 2022, 62, ezac307. [Google Scholar] [CrossRef]
- Vilalta, V.; Cediel, G.; Mohammadi, S.; López, H.; Kalavrouziotis, D.; Resta, H.; Dumont, E.; Voisine, P.; Philippon, F.; Escabia, C.; et al. New-Onset Persistent Left Bundle Branch Block Following Sutureless Aortic Valve Replacement. Heart 2022, 109, 143–150. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Tabata, M. Modified Annular Suturing Technique for Minimizing Postoperative Pacemaker Use after Surgical Aortic Valve Replacement. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 254–259. [Google Scholar] [CrossRef]
- Kunioka, S.; Fujita, K.; Iwasa, S.; Kamiya, H.; Yamazaki, K.; Tsukui, H. Non-Pledget Commissural Suture Technique to Avoid Atrioventricular Block. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Yagel, O.; Belhassen, B.; Planer, D.; Amir, O.; Elbaz-Greener, G. The QRS Frontal Plane Axis Changes during Left Bundle Branch Block after Transcatheter Aortic Valve Replacement. Pacing. Clin. Electrophysiol. 2023, 46, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodés-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Mas-Peiro, S.; Lhermusier, T.; Urena, M.; Nombela-Franco, L.; Vilalta, V.; Munoz-Garcia, A.; Amat-Santos, I.; Atienza, F.; Kleiman, N.; Chamandi, C.; et al. Late Arrhythmic Burden in Patients with Left Bundle Branch Block After TAVR with the Evolut Valve. Europace 2025, 27, euaf057. [Google Scholar] [CrossRef] [PubMed]
- Muntané-Carol, G.; Nombela-Franco, L.; Serra, V.; Urena, M.; Amat-Santos, I.; Vilalta, V.; Chamandi, C.; Lhermusier, T.; Veiga-Fernandez, G.; Kleiman, N.; et al. Late Arrhythmias in Patients with New-Onset Persistent Left Bundle Branch Block after Transcatheter Aortic Valve Replacement Using a Balloon-Expandable Valve. Heart Rhythm 2021, 18, 1733–1740. [Google Scholar] [CrossRef]
- Kawamura, I.; Batul, S.A.; Vijayaraman, P.; Needelman, B.; Choy, A.; Martinez, J.; Tung, R.; Khera, S.; Kini, A.; Sharma, S.; et al. ECG Characteristics of “True” Left Bundle Branch Block: Insights from Transcatheter Aortic Valve-Related LBBB and His-Purkinje Conduction System Pacing-Correctable LBBB. Heart Rhythm 2023, 20, 1659–1666. [Google Scholar] [CrossRef]
- Cecchini, E.; Massaro, G.; Carbone, V.; Sangiorgi, G. Intermittent Normal Ventricular Conduction in Left Bundle Branch Block After TAVR. JACC Case Rep. 2023, 15, 101865. [Google Scholar] [CrossRef]
- Alqarawi, W.; Sadek, M.M.; Golian, M.; Hibbert, B.; Redpath, C.J.; Nair, G.M.; Nery, P.B.; Davis, D.R.; Klein, A.; Birnie, D.H.; et al. A New Electrocardiographic Definition of Left Bundle Branch Block (LBBB) in Patients after Transcatheter Aortic Valve Replacement (TAVR). J. Electrocardiol. 2020, 63, 167–172. [Google Scholar] [CrossRef]
- Ko, T.-Y.; Kao, H.-L.; Liu, Y.-J.; Yeh, C.-F.; Huang, C.-C.; Chen, Y.-H.; Hung, C.S.; Chan, C.-Y.; Lin, L.C.; Chen, Y.-S.; et al. Impact of Conduction Disturbances on Left Ventricular Mass Regression and Geometry Change Following Transcatheter Aortic Valve Replacement. Sc.i Rep. 2021, 11, 16778. [Google Scholar] [CrossRef]
- Merdler, I.; Case, B.C.; Ben-Dor, I.; Chitturi, K.R.; Fahey, H.; Hayat, F.; Isaac, I.; Satler, L.F.; Rogers, T.; Waksman, R. Impact of Left Bundle Branch Block or Permanent Pacemaker after Transcatheter Aortic Valve Replacement on Mid-Term Left Ventricular Ejection Fraction. Cardiovasc. Revasc. Med. 2024, 73, 8–14. [Google Scholar] [CrossRef]
- Klaeboe, L.G.; Brekke, P.H.; Lie, Ø.H.; Aaberge, L.; Haugaa, K.H.; Edvardsen, T. Classical Mechanical Dyssynchrony Is Rare in Transcatheter Aortic Valve Implantation-Induced Left Bundle Branch Block. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Calle, S.; Coeman, M.; Desmet, K.; De Backer, T.; De Buyzere, M.; De Pooter, J.; Timmermans, F. Septal Flash Is a Prevalent and Early Dyssynchrony Marker in Transcatheter Aortic Valve Replacement-Induced Left Bundle Branch Block. Int. J. Cardiovasc. Imaging. 2020, 36, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.M.; Araujo Silva, B.; Marum, A.A.; Bortolotto, A.L.; Nearing, B.D.; Chen, M.J.; Fostello, S.; Popma, J.J.; Verrier, R.L.; Chang, J.D. Speckle Tracking Strain and ECG Heterogeneity Correlate in Transcatheter Aortic Valve Replacement-Induced Left Bundle Branch Blocks and Right Ventricular Paced Rhythms. Open Heart 2021, 8, e001542. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.R.; Sundh, F.; Simlund, J.; Harrison, J.K.; Jackson, K.P.; Hughes, G.C.; Wagner, G.S.; Risum, N.; Søgaard, P.; Strauss, D.G.; et al. Immediate Mechanical Effects of Acute Left Bundle Branch Block by Speckle Tracked Strain. J. Electrocardiol. 2015, 48, 643–651. [Google Scholar] [CrossRef]
- Klaeboe, L.G.; Brekke, P.H.; Aaberge, L.; Haugaa, K.; Edvardsen, T. Impact of Transcatheter Aortic Valve Implantation on Mechanical Dispersion. Open Heart 2020, 7, e001199. [Google Scholar] [CrossRef]
- Kaya, E.; Andresen, K.; Lie, Ø.H.; Aaberge, L.; Haugaa, K.H.; Edvardsen, T.; Skulstad, H. Left Ventricular Mechanical Dispersion as a Predictor of the Need for Pacemaker Implantation after Transcatheter Aortic Valve Implantation: MeDiPace TAVI Study. Eur. Heart J. Cardiovasc. Imaging. 2024, 25, 539–547. [Google Scholar] [CrossRef]
- Kawashima, T.; Sato, F. Visualizing Anatomical Evidences on Atrioventricular Conduction System for TAVI. Int. J. Cardiol. 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Kharche, S.R.; Bateman, M.G.; Iaizzo, P.A.; Dobrzynski, H. 3D Anatomical Reconstruction of Human Cardiac Conduction System and Simulation of Bundle Branch Block after TAVI Procedure. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 5583–5586. [Google Scholar] [CrossRef]
- Ananwattanasuk, T.; Atreya, A.R.; Teerawongsakul, P.; Ghannam, M.; Lathkar-Pradhan, S.; Latchamsetty, R.; Jame, S.; Patel, H.J.; Grossman, P.M.; Oral, H.; et al. Outcomes in Patients with Electrocardiographic Left Ventricular Dyssynchrony Following Transcatheter Aortic Valve Replacement. Heart Rhythm 2023, 20, 22–28. [Google Scholar] [CrossRef]
- Margulescu, A.D.; Thomas, D.E.; Awadalla, M.; Shah, P.; Khurana, A.; Aldalati, O.; Obaid, D.R.; Chase, A.J.; Smith, D. Prevalence and Progression of LV Dysfunction and Dyssynchrony in Patients with New-Onset LBBB Post TAVR. Cardiovasc. Revasc. Med. 2024, 68, 23–29. [Google Scholar] [CrossRef]
- Kikuchi, S.; Minamimoto, Y.; Matsushita, K.; Cho, T.; Terasaka, K.; Hanajima, Y.; Nakahashi, H.; Gohbara, M.; Kimura, Y.; Yasuda, S.; et al. Impact of New-Onset Right Bundle-Branch Block After Transcatheter Aortic Valve Replacement on Permanent Pacemaker Implantation. J. Am. Heart Assoc. 2024, 13, e032777. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.; Adedinsewo, D.; El Sabbagh, A.; Sayed Ahmed, A.F.; Carolina Morales-Lara, A.; Wieczorek, M.; Madhavan, M.; Mulpuru, S.K.; Deshmukh, A.J.; Asirvatham, S.J.; et al. Incidence and Outcomes of New-Onset Right Bundle Branch Block Following Transcatheter Aortic Valve Replacement. Circ. Arrhythm. Electrophysiol. 2024, 17, e012377. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Ye, H.; Jiang, L. Outcomes of Non-Left Bundle Branch Block Conduction Abnormalities after Transcatheter Aortic Valve Replacement. Pacing. Clin. Electrophysiol. 2021, 44, 2124–2126. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, G.; Gupta, S.; Pravda, N.S.; Pérez-Riera, A.R.; Baranchuk, A. A Case of Post-TAVI: Left Septal Fascicular Block & Right Bundle Branch Block. J. Electrocardiol. 2025, 88, 153827. [Google Scholar] [CrossRef]
- Kulkarni, V.T.; Pothineni, N.V.K.; Kumareswaran, R. Alternating QRS Morphologies and PR Intervals After Transcatheter Aortic Valve Replacement. JACC Case Rep. 2020, 2, 1742–1744. [Google Scholar] [CrossRef]
- Ishiguchi, H.; Okamura, T.; Kobayashi, S.; Yano, M. Alternating Bundle Branch Block with Paroxysmal Atrioventricular Block 22 Months After Valve-in-Valve Transcatheter Aortic Valve Replacement. Circ. J. 2021, 86, 170. [Google Scholar] [CrossRef]
- Bhullar, A.; Sharma, N.; Ma, R.; Bimal, T.; Ansari, U.; Mountantonakis, S. A Case of Bundle Branch Re-Entrant Ventricular Tachycardia 1 Year After Transcatheter Aortic Valve Replacement. J. Innov. Card. Rhythm Manag. 2022, 13, 5126–5130. [Google Scholar] [CrossRef]
- Workman, V.; Forrest, J.K.; Enriquez, A. A Case of Presyncope After Transcatheter Aortic Valve Replacement. Circulation 2021, 143, 857–861. [Google Scholar] [CrossRef]
- Ma, J.-F.; Zhou, Y.; Fu, H.-X. Delayed Bundle Branch Reentry Ventricular Tachycardia Following Transcatheter Aortic Valve Replacement. Heart Rhythm Case Rep. 2024, 10, 879–881. [Google Scholar] [CrossRef]
- Belhassen, B.; Shauer, A.; Biton, Y. Left Bundle-Branch Block Tachycardia After Transcatheter Aortic Valve Replacement. Circulation 2021, 144, 1444–1448. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Urena, M.; Nombela-Franco, L.; Amat-Santos, I.; Kleiman, N.; Munoz-Garcia, A.; Atienza, F.; Serra, V.; Deyell, M.W.; Veiga-Fernandez, G.; et al. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients with New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Replacement: The MARE Study. JACC Cardiovasc. Interv. 2018, 11, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, 382–482. [Google Scholar] [CrossRef]
- Hamandi, M.; Tabachnick, D.; Lanfear, A.T.; Baxter, R.; Shin, K.; Zingler, B.; Mack, M.J.; DiMaio, J.M.; Kindsvater, S. Effect of New and Persistent Left Bundle Branch Block after Transcatheter Aortic Valve Replacement on Long-Term Need for Pacemaker Implantation. Proceedings 2020, 33, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mesnier, J.; Ternacle, J.; Cheema, A.N.; Campelo-Parada, F.; Urena, M.; Veiga-Fernandez, G.; Nombela-Franco, L.; Munoz-Garcia, A.J.; Vilalta, V.; Regueiro, A.; et al. Cardiac Death After Transcatheter Aortic Valve Replacement with Contemporary Devices. JACC Cardiovasc. Interv. 2023, 16, 2277–2290. [Google Scholar] [CrossRef]
- Echivard, M.; Vaxelaire, N.; Pibarot, P.; Lamiral, Z.; Freysz, L.; Popovic, B.; Monzo, L.; Baudry, G.; Phamisith, E.; Maureira, J.-P.; et al. Factors Associated with Heart Failure Events in Patients with New-Onset Persistent Left Bundle Branch Block at Discharge after Transcatheter Aortic Valve Replacement. Heart Rhythm 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Sasaki, K.; Izumo, M.; Kuwata, S.; Ishibashi, Y.; Kamijima, R.; Watanabe, M.; Kaihara, T.; Okuyama, K.; Koga, M.; Nishikawa, H.; et al. Clinical Impact of New-Onset Left Bundle-Branch Block After Transcatheter Aortic Valve Implantation in the Japanese Population—A Single High-Volume Center Experience. Circ. J. 2020, 84, 1012–1019. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; De Marco, F.; De Carlo, M.; Agnifili, M.; Latini, R.A.; Petronio, A.S.; Ettori, F.; Poli, A.; De Servi, S.; et al. Clinical Impact of Persistent Left Bundle-Branch Block after Transcatheter Aortic Valve Implantation with CoreValve Revalving System. Circulation 2013, 127, 1300–1307. [Google Scholar] [CrossRef]
- Nazif, T.M.; Williams, M.R.; Hahn, R.T.; Kapadia, S.; Babaliaros, V.; Rodés-Cabau, J.; Szeto, W.Y.; Jilaihawi, H.; Fearon, W.F.; Dvir, D.; et al. Clinical Implications of New-Onset Left Bundle Branch Block after Transcatheter Aortic Valve Replacement: Analysis of the PARTNER Experience. Eur. Heart J. 2014, 35, 1599–1607. [Google Scholar] [CrossRef]
- Leone, A.; Castiello, D.S.; Angellotti, D.; Mariani, A.; Manzo, R.; Avvedimento, M.; Ilardi, F.; Piccolo, R.; Esposito, G.; Franzone, A. Incidence, Predictors, and Prognostic Impact of Temporary Left Bundle Branch Block after Transcatheter Aortic Valve Replacement. J. Electrocardiol. 2022, 74, 114–115. [Google Scholar] [CrossRef]
- Hodel, C.; Moccetti, F.; Brunner, S.; Sandoz, V.; Loretz, L.; Wolfrum, M.; Toggweiler, S. Long-Term Outcomes After Transcatheter Aortic Valve Replacement Complicated by New-Onset Persistent Left Bundle Branch Block. Catheter. Cardiovasc. Interv. 2025, 105, 1545–1551. [Google Scholar] [CrossRef]
- Chamandi, C.; Barbanti, M.; Munoz-Garcia, A.; Latib, A.; Nombela-Franco, L.; Gutiérrez-Ibanez, E.; Veiga-Fernandez, G.; Cheema, A.N.; Cruz-Gonzalez, I.; Serra, V.; et al. Long-Term Outcomes in Patients with New-Onset Persistent Left Bundle Branch Block Following TAVR. JACC Cardiovasc. Interv. 2019, 12, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.M.; Nazif, T.M.; Hess, P.L.; Biviano, A.; Garan, H.; Douglas, P.S.; Kapadia, S.; Babaliaros, V.; Herrmann, H.C.; Szeto, W.Y.; et al. Chronic Pacing and Adverse Outcomes after Transcatheter Aortic Valve Implantation. Heart 2015, 101, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Pagnesi, M.; Kim, W.-K.; Baggio, S.; Scotti, A.; Barbanti, M.; De Marco, F.; Adamo, M.; Eitan, A.; Estévez-Loureiro, R.; Conradi, L.; et al. Incidence, Predictors, and Prognostic Impact of New Permanent Pacemaker Implantation After TAVR with Self-Expanding Valves. JACC Cardiovasc. Interv. 2023, 16, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Tzou, W.S. A Disruptive Technology: Determining Need for Permanent Pacing After TAVR. Curr. Cardiol. Rep. 2021, 23, 53. [Google Scholar] [CrossRef]
- Kagase, A.; Yamamoto, M.; Shimura, T.; Kodama, A.; Kano, S.; Koyama, Y.; Tada, N.; Takagi, K.; Araki, M.; Yamanaka, F.; et al. Evaluation of the Incidence, Timing, and Potential Recovery Rates of Complete Atrioventricular Block after Transcatheter Aortic Valve Implantation: A Japanese Multicenter Registry Study. Cardiovasc. Interv. Ther. 2021, 36, 246–255. [Google Scholar] [CrossRef]
- Qi, Y.; Lin, X.; Pan, W.; Zhang, X.; Ding, Y.; Chen, S.; Zhang, L.; Zhou, D.; Ge, J. A Prediction Model for Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Eur. J. Med. Res. 2023, 28, 262. [Google Scholar] [CrossRef]
- Bianchini, F.; Bianchini, E.; Romagnoli, E.; Aurigemma, C.; Zito, A.; Busco, M.; Nesta, M.; Bruno, P.; Laezza, D.; Giambusso, N.; et al. Anatomical Annulus Predictors of New Permanent Pacemaker Implantation Risk After Balloon-Expandable Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2024, 224, 26–35. [Google Scholar] [CrossRef]
- Sharma, E.; McCauley, B.; Ghosalkar, D.S.; Atalay, M.; Collins, S.; Parulkar, A.; Sheikh, W.; Ahmed, M.B.; Chu, A. Aortic Valve Calcification as a Predictor of Post-Transcatheter Aortic Valve Replacement Pacemaker Dependence. Cardiol. Res. 2020, 11, 155–167. [Google Scholar] [CrossRef]
- Barbe, T.; Fauvel, C.; Hemery, T.; Le Pessec, G.; Tron, C.; Bouhzam, N.; Bettinger, N.; Burdeau, J.; Makke, J.; Laissac, Q.; et al. Can Aortic Valve Calcium Score Predict a Need for Permanent Pacemaker Implantation after Transcatheter Aortic Valve Implantation? Open Heart 2025, 12, e002934. [Google Scholar] [CrossRef]
- Ishizu, K.; Murakami, N.; Morinaga, T.; Hayashi, M.; Isotani, A.; Arai, Y.; Ohno, N.; Kakumoto, S.; Shirai, S.; Ando, K. Impact of Tapered-Shape Left Ventricular Outflow Tract on Pacemaker Rate after Transcatheter Aortic Valve Replacement. Heart Vessel. 2022, 37, 1055–1065. [Google Scholar] [CrossRef]
- Nwaedozie, S.; Zhang, H.; Najjar Mojarrab, J.; Sharma, P.; Yeung, P.; Umukoro, P.; Soodi, D.; Gabor, R.; Anderson, K.; Garcia-Montilla, R. Novel Predictors of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement. World J. Cardiol. 2023, 15, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Abu Rmilah, A.A.; Al-Zu’bi, H.; Haq, I.-U.; Yagmour, A.H.; Jaber, S.A.; Alkurashi, A.K.; Qaisi, I.; Kowlgi, G.N.; Cha, Y.-M.; Mulpuru, S.; et al. Predicting Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: A Contemporary Meta-Analysis of 981,168 Patients. Heart Rhythm 2022, 3, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Portugal, G.; Patrício, L.; Abreu, A.; Cacela, D.; Oliveira, M.; Ferreira, R. Conventional and Novel Predictors of Permanent Pacemaker after TAVI in Atrial Fibrillation Patients. J. Heart Valve Dis. 2016, 25, 397–402. [Google Scholar] [PubMed]
- Minha, S.; Yarkoni, Y.; Segev, A.; Finkelstein, A.; Danenberg, H.; Fefer, P.; Orvin, K.; Steinvil, A.; Maor, E.; Beinart, R.; et al. Comparison of Permanent Pacemaker Implantation Rate after First and Second Generation of Transcatheter Aortic Valve Implantation-A Retrospective Cohort Study. Catheter. Cardiovasc. Interv. 2021, 98, E990–E999. [Google Scholar] [CrossRef]
- Güzel, T.; Demir, M.; Aktan, A.; Arık, B.; Argun, L.; İldırımlı, K.; Sütcü, M.; Arslan, B.; Özbek, M.; Kılıç, R.; et al. A New Trend to Reduce Adverse Events in Patients Undergoing Transcatheter Aortic Valve Implantation: Cusp Overlap Technique: A Cross Sectional Study. Aging. Clin. Exp. Res. 2023, 35, 375–385. [Google Scholar] [CrossRef]
- Bergmann, M.W.; Krause, J.M.; Schofer, N.; Meincke, F.; Paitazoglou, C.; Heeger, C.-H.; Willems, S.; Hakmi, S.; Tigges, E. ALSTER-TAVR 2024: Clinical Results at One Year Following Optimized Self-Expanding, Transcatheter Aortic Valve Peplacement Employing the Cusp-Overlay Technique. J. Invasive Cardiol. 2024, 36. [Google Scholar] [CrossRef]
- Hein-Rothweiler, R.; Jochheim, D.; Rizas, K.; Egger, A.; Theiss, H.; Bauer, A.; Massberg, S.; Mehilli, J. Aortic Annulus to Left Coronary Distance as a Predictor for Persistent Left Bundle Branch Block after TAVI. Catheter. Cardiovasc. Interv. 2017, 89, E162–E168. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Muntané-Carol, G.; Okoh, A.K.; Chen, C.; Nault, I.; Kassotis, J.; Mohammadi, S.; Coromilas, J.; Lee, L.Y.; Alperi, A.; Philippon, F.; et al. Ambulatory Electrocardiographic Monitoring Following Minimalist Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 2711–2722. [Google Scholar] [CrossRef]
- Egger, F.; Nürnberg, M.; Rohla, M.; Weiss, T.W.; Unger, G.; Smetana, P.; Geppert, A.; Gruber, S.C.; Bambazek, A.; Falkensammer, J.; et al. High-Degree Atrioventricular Block in Patients with Preexisting Bundle Branch Block or Bundle Branch Block Occurring during Transcatheter Aortic Valve Implantation. Heart Rhythm 2014, 11, 2176–2182. [Google Scholar] [CrossRef]
- Pagnoni, M.; Meier, D.; Luca, A.; Fournier, S.; Aminfar, F.; Haddad, C.; Maurizi, N.; Domenichini, G.; Le Bloa, M.; Herrera Siklody, C.; et al. Role of Routine Electrophysiological Study Performed During Transcatheter Aortic Valve Replacement to Predict AV Block. Pacing. Clin. Electrophysiol. 2025, 48, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hokken, T.W.; Muhemin, M.; Okuno, T.; Veulemans, V.; Lopes, B.B.; Beneduce, A.; Vittorio, R.; Ooms, J.F.; Adrichem, R.; Neleman, T.; et al. Impact of Membranous Septum Length on Pacemaker Need with Different Transcatheter Aortic Valve Replacement Systems: The INTERSECT Registry. J. Cardiovasc. Comput. Tomogr. 2022, 16, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aranzulla, T.C. Choose to Fly Higher Rather than Pacing Around. Catheter. Cardiovasc. Interv. 2020, 95, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, S.; Hidalgo, F.; Romero, M.; Mazuelos, F.; Suárez de Lezo, J.; Martín, E.; Lostalo, A.; Luque, A.; González, R.; Fernández, A.; et al. Impact of the Repositionable Evolut R CoreValve System on the Need for a Permanent Pacemaker after Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis. Catheter. Cardiovasc. Interv. 2020, 95, 783–790. [Google Scholar] [CrossRef]
- Iacovelli, F.; Giugliano, G.; Gerardi, D.; Salemme, L.; Cioppa, A.; Pucciarelli, A.; Popusoi, G.; Loizzi, F.; Schettino, G.; Favale, S.; et al. Conduction Delays after Transcatheter Aortic Valve Implantation with Balloon-Expandable Prosthesis and High Implantation Technique. Heart Vessel. 2022, 37, 337–346. [Google Scholar] [CrossRef]
- Grubb, K.J.; Gada, H.; Mittal, S.; Nazif, T.; Rodés-Cabau, J.; Fraser, D.G.W.; Lin, L.; Rovin, J.D.; Khalil, R.; Sultan, I.; et al. Clinical Impact of Standardized TAVR Technique and Care Pathway: Insights from the Optimize PRO Study. JACC Cardiovasc. Interv. 2023, 16, 558–570. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Liu, X.; Wu, W.; Chai, H.; Tao, M.; Kong, D.; Li, Y.; Wang, L. Comparison of Cusp-Overlap Projection and Standard Three-Cusp Coplanar View during Self-Expanding Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 927642. [Google Scholar] [CrossRef]
- Wienemann, H.; Maier, O.; Beyer, M.; Portratz, M.; Tanaka, T.; Mauri, V.; Ernst, A.; Waldschmidt, L.; Kuhn, E.; Bleiziffer, S.; et al. Cusp Overlap versus Standard Three-Cusp Technique for Self-Expanding Evolut Transcatheter Aortic Valves. EuroIntervention 2023, 19, 176–187. [Google Scholar] [CrossRef]
- Mendiz, O.A.; Noč, M.; Fava, C.M.; Gutiérrez Jaikel, L.A.; Sztejfman, M.; Pleskovič, A.; Gamboa, P.; Valdivieso, L.R.; Gada, H.; Tang, G.H.L. Impact of Cusp-Overlap View for TAVR with Self-Expandable Valves on 30-Day Conduction Disturbances. J. Interv. Cardiol. 2021, 2021, 9991528. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Tsao, T.-P.; Lee, K.-C.; Lin, H.-C.; Liu, C.-T.; Hsiung, M.-C.; Yin, W.-H.; Wei, J. Predictors of Permanent Pacemaker Requirement in Aortic Stenosis Patients Undergoing Self-Expanding Valve Transcatheter Aortic Valve Replacement Using the Cusp Overlap Technique. Front. Cardiovasc. Med. 2025, 12, 1486375. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, Y.; Pan, W.; Zhang, X.; Lin, X.; Chen, S.; Zhang, L.; Zhou, D.; Ge, J. Mean Compression Ratio of a Self-Expandable Valve Is Associated with the Need for Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Eur. J. Med. Res. 2024, 29, 85. [Google Scholar] [CrossRef] [PubMed]
- Klambauer, K.; Puhr-Westerheide, D.; Fabritius, M.P.; Kunz, W.G.; Dinkel, J.; Schmid-Tannwald, C.; Utz, C.; Grathwohl, F.; Fink, N.; Rizas, K.D.; et al. ECG, Clinical and Novel CT-Imaging Predictors of Necessary Pacemaker Implantation after Transfemoral Aortic Valve Replacement. Eur. J. Radiol. 2025, 182, 111835. [Google Scholar] [CrossRef] [PubMed]
- Bar-Moshe, A.; Abu-Salman, A.; Frumkin, E.; Cafri, C.; Merkin, M.; Bereza, S.; Kezerle, L.; Haim, M.; Konstantino, Y. A Proposed Algorithm for Management of Patients with Left Bundle Branch Block Post-TAVR: 1-Year Follow-Up. Heart Rhythm 2024, 5, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Guthoff, H.; Abdel-Wahab, M.; Kim, W.-K.; Witberg, G.; Wienemann, H.; Thurow, M.; Shamekhi, J.; Eckel, C.; von der Heide, I.; Veulemans, V.; et al. Impact of Measured and Predicted Prosthesis-Patient Mismatch After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2024, 17, 2626–2635. [Google Scholar] [CrossRef]
- Chen, S.; Dizon, J.M.; Hahn, R.T.; Pibarot, P.; George, I.; Zhao, Y.; Blanke, P.; Kapadia, S.; Babaliaros, V.; Szeto, W.Y.; et al. Predictors and 5-Year Clinical Outcomes of Pacemaker After TAVR: Analysis from the PARTNER 2 SAPIEN 3 Registries. JACC Cardiovasc. Interv. 2024, 17, 1325–1336. [Google Scholar] [CrossRef]
- Prajapathi, S.; Pradhan, A. Predictors of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement-the Search Is Still On! World J. Cardiol. 2024, 16, 104–108. [Google Scholar] [CrossRef]
- Park, S.; Kang, D.Y.; Ahn, J.M.; Kim, D.H.; Park, D.W.; Park, S.J.; Kang, J.W.; Yang, D.H.; Lee, S.A.; Koo, H.J. Impact of New-Onset Arrhythmia on Cardiac Reverse Remodeling Following Transcatheter Aortic Valve Replacement: Computed Tomography-Derived Left Ventricular and Atrial Strains. Eur. Radiol. 2023, 33, 8454–8463. [Google Scholar] [CrossRef]
- Loewenstein, I.; Finkelstein, A.; Banai, S.; Halkin, A.; Konigstein, M.; Ben-Shoshan, J.; Arbel, Y.; Barbash, I.; Segev, A.; David, P.; et al. Conduction Disorders Following Transcatheter Aortic Valve Replacement Using Acurate Neo2 Transcatheter Heart Valve: A Propensity Matched Analysis. Cardiovasc. Revasc. Med. 2024, 68, 17–22. [Google Scholar] [CrossRef]
- Roten, L.; Stortecky, S.; Scarcia, F.; Kadner, A.; Tanner, H.; Delacrétaz, E.; Meier, B.; Windecker, S.; Carrel, T.; Wenaweser, P. Atrioventricular Conduction after Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement. J. Cardiovasc. Electrophysiol. 2012, 23, 1115–1122. [Google Scholar] [CrossRef]
- Reiter, C.; Lambert, T.; Kellermair, J.; Blessberger, H.; Fellner, A.; Strasser, B.; Grund, M.; Nahler, A.; Steinwender, C. Intraprocedural Dynamics of Cardiac Conduction during Transcatheter Aortic Valve Implantation: Assessment by Simultaneous Electrophysiological Testing. Heart Rhythm 2021, 18, 419–425. [Google Scholar] [CrossRef]
- Bernardi, F.L.M.; Ribeiro, H.B.; Carvalho, L.A.; Sarmento-Leite, R.; Mangione, J.A.; Lemos, P.A.; Abizaid, A.; Grube, E.; Rodés-Cabau, J.; de Brito, F.S. Direct Transcatheter Heart Valve Implantation Versus Implantation with Balloon Predilatation: Insights from the Brazilian Transcatheter Aortic Valve Replacement Registry. Circ. Cardiovasc. Interv. 2016, 9, e003605. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Waksman, R.; Harrison, J.K.; Deeb, G.M.; Zhang, A.Q.; Hermiller, J.B.; Popma, J.J.; Reardon, M.J. Impact of Balloon Predilatation on Hemodynamics and Outcomes After Transcatheter Aortic Valve Implantation with the Self-Expanding CoreValve Prosthesis. Am. J. Cardiol. 2018, 121, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Laynez, A.; Ben-Dor, I.; Hauville, C.; Xue, Z.; Satler, L.F.; Kent, K.M.; Pichard, A.D.; Lindsay, J.; Waksman, R. Frequency of Cardiac Conduction Disturbances after Balloon Aortic Valvuloplasty. Am. J. Cardiol. 2011, 108, 1311–1315. [Google Scholar] [CrossRef]
- Panchal, H.B.; Barry, N.; Bhatheja, S.; Albalbissi, K.; Mukherjee, D.; Paul, T. Mortality and Major Adverse Cardiovascular Events after Transcatheter Aortic Valve Replacement Using Edwards Valve versus CoreValve: A Meta-Analysis. Cardiovasc. Revasc. Med. 2016, 17, 24–33. [Google Scholar] [CrossRef]
- Tamargo, M.; Gutiérrez-Ibañes, E. Left Bundle Branch Block in Aortic Stenosis: Implications Beyond Pacemaker Implantation. JACC Asia 2024, 4, 320–322. [Google Scholar] [CrossRef]
- Faroux, L.; Muntané-Carol, G.; Urena, M.; Nombela-Franco, L.; Amat-Santos, I.; Kleiman, N.; Munoz-Garcia, A.; Atienza, F.; Serra, V.; Deyell, M.W.; et al. Late Electrocardiographic Changes in Patients with New-Onset Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 795–802. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, Z.; Li, B.; Zhang, H.; Cai, C.; Tao, Y.; Qiao, F.; Lu, F.; Han, L.; Xu, Z. Wider Means Worsen? Influence of QRS Duration of Left Bundle Branch Block on Prognosis of Patients after Transcatheter Aortic Valve Replacement. Medicine 2025, 104, e41940. [Google Scholar] [CrossRef]
- Boonyakiatwattana, W.; Maneesai, A.; Chaithiraphan, V.; Jakrapanichakul, D.; Sakiyalak, P.; Chunhamaneewat, N.; Slisatkorn, W.; Chotinaiwattarakul, C.; Pongakasira, R.; Wongpraparut, N. Preprocedural and Procedural Variables That Predict New-Onset Conduction Disturbances after Transcatheter Aortic Valve Replacement. BMC Cardiovasc. Disord. 2022, 22, 135. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ohno, Y.; Sakai, K.; Murakami, T.; Horinouchi, H.; Hasegawa, M.; Natsumeda, M.; Torii, S.; Okada, K.; Cho, Y.; et al. Novel Strategy for Patients with Pre-Existing Right Bundle Branch Block: Membranous Septum Guided TAVR. JACC Cardiovasc. Interv. 2020, 13, 2184–2185. [Google Scholar] [CrossRef]
- Mailey, J.A.; Brennan, P.F.; Kearney, A.; Hogg, M.C.; McNeice, A.H.; Jeganathan, R.; Manoharan, G.; Owens, C.G.; Spence, M.S. Reframing Optimal Implantation of the Sapien 3 Transcatheter Heart Valve. J. Invasive Cardiol. 2022, 34, 380–389. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Zhao, Z.; Du, R.; Staniloae, C.; Saric, M.; Neuburger, P.J.; Querijero, M.; Vainrib, A.; Hisamoto, K.; Ibrahim, H.; et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Moriyama, N.; Sugiyama, Y.; Jalanko, M.; Dahlbacka, S.; Vähäsilta, T.; Vainikka, T.; Viikilä, J.; Laine, M. Conduction Disturbance After Transcatheter Aortic Valve Implantation with Self-or Balloon-Expandable Valve According to the Implantation Depth. Am. J. Cardiol. 2023, 203, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Layoun, H.; Kassab, J.; Helou, M.C.E.; Dahdah, J.E.; Iskandar, O.; Saidan, M.M.A.M.; Abushouk, A.; Isogai, T.; Reed, G.; Puri, R.; et al. Aortic Root Anatomy and Impact on New-Onset Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. Catheter. Cardiovasc. Interv. 2025, 105, 1375–1380. [Google Scholar] [CrossRef]
- Aljabbary, T.F.; Komatsu, I.; Ochiai, T.; Fremes, S.E.; Ali, N.; Burke, L.; Peterson, M.D.; Fam, N.P.; Wijeysundera, H.C.; Radhakrishnan, S. Cusp Overlap Method for Self-Expanding Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2024, 103, 202–208. [Google Scholar] [CrossRef]
- Panagides, V.; Cheema, A.N.; Urena, M.; Nombela-Franco, L.; Veiga-Fernandez, G.; Vilalta, V.; Regueiro, A.; Del Val, D.; Asmarats, L.; Del Trigo, M.; et al. Optimal Degree of Balloon-Expandable Transcatheter Valve Oversizing in Patients with Borderline Aortic Annulus Measurements: Insights from a Multicenter Real-World Experience. Circ. Cardiovasc. Interv. 2023, 16, e012554. [Google Scholar] [CrossRef]
- Chandrasekar, B.; AlMerri, K.; AlEnezi, A.; AlRashdan, I.; AlKhdair, D.; AlKandari, F. Native Aortic Leaflets and Permanent Pacemaker Implantation Risk Following Balloon-Expandable Transcatheter Aortic Valve Implantation. Indian Heart J. 2023, 75, 268–273. [Google Scholar] [CrossRef]
- Barone, L.; Muscoli, S.; Belli, M.; Di Luozzo, M.; Sergi, D.; Marchei, M.; Prandi, F.R.; Uccello, G.; Romeo, F.; Barillà, F. Effect of Acute CORticosteroids on Conduction Defects after Transcatheter Aortic Valve Implantation: The CORTAVI Study. J. Cardiovasc. Med. 2023, 24, 676–679. [Google Scholar] [CrossRef]
- Chiabrando, J.G.; Lombardi, M.; Seropian, I.M.; Valle Raleigh, J.M.; Vergallo, R.; Larribau, M.; Agatiello, C.R.; Trani, C.; Burzotta, F. Chronic Systemic Glucocorticoid Therapy Is Associated with Increased Risk of Major Vascular Complications and Cardiac Tamponade after Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Minerva Cardiol. Angiol. 2024, 72, 284–291. [Google Scholar] [CrossRef]
- Oestreich, B.; Gurevich, S.; Adabag, S.; Kelly, R.; Helmer, G.; Raveendran, G.; Yannopoulos, D.; Biring, T.; Garcia, S. Exposure to Glucocorticoids Prior to Transcatheter Aortic Valve Replacement Is Associated with Reduced Incidence of High-Degree AV Block and Pacemaker. Cardiovasc. Revasc. Med. 2019, 20, 328–331. [Google Scholar] [CrossRef]
- Bendandi, F.; Taglieri, N.; Ciurlanti, L.; Mazzapicchi, A.; Foroni, M.; Lombardi, L.; Palermo, F.; Filice, F.; Ghetti, G.; Bruno, A.G.; et al. Development and Validation of the D-PACE Scoring System to Predict Delayed High-Grade Conduction Disturbances after Transcatheter Aortic Valve Implantation. EuroIntervention 2025, 21, 119–129. [Google Scholar] [CrossRef]
- Barrett, C.D.; Nickel, A.; Rosenberg, M.A.; Ream, K.; Tzou, W.S.; Aleong, R.; Tumolo, A.; Garg, L.; Zipse, M.; West, J.J.; et al. PRIME Score for Prediction of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2023, 102, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Tsai, C.; Atallah, I.; Ahmad, A.; Bedi, R.; Golemi, L.; Mikhail, S.; Karickal, J.; Azrak, E.; Bishara, S.; et al. Electrophysiological Evaluation Following Development of New and Persistent Left Bundle Branch Block after Transcatheter Aortic Valve Replacement: A Single Center Pilot Study. Pacing Clin. Electrophysiol. 2023, 46, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Schaer, B.; Reichlin, T.; Spies, F.; Madaffari, A.; Vischer, A.; Fahrni, G.; Jeger, R.; Kaiser, C.; Osswald, S.; et al. Electrophysiology Testing to Stratify Patients with Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020, 9, e014446. [Google Scholar] [CrossRef] [PubMed]
- Serban, T.; Knecht, S.; du Fay du Lavallaz, J.; Nestelberger, T.; Kaiser, C.; Leibundgut, G.; Osswald, S.; Schaer, B.; Sticherling, C.; Kühne, M.; et al. Ventricular Pacing Burden in Patients with Left Bundle Branch Block after Transcatheter Aortic Valve Replacement Therapy. J. Cardiovasc. Electrophysiol. 2023, 34, 1464–1468. [Google Scholar] [CrossRef]
- Badertscher, P.; Knecht, S.; Spies, F.; Auberson, C.; Salis, M.; Jeger, R.V.; Fahrni, G.; Kaiser, C.; Schaer, B.; Osswald, S.; et al. Value of Periprocedural Electrophysiology Testing During Transcatheter Aortic Valve Replacement for Risk Stratification of Patients with New-Onset Left Bundle-Branch Block. J. Am. Heart Assoc. 2022, 11, e026239. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Luosang, G.; Wang, J.; Peng, G.; Pu, X.; Jiang, W.; Li, W.; Zhao, Z.; Peng, Y.; et al. Electrocardiogram-Based Prediction of Conduction Disturbances after Transcatheter Aortic Valve Replacement with Convolutional Neural Network. Eur. Heart J. Digit. Health 2024, 5, 219–228. [Google Scholar] [CrossRef]
- Özcan, C.; Raunsø, J.; Lamberts, M.; Køber, L.; Lindhardt, T.B.; Bruun, N.E.; Laursen, M.L.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, M.L. Infective Endocarditis and Risk of Death after Cardiac Implantable Electronic Device Implantation: A Nationwide Cohort Study. Europace 2017, 19, 1007–1014. [Google Scholar] [CrossRef]
- Cacoub, P.; Leprince, P.; Nataf, P.; Hausfater, P.; Dorent, R.; Wechsler, B.; Bors, V.; Pavie, A.; Piette, J.C.; Gandjbakhch, I. Pacemaker Infective Endocarditis. Am. J. Cardiol. 1998, 82, 480–484. [Google Scholar] [CrossRef]
- Urena, M.; Hayek, S.; Cheema, A.N.; Serra, V.; Amat-Santos, I.J.; Nombela-Franco, L.; Ribeiro, H.B.; Allende, R.; Paradis, J.-M.; Dumont, E.; et al. Arrhythmia Burden in Elderly Patients with Severe Aortic Stenosis as Determined by Continuous Electrocardiographic Recording: Toward a Better Understanding of Arrhythmic Events after Transcatheter Aortic Valve Replacement. Circulation 2015, 131, 469–477. [Google Scholar] [CrossRef]
- Poels, T.T.; Stassen, R.; Kats, S.; Veenstra, L.; van Ommen, V.; Kietselaer, B.; Houthuizen, P.; Maessen, J.G.; Prinzen, F.W. Effective Distance between Aortic Valve and Conduction System Is an Independent Predictor of Persistent Left Bundle Branch Block during Transcatheter Aortic Valve Implantation. Medicina 2021, 57, 476. [Google Scholar] [CrossRef]
- Verhemel, S.; Nuis, R.-J.; van den Dorpel, M.; Adrichem, R.; de Sá Marchi, M.F.; Hirsch, A.; Daemen, J.; Budde, R.P.J.; Van Mieghem, N.M. Computed Tomography to Predict Pacemaker Need after Transcatheter Aortic Valve Replacement. J. Cardiovasc. Comput. Tomogr. 2024, 18, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Gooley, R.; McCormick, L.; Rashid, H.N.; Dargan, J.; Khan, F.; Firoozi, S.; Brecker, S.J. Patient-Specific Computer Simulation to Predict Conduction Disturbance with Current-Generation Self-Expanding Transcatheter Heart Valves. Struct. Heart 2022, 6, 100010. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, B.A.; Mbai, M.; Gurevich, S.; Nijjar, P.S.; Adabag, S.; Bertog, S.; Kelly, R.; Garcia, S. Computed Tomography (CT) Assessment of the Membranous Septal Anatomy Prior to Transcatheter Aortic Valve Replacement (TAVR) with the Balloon-Expandable SAPIEN 3 Valve. Cardiovasc. Revasc. Med. 2018, 19, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Feroze, R.; Kang, P.; Dallan, L.A.P.; Akula, N.; Galo, J.; Yoon, S.-H.; Ukaigwe, A.; Filby, S.J.; Baeza, C.; Pelletier, M.; et al. Elevated Myocardial Extracellular Volume Fraction Is Associated with the Development of Conduction Pathway Defects Following Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2024, 104, 1119–1128. [Google Scholar] [CrossRef]
- Murakami, T.; Horinouchi, H.; Noda, S.; Hashimoto, K.; Miyamoto, J.; Kamioka, N.; Nagai, T.; Sakai, K.; Torii, S.; Tanaka, S.; et al. Feasibility and Outcome of Transjugular Intracardiac Echocardiography-Guided Transcatheter Aortic Valve Replacement. JACC Asia 2023, 3, 925–934. [Google Scholar] [CrossRef]
- Reza, S.; Kovarovic, B.; Bluestein, D. Assessing Post-TAVR Cardiac Conduction Abnormalities Risk Using an Electromechanically Coupled Beating Heart. Biomech. Model Mechanobiol. 2025, 24, 29–45. [Google Scholar] [CrossRef]
- Leclercq, F.; Iemmi, A.; Lattuca, B.; Macia, J.-C.; Gervasoni, R.; Roubille, F.; Gandet, T.; Schmutz, L.; Akodad, M.; Agullo, A.; et al. Feasibility and Safety of Transcatheter Aortic Valve Implantation Performed Without Intensive Care Unit Admission. Am. J. Cardiol. 2016, 118, 99–106. [Google Scholar] [CrossRef]
- Waksman, R.; Khan, J.M. Left Bundle Branch Block After TAVR: Bubble or Trouble? JACC Cardiovasc. Interv. 2019, 12, 1185–1187. [Google Scholar] [CrossRef]
- Badertscher, P.; Knecht, S.; Zeljković, I.; Sticherling, C.; de Asmundis, C.; Conte, G.; Barra, S.; Jedrzej, K.; Kühne, M.; Boveda, S. Management of Conduction Disorders after Transcatheter Aortic Valve Implantation: Results of the EHRA Survey. Europace 2022, 24, 1179–1185. [Google Scholar] [CrossRef]
- Natarajan, M.K.; Sheth, T.N.; Wijeysundera, H.C.; Chavarria, J.; Rodes-Cabau, J.; Velianou, J.L.; Radhakrishnan, S.; Newman, T.; Smith, A.; Wong, J.A.; et al. Remote ECG Monitoring to Reduce Complications Following Transcatheter Aortic Valve Implantations: The Redirect TAVI Study. Europace 2022, 24, 1475–1483. [Google Scholar] [CrossRef]
- Akdemir, B.; Roukoz, H. A Single-Centre Cohort and Short-Term Follow-up of Patients Who Developed Persistent New Onset Left Bundle Branch Block after Transcatheter Aortic Valve Replacement. Acta Cardiol. 2020, 75, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, X.; Lu, Z.-N.; Yao, J.; Yin, C.; Wu, W.; Yuan, F.; Luo, T.; Liu, R.; Yan, Y.; et al. Feasibility Study of Temporary Permanent Pacemaker in Patients with Conduction Block after TAVR. Front. Cardiovasc. Med. 2023, 10, 978394. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Jiang, Z.; Liu, X.; Tang, Y.; Bai, M.; Xu, J.; Wang, H.; Chen, Y.; Li, C.; Chen, Y.; et al. Permanent Pacemaker Reduction Using Temporary-Permanent Pacemaker as a 1-Month Bridge after Transcatheter Aortic Valve Replacement: A Prospective, Multicentre, Single-Arm, Observational Study. EClinicalMedicine 2024, 72, 102603. [Google Scholar] [CrossRef] [PubMed]
- Kassier, A.; Velagapudi, P.; Shrestha, N.M.; Schuitema, J.; Gauri, A.; Frost, J.; Merhi, W.; Jovinge, S.; Chalfoun, N. Optimizing Care of Patients with Right Bundle Branch Block Undergoing Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc. Med. 2022, 42, 17–25. [Google Scholar] [CrossRef]
- Hilling-Smith, R.; Smethurst, J.; Cockburn, J.; Williams, T.; Trivedi, U.; Banning, A.; Redwood, S.; de Belder, A.; MacCarthy, P.; Khogali, S.; et al. Pre-Procedural Pacing Bias among Transcatheter Aortic Valves with Higher Post-Procedure Pacing Rates: Evidence from the UK TAVI Registry. Heart Vessel. 2021, 36, 408–413. [Google Scholar] [CrossRef]
- Zorman, M.; Bamford, P.; Coronelli, M.; Barnes, C.; Saunderson, C.; Gamble, J.; Dawkins, S.; Kharbanda, R.K.; Newton, J.; Banning, A.P.; et al. Prophylactic Permanent Pacemaker Implantation for Baseline Right Bundle Branch Block in Patients Undergoing Transcatheter Aortic Valve Replacement: Clinical Efficacy, Safety, and Long-Term Pacing Requirement. Struct. Heart 2024, 8, 100326. [Google Scholar] [CrossRef]
- Fukutomi, M.; Hokken, T.; Wong, I.; Bieliauskas, G.; Daemen, J.; de Jaegere, P.; Van Mieghem, N.; Søndergaard, L.; De Backer, O. Prophylactic Permanent Pacemaker Strategy in Patients with Right Bundle Branch Block Undergoing Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2021, 98, 1017–1025. [Google Scholar] [CrossRef]
- Pavitt, C.; Waleed, M.; Arunothayaraj, S.; Michail, M.; Cockburn, J.; de Belder, A.; Hildick-Smith, D. Transcatheter Aortic Valve Implantation in Patients with Right Bundle-Branch Block: Should Prophylactic Pacing Be Undertaken? J. Invasive Cardiol. 2023, 35, 37–45. [Google Scholar] [CrossRef]
- Ziacchi, M.; Spadotto, A.; Palmisano, P.; Guerra, F.; De Ponti, R.; Zanotto, G.; Bertini, M.; Biffi, M.; Boriani, G. Conduction System Disease Management in Patients Candidate and/or Treated for the Aortic Valve Disease: An Italian Survey Promoted by Italian Association of Arrhythmology and Cardiac Pacing (AIAC). Acta Cardiol. 2024, 79, 367–373. [Google Scholar] [CrossRef]
- Mazzella, A.J.; Sanders, M.; Yang, H.; Li, Q.; Vavalle, J.P.; Gehi, A. Predicting Need for Pacemaker Implantation Early and Late after Transcatheter Aortic Valve Implantation. Catheter. Cardiovasc. Interv. 2021, 97, E588–E596. [Google Scholar] [CrossRef]
- Muntané-Carol, G.; Urena, M.; Nombela-Franco, L.; Amat-Santos, I.; Kleiman, N.; Munoz-Garcia, A.; Atienza, F.; Serra, V.; Deyell, M.W.; Veiga-Fernandez, G.; et al. Arrhythmic Burden in Patients with New-Onset Persistent Left Bundle Branch Block after Transcatheter Aortic Valve Replacement: 2-Year Results of the MARE Study. Europace 2021, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Cano, Ó.; Koruth, J.S.; Subzposh, F.A.; Nanda, S.; Pugliese, J.; Ravi, V.; Naperkowski, A.; Sharma, P.S. His-Purkinje Conduction System Pacing Following Transcatheter Aortic Valve Replacement: Feasibility and Safety. JACC Clin. Electrophysiol. 2020, 6, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.-X.; Liu, X.; Gu, M.; Chen, X.; Cai, C.; Cai, M.; Zhang, S.; Hua, W. Conduction System Pacing for Post Transcatheter Aortic Valve Replacement Patients: Comparison with Right Ventricular Pacing. Front. Cardiovasc. Med. 2021, 8, 772548. [Google Scholar] [CrossRef]
- Dell’Era, G.; Baroni, M.; Frontera, A.; Ghiglieno, C.; Carbonaro, M.; Penela, D.; Romano, C.; Giordano, F.; Del Monaco, G.; Galimberti, P.; et al. Left Bundle Branch Area versus Conventional Pacing after Transcatheter Valve Implant for Aortic Stenosis: The LATVIA Study. J. Cardiovasc. Med. 2024, 25, 450–456. [Google Scholar] [CrossRef]
- Wei, H.-Q.; Li, H.; Liao, H.; Liang, Y.; Zhan, X.; Zhang, Q.; Deng, H.; Wei, W.; Liao, Z.; Liu, Y.; et al. Feasibility and Safety of Permanent Left Bundle Branch Pacing in Patients with Conduction Disorders Following Prosthetic Cardiac Valves. Front. Cardiovasc. Med. 2021, 8, 705124. [Google Scholar] [CrossRef]
- Mechulan, A.; Prevot, S.; Peret, A.; Nait-Saidi, L.; Miliani, I.; Leong-Feng, L.; Leude-Vaillant, E.; Vaillant, A.; Cornen, A.; Latiere, B.; et al. Micra AV Leadless Pacemaker Implantation after Transcatheter Aortic Valve Implantation. Pacing Clin. Electrophysiol. 2022, 45, 1310–1315. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, M.Z.; Ullah, W.; Tanveer Ud Din, M.; Abbas, S.; Ubaid, A.; Khan, M.U.; Rai, D.; Baibhav, B.; Rao, M.; et al. In-Hospital Outcomes of TAVR Patients with a Bundle Branch Block: Insights from the National Inpatient Sample 2011-2018. Catheter. Cardiovasc. Interv. 2022, 100, 424–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, T.-Y.; Yang, X.-M.; Chen, D.-F.; Li, Y.-M.; Bao, Y.; Chen, M. Ambulatory Smartwatch ECG Monitoring among Patients Undergoing Transcatheter Aortic Valve Replacement Early after Discharge: An Observational Study. Rev. Cardiovasc. Med. 2023, 24, 11. [Google Scholar] [CrossRef]
- Fan, J.; Dai, H.; Guo, Y.; Xu, J.; Wang, L.; Jiang, J.; Lin, X.; Li, C.; Zhou, D.; Li, H.; et al. Smartwatch-Detected Arrhythmias in Patients After Transcatheter Aortic Valve Replacement (TAVR): Analysis of the SMART TAVR Trial. J. Med. Internet Res. 2024, 26, e41843. [Google Scholar] [CrossRef]
- Liu, X.; Fan, J.; Guo, Y.; Dai, H.; Xu, J.; Wang, L.; Hu, P.; Lin, X.; Li, C.; Zhou, D.; et al. Wearable Smartwatch Facilitated Remote Health Management for Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2022, 11, e023219. [Google Scholar] [CrossRef]
- Cammalleri, V.; Ussia, G.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Advances in Transcatheter Aortic Valve Therapy in Bicuspid Aortic Valve Disease: Insight into Patient Selection, Management, and Future Directions. Surg. Technol. Int. 2021, 39, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, X.; Li, R.; Ng, S.; Liu, Q.; Wang, L.; Hu, P.; Ren, K.; Jiang, J.; Fan, J.; et al. Comparison of Downsizing Strategy (HANGZHOU Solution) and Standard Annulus Sizing Strategy in Type 0 Bicuspid Aortic Stenosis Patients Undergoing Transcatheter Aortic Valve Replacement: Rationale and Design of a Randomized Clinical Trial. Am. Heart J. 2024, 274, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Süygün, H.; Kasapkara, H.A.; Güney, M.C.; Polat, M.; Bozkurt, E. Incidence and Predictors of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Implantation with a Balloon-Expandable Biosprosthesis in Patients with Bicuspid Aortic Valves. Postep Kardiol. Interwencyjnej 2024, 20, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Shiyovich, A.; Kornowski, R.; Plakht, Y.; Aviv, Y.; Assa, H.V.; Assali, A.; Bental, T.; Lessick, J.; Kerner, A.; Segev, A.; et al. Increased Rate of New-Onset Left Bundle Branch Block in Patients with Bicuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation (From a National Registry). Am. J. Cardiol. 2021, 156, 101–107. [Google Scholar] [CrossRef]
- Hamdan, A.; Nassar, M.; Schwammenthal, E.; Perlman, G.; Arow, Z.; Lessick, J.; Kerner, A.; Barsheshet, A.; Assa, H.V.; Assali, A.; et al. Short Membranous Septum Length in Bicuspid Aortic Valve Stenosis Increases the Risk of Conduction Disturbances. J. Cardiovasc. Comput. Tomogr. 2021, 15, 339–347. [Google Scholar] [CrossRef]
- Barfuss, S.B.; Boucek, D.M.; McFarland, C.A.; Martin, M.H.; LuAnn Minich, L.; Eckhauser, A.W.; Ou, Z.; Gray, R.G.; Tani, L.Y. Short-Term Left Ventricular Reverse Remodeling after Transcatheter Aortic Valve Replacement in Children. J. Am. Soc. Echocardiogr. 2022, 35, 1077–1083. [Google Scholar] [CrossRef]
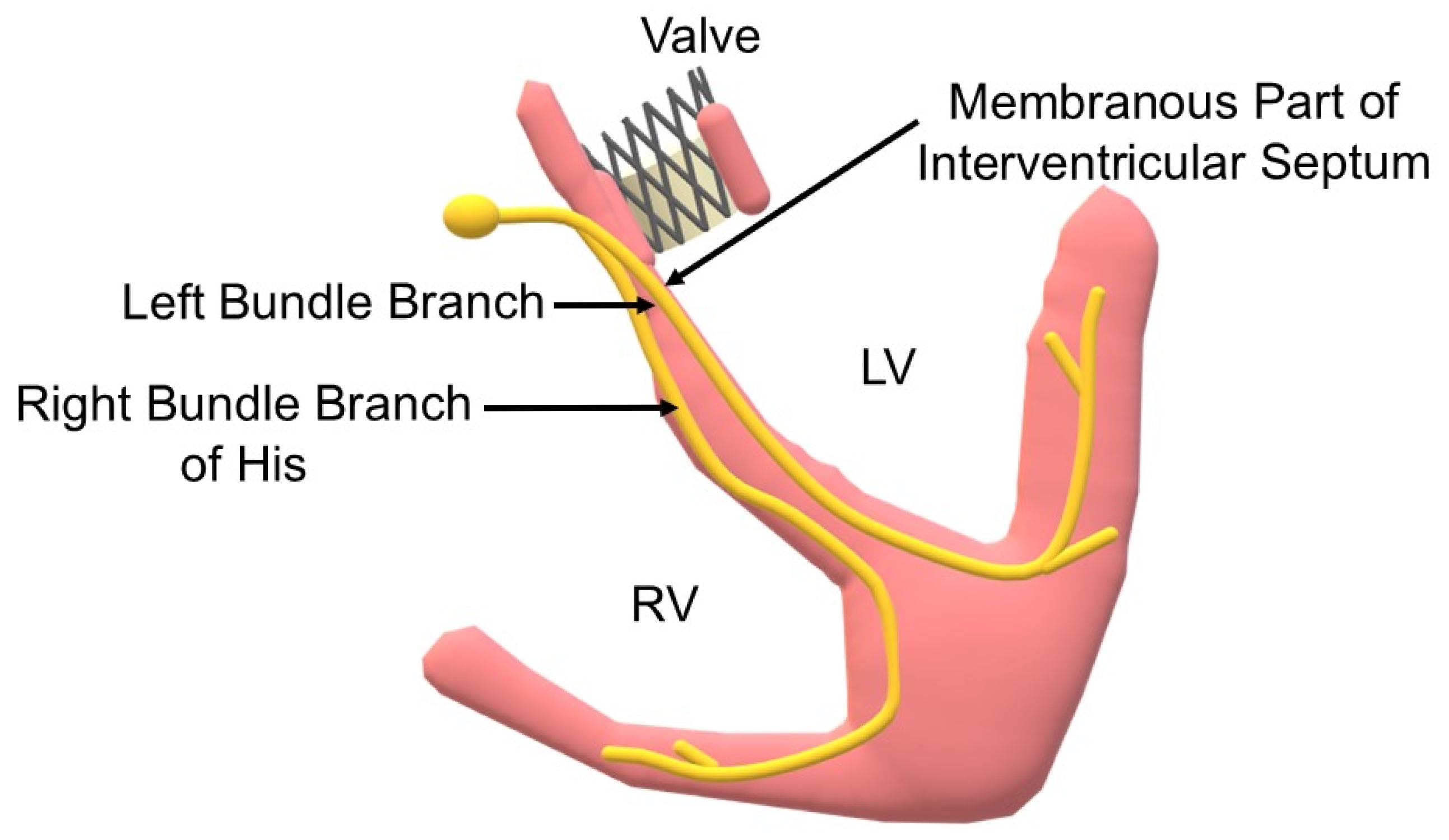
| Risk Factor | OR | Estimated Increase in Risk (%) |
|---|---|---|
| Right bundle branch block (RBBB) | 2.739 | +174% |
| AV block I° | 2.091 | +109% |
| Valve diameter | 1.351 | +35% |
| Atrial fibrillation (AF) | 1.255 | +26% |
| Hypertension (HT) | 1.215 | +21% |
| Coronary artery disease (CAD) | 1.070 | +7% |
| Angle of axis of the ventricle and aortic root—CT | 1.030 | +3% |
| Height of the sinus–cephalic junction—CT | 1.014 | +1.4% |
| Calcification of the left coronary valve—CT | 1.007 | +0.7% |
| Risk Factor | Points Scored | |
|---|---|---|
| Self-expanding valve | Yes | 1 |
| No | 0 | |
| Preprocedural RBBB | Yes | 2 |
| No | 0 | |
| New-onset persistent LBBB | Yes | 3 |
| No | 0 | |
| New-onset persistent RBBB | Yes | 4 |
| No | 0 | |
| Implantation depth | <3.0 mm | 0 |
| 3.0–4.9 mm | 1 | |
| 5.0–6.9 mm | 2 | |
| ≥7.0 mm | 4 | |
| Preprocedural PR duration | <150 ms | 0 |
| 150–199 ms | 1 | |
| 200–249 ms | 2 | |
| ≥250 ms | 3 | |
| Next-day PR interval increase | <1 ms | 0 |
| 1–19 ms | 1 | |
| ≥20 ms | 3 | |
| Acronym | Risk Factor | Points Scored |
|---|---|---|
| P | PR interval > 200 ms | 1 |
| R | Right bundle branch block | 3 |
| I | Valve In Valve procedurę | −3 |
| M | Myocardial infarction | −1 |
| E | Self-Expanding valve | 1 |
| Group Number | Patient Characteristics | Recommendations |
|---|---|---|
| Group 1. | No significant changes in ECG before and after surgery. | Exclusive telemetry monitoring for 24 h after the procedure. Removal of temporary pacemaker (if used during TAVR) immediately after the procedure. |
| Group 2. | RBBB existing before the procedure. | Highest risk group for high atrioventricular block (HAVB) or complete heart block (CHB). Follow-up for a minimum of 48 h after the procedure along with a temporary pacemaker for at least the first 24 h. |
| Group 3. | QRS complex duration >120 ms before the procedure or 1st degree atrioventricular block before the procedure with prolongation of the QRS complex or PR interval by ≥20 ms after the procedure. | Telemetry follow-up for 24–48 h after the procedure. Retention of temporary pacemaker until regression of QRS or PR length. If no improvement, perform invasive electrophysiology study (EPS). |
| Group 4. | Appearance of LBBB after TAVR. | Telemetry follow-up for 24 h after the procedure with the use of a temporary pacemaker. Removal of pacemaker if no progression of LBBB, QRS complexes or intervals of PR. If deterioration, conduct EPS, ambulatory monitoring or implantation of a permanent pacemaker (PPM). |
| Group 5. | Appearance of transient high-grade atrioventricular block or complete heart block during the procedure. | Telemetric monitoring for 24 h after the procedure with the use of a temporary pacemaker. If no improvement, use of permanent pacemaker. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Serafin, I.; Aebisher, D. New-Onset Left Bundle Branch Block and Other Conduction Disturbances After TAVR: Incidence, Predictors, and Clinical Implications. Prosthesis 2025, 7, 71. https://doi.org/10.3390/prosthesis7040071
Bartusik-Aebisher D, Serafin I, Aebisher D. New-Onset Left Bundle Branch Block and Other Conduction Disturbances After TAVR: Incidence, Predictors, and Clinical Implications. Prosthesis. 2025; 7(4):71. https://doi.org/10.3390/prosthesis7040071
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Iga Serafin, and David Aebisher. 2025. "New-Onset Left Bundle Branch Block and Other Conduction Disturbances After TAVR: Incidence, Predictors, and Clinical Implications" Prosthesis 7, no. 4: 71. https://doi.org/10.3390/prosthesis7040071
APA StyleBartusik-Aebisher, D., Serafin, I., & Aebisher, D. (2025). New-Onset Left Bundle Branch Block and Other Conduction Disturbances After TAVR: Incidence, Predictors, and Clinical Implications. Prosthesis, 7(4), 71. https://doi.org/10.3390/prosthesis7040071








