Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
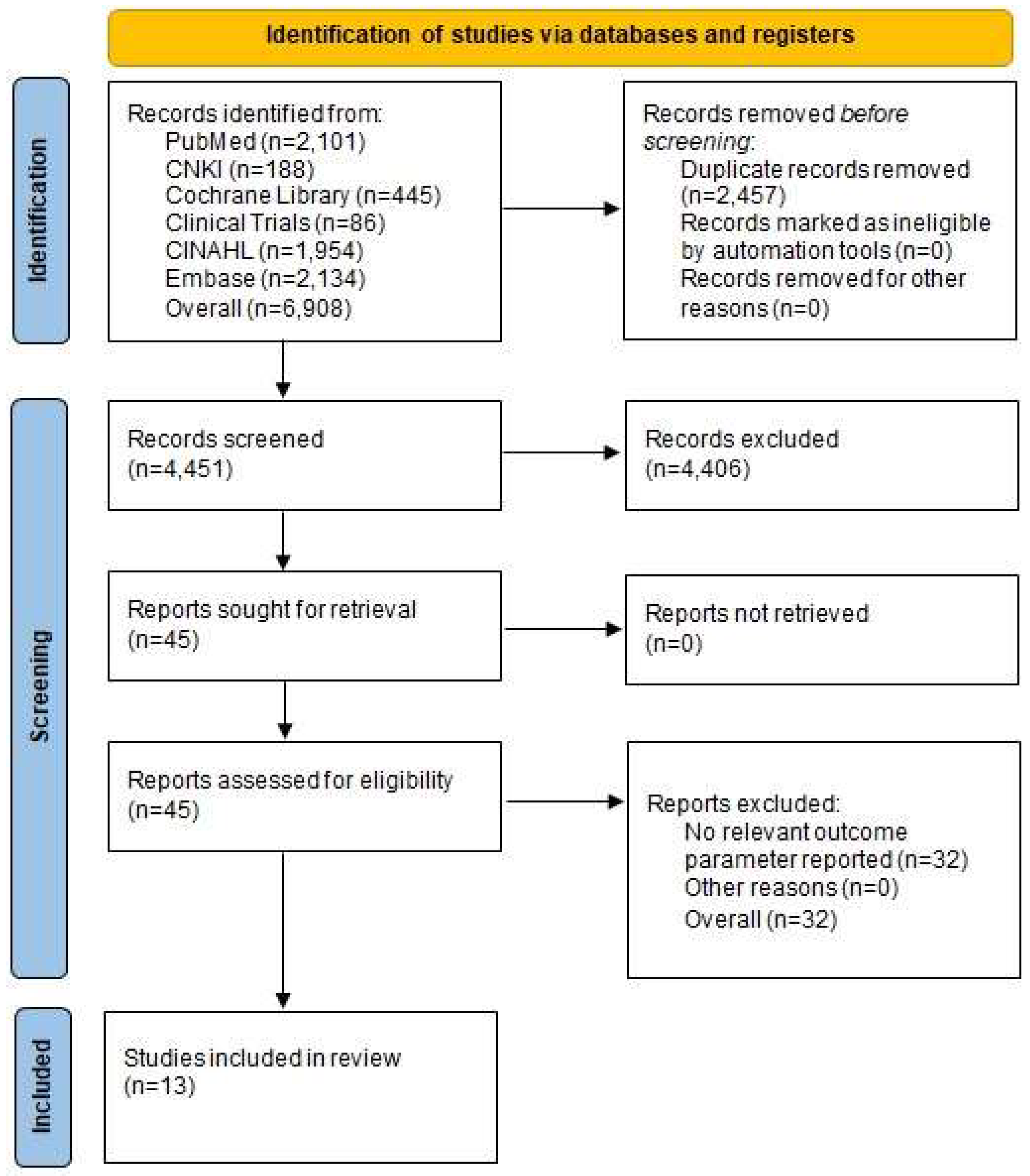
2.2. Study Screening and Selection
2.3. Inclusion Criteria
- randomized controlled trials (RCTs).
- human participants with hip conditions such as osteoarthritis, femoral neck fracture, dysplasia, and ANFH.
- MI THA or CA THA.
- creatine kinase (CK);
- C-reactive protein (CRP);
- hemoglobin (Hb).
2.4. Statistical Analysis
2.4.1. Data Extraction and Quality Assessment
2.4.2. Measures of Treatment Effect
2.4.3. Missing Data
3. Results
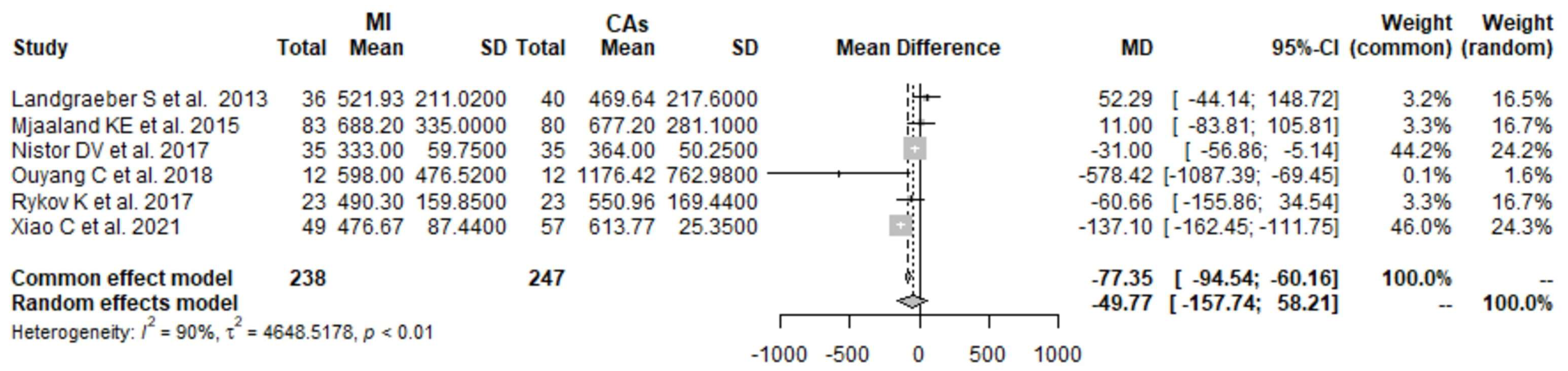
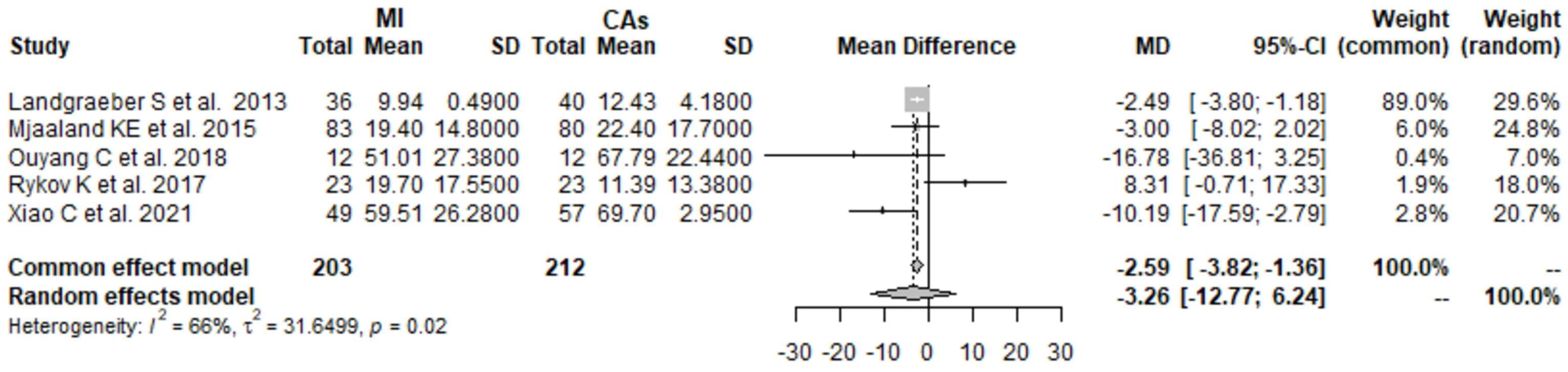
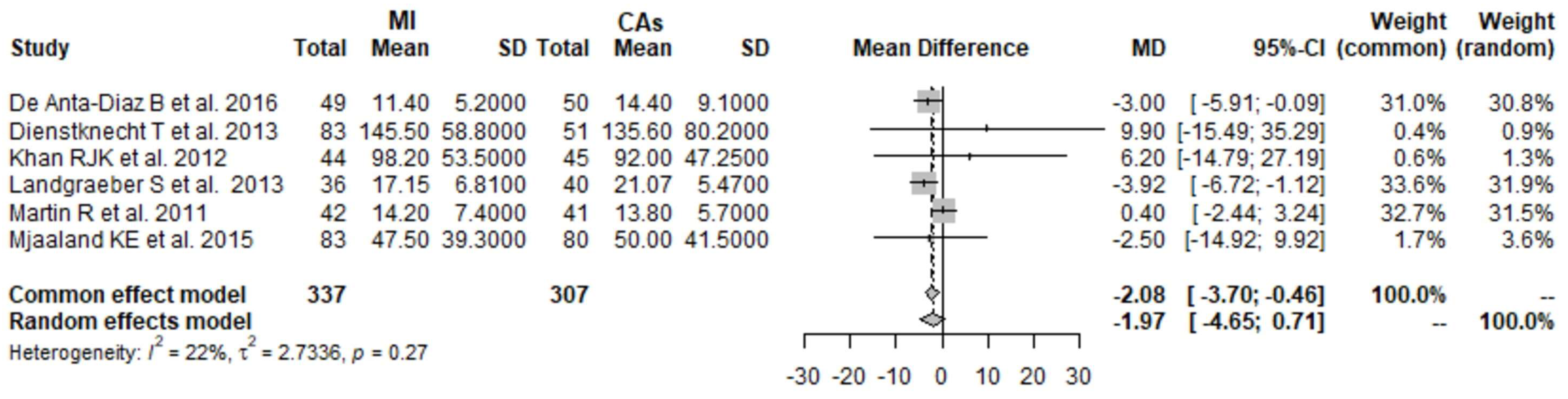
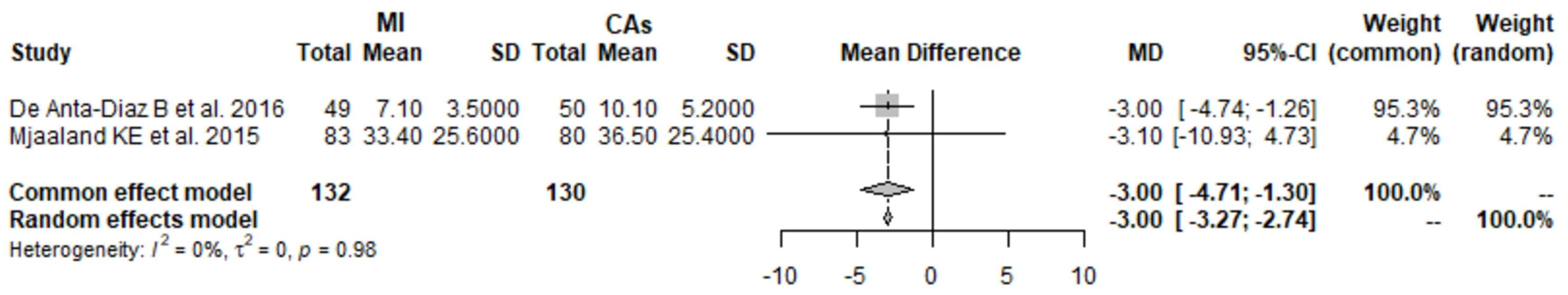
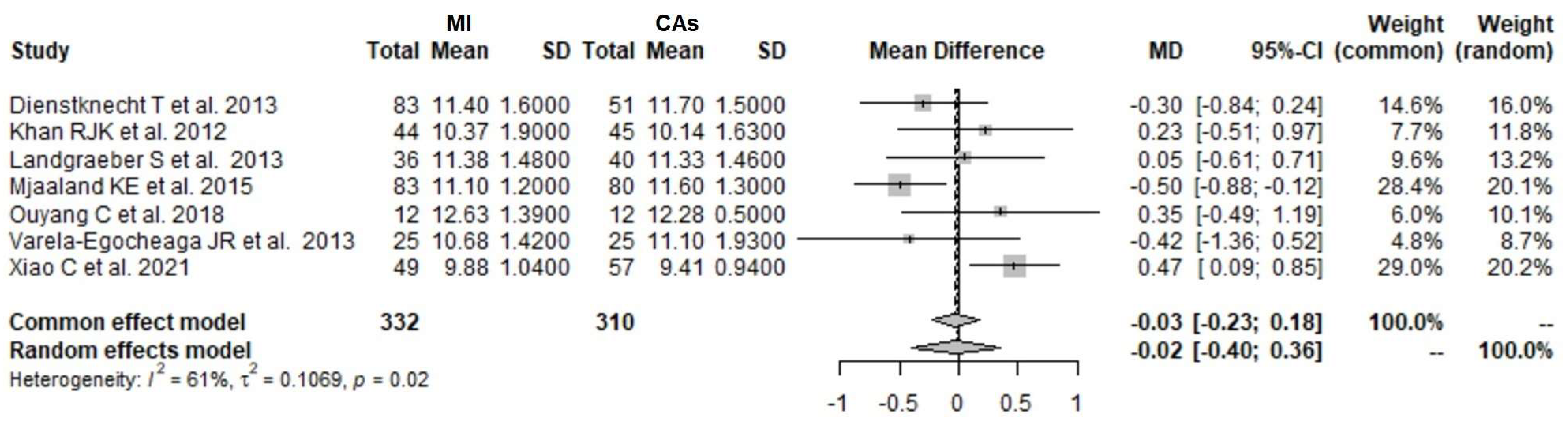
3.1. CK 1 Day Postoperatively: MI THA vs. CA THA
3.2. CK 2 Days Postoperatively: MI THA vs. CA THA
3.3. CK 3 Days Postoperatively: MI THA vs. CA THA
3.4. CK 4 Days Postoperatively: MI THA vs. CA THA
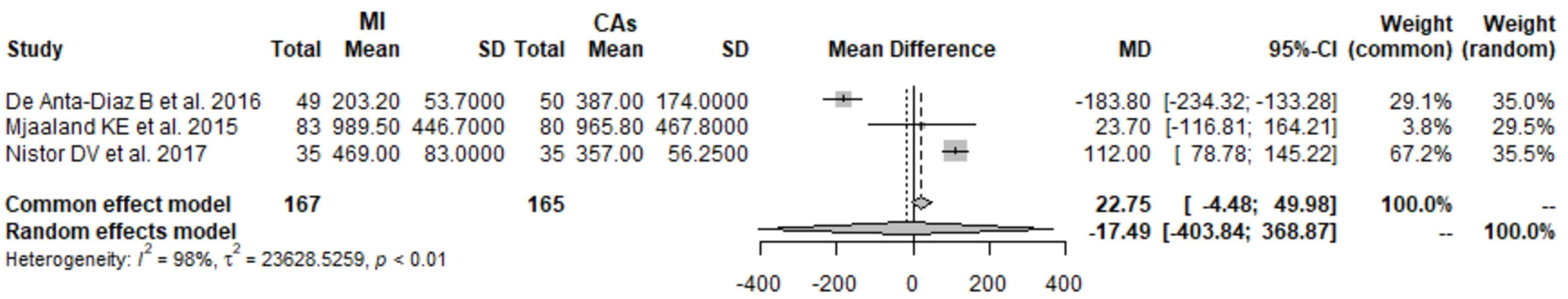
3.5. CRP 1 Day Postoperatively: MI THA vs. CA THA
3.6. CRP 2 Days Postoperatively: MI THA vs. CA THA
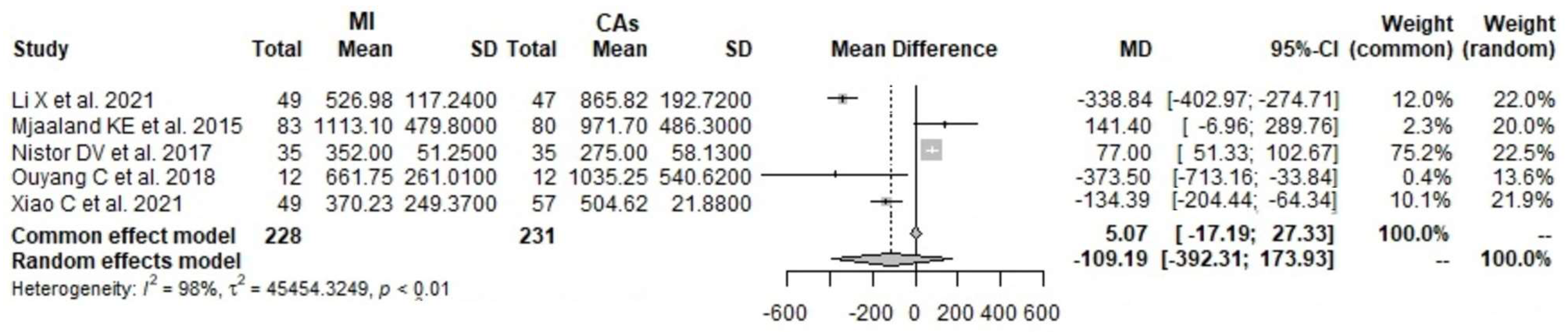
3.7. CRP 3 Days Postoperatively: MI THA vs. CA THA
3.8. CRP 4 Days Postoperatively: MI THA vs. CA THA
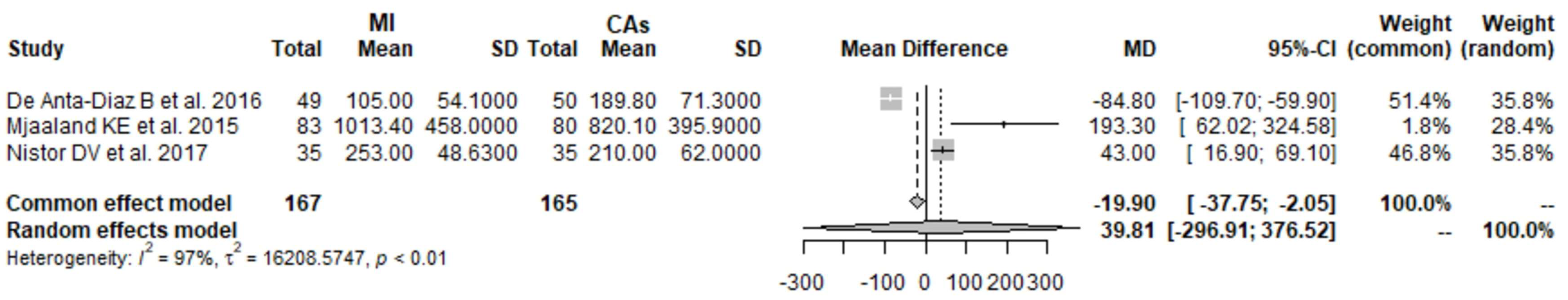
3.9. Hb 1 Day Postoperatively: MI THA vs. CA THA
3.10. Hb 2 Days Postoperatively: MI THA vs. CA THA
3.11. Hb 3 Days Postoperatively: MI THA vs. CA THA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANFH | avascular necrosis of the femoral head |
| BMI | body mass index |
| CA | conventional approach |
| CI | confidence interval |
| CK | creatine kinase |
| CNKI | China National Knowledge Infrastructure |
| CRP | C-reactive protein |
| Hb | hemoglobin |
| HHS | Harris Hip Score |
| ITT | intention to treat |
| MCID | minimal clinically important difference |
| MD | mean difference |
| MI | minimally invasive |
| PP | per protocol analysis |
| RCT | randomized controlled trials |
| SD | standard deviation |
| THA | total hip arthroplasty |
References
- Konarski, W.; Poboży, T.; Śliwczyński, A.; Kotela, I.; Krakowiak, J.; Hordowicz, M.; Kotela, A. Avascular Necrosis of Femoral Head—Overview and Current State of the Art. Int. J. Environ. Res. Public Health 2022, 19, 7348. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Vittori, J.; Grosso, E.; Tarello, M.; Artiaco, S.; Massè, A. Contralateral non-simultaneous proximal femoral fractures in patients over 65 years old. Eur. J. Orthop. Surg. Traumatol. 2021, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Petis, S.; Howard, J.L.; Lanting, B.L.; Vasarhelyi, E.M. Surgical approach in primary total hip arthroplasty: Anatomy, technique and clinical outcomes. Can. J. Surg. 2015, 58, 128–139. [Google Scholar] [CrossRef]
- Shigemura, T.; Murata, Y.; Yamamoto, Y.; Shiratani, Y.; Hamano, H.; Wada, Y. Minimally invasive anterolateral approach versus lateral transmuscular approach for total hip arthroplasty: A systematic review and meta-analysis. Surg. 2021, 20, e254–e261. [Google Scholar] [CrossRef]
- Clesham, K.; Sheridan, G.A.; Greidanus, N.V.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Howard, L.C. Minimally Invasive Intermuscular Approaches Versus Conventional Approaches in Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2022, 37, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Vavken, P.; Kotz, R.; Dorotka, R. Der minimalinvasive Hüftersatz—Eine Metaanalyse [Minimally invasive hip replacement—A meta-analysis]. Z. Orthop. Unfall. 2007, 145, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Biagini, M.; Rath, B.; Meisen, N.; Tingart, M.; Eschweiler, J. Total hip arthroplasty: Minimally invasive surgery or not? Meta-analysis of clinical trials. Int. Orthop. 2018, 43, 1573–1582. [Google Scholar] [CrossRef]
- Smith, T.O.; Blake, V.; Hing, C.B. Minimally invasive versus conventional exposure for total hip arthroplasty: A systematic review and meta-analysis of clinical and radiological outcomes. Int. Orthop. 2010, 35, 173–184. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Neher, M.D.; Weckbach, S.; Flierl, M.A.; Huber-Lang, M.S.; Stahel, P.F. Molecular mechanisms of inflammation and tissue injury after major trauma-is complement the “bad guy”? J. Biomed. Sci. 2011, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Miao, K.; Ni, S.; Zhou, X.; Xu, N.; Sun, R.; Zhuang, C.; Wang, Y. Hidden blood loss and its influential factors after total hip arthroplasty. J. Orthop. Surg. Res. 2015, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, M.G.; Mandrekas, P.I.; Stasi, S.; Makris, K.; Macheras, G.A.; Mavrogenis, A.F.; Babis, G.C.; Nikolaou, V.S. Serum biomarkers for the assessment of muscle damage in various surgical approaches in primary total hip arthroplasty: A systematic review of comparative studies. Int. Orthop. 2022, 46, 1681–1692. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Ramadanov, N.; Bueschges, S.; Liu, K.; Klein, R.; Schultka, R. Comparison of short-term outcomes between SuperPATH approach and conventional approaches in hip replacement: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2020, 15, 420. [Google Scholar] [CrossRef]
- Ramadanov, N. An Updated Meta-Analysis of Randomized Controlled Trials on Total Hip Arthroplasty through SuperPATH versus Conventional Approaches. Orthop. Surg. 2022, 14, 807–823. [Google Scholar] [CrossRef]
- Ramadanov, N.; Bueschges, S.; Liu, K.; Lazaru, P.; Marintschev, I. Comparison of short-term outcomes between direct anterior approach (DAA) and SuperPATH in total hip replacement: A systematic review and network meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 324. [Google Scholar] [CrossRef]
- Ramadanov, N.; Bueschges, S.; Liu, K.; Lazaru, P.; Marintschev, I. Direct anterior approach vs. SuperPATH vs. conventional approaches in total hip replacement: A network meta-analysis of randomized controlled trials. Orthop. Traumatol. Surg. Res. 2021, 107, 103058. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Glob. Spine, J. 2022, 12, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Deeks, J.; Altman, D. Special topics in statistics. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; Cochrane Collaboration: Oxford, UK, 2011; Chapter 16; Available online: https://handbook-5-1.cochrane.org/chapter_16/16_1_3_1imputing_standard_deviations.htm. (accessed on 31 March 2023).
- Barrett, W.P.; Turner, S.E.; Leopold, J.P. Prospective Randomized Study of Direct Anterior vs Postero-Lateral Approach for Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Bon, G.; Ben Kacem, E.; Lepretre, P.M.; Weissland, T.; Mertl, P.; Dehl, M.; Gabrion, A. Does the direct anterior approach allow earlier recovery of walking following total hip arthroplasty? A randomized prospective trial using accelerometry. Orthop. Traumatol. Surg. Res. 2019, 105, 445–452. [Google Scholar] [CrossRef]
- Brismar, B.H.; Hallert, O.; Tedhamre, A.; Lindgren, J.U. Early gain in pain reduction and hip function, but more complications following the direct anterior minimally invasive approach for total hip arthroplasty: A randomized trial of 100 patients with 5 years of follow up. Acta Orthop. 2018, 89, 484–489. [Google Scholar] [CrossRef]
- Cheng, T.E.; Wallis, J.A.; Taylor, N.F.; Holden, C.T.; Marks, P.; Smith, C.L.; Armstrong, M.S.; Singh, P.J. A Prospective Randomized Clinical Trial in Total Hip Arthroplasty—Comparing Early Results Between the Direct Anterior Approach and the Posterior Approach. J. Arthroplast. 2017, 32, 883–890. [Google Scholar] [CrossRef]
- D’arrigo, C.; Speranza, A.; Monaco, E.; Carcangiu, A.; Ferretti, A. Learning curve in tissue sparing total hip replacement: Comparison between different approaches. J. Orthop. Traumatol. 2009, 10, 47–54. [Google Scholar] [CrossRef]
- Fink, B.; Mittelstaedt, A.; Schulz, M.S.; Sebena, P.; Singer, J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty A prospective and comparative study. J. Orthop. Surg. Res. 2010, 5, 46. [Google Scholar] [CrossRef]
- Gao, P.; Shi, X. The effect of total hip replacement with minimally invasive SuperPATH approach in the treatment of femoral neck fractures in the elderly. Henan Med. Res. 2020, 29, 3715–3717. (In Chinese) [Google Scholar]
- Hou, J.Z.; Bao, H.; Cheng, Y. Early effect observation of total hip arthroplasty by using SuperPATH technique. J. Clin. Orthop. 2017, 20, 50–53. (In Chinese) [Google Scholar]
- Huang, K.; Xie, K.; Shi, Y.; Lu, X.; Chen, J.; Lu, L.; Liu, J.; Lu, M.; Pan, S.; Tang, Y. Analysis of early clinical efficacy of SuperPATH approach and lateral approach for initial total hip arthroplasty. Youjiang Med. J. 2021, 49, 646–651. (In Chinese) [Google Scholar] [CrossRef]
- Ling, Z.; Zhou, P.; Fu, Y. Analysis of the effect of total hip replacement via SuperPATH approach on the prognosis of elderly patients with femoral neck fracture. Chin. J. Front. Med. Science. 2020, 12, 66–70. (In Chinese) [Google Scholar]
- Liu, Y.; Hu, P.; Zhu, J.; She, H.; Zhang, Y. Efficacy of minimally invasive total hip arthroplasty in the treatment of elderly femoral neck fractures. Pract. J. Med. Pharm. 2021, 38, 226–231. (In Chinese) [Google Scholar]
- Liu, W.; Liu, X.; Gao, H.; Wang, G.; Li, J. Comparison of the curative effect, pain degree, and hip joint function between SuperPATH hip replacement and total hip replacement. Mod. Chin. Doc. 2022, 60, 78–84. (In Chinese) [Google Scholar]
- Meng, W.; Huang, Z.; Wang, H.; Wang, D.; Luo, Z.; Bai, Y.; Gao, L.; Wang, G.; Zhou, Z. Supercapsular percutaneously-assisted total hip (SuperPath) versus posterolateral total hip arthroplasty in bilateral osteonecrosis of the femoral head: A pilot clinical trial. BMC Musculoskelet. Disord. 2019, 21, 2. [Google Scholar] [CrossRef]
- Mjaaland, K.E.; Kivle, K.; Svenningsen, S.; Nordsletten, L. Do Postoperative Results Differ in a Randomized Trial Between a Direct Anterior and a Direct Lateral Approach in THA? Clin. Orthop. Relat. Res. 2019, 477, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Moerenhout, K.; Derome, P.; Laflamme, G.Y.; Leduc, S.; Gaspard, H.S.; Benoit, B. Direct anterior versus posterior approach for total hip arthroplasty: A multicentre, prospective, randomized clinical trial. Can. J. Surg. 2020, 63, E412–E417. [Google Scholar] [CrossRef]
- Müller, M.; Tohtz, S.; Springer, I.; Dewey, M.; Perka, C. Randomized controlled trial of abductor muscle damage in relation to the surgical approach for primary total hip replacement: Minimally invasive anterolateral versus modified direct lateral approach. Arch. Orthop. Trauma. Surg. 2011, 131, 179–189. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Yan, X.; Chang, X.; Li, J.; Tang, B. Comparison of SuperPATH and posterolateral total hip replacement. Orthop. J. China 2020, 28, 1176–1180. (In Chinese) [Google Scholar]
- Parvizi, J.; Restrepo, C.; Maltenfort, M.G. Total Hip Arthroplasty Performed Through Direct Anterior Approach Provides Su-perior Early Outcome: Results of a Randomized, Prospective Study. Orthop Clin. N. Am. 2016, 47, 497–504. [Google Scholar] [CrossRef]
- Reichert, J.C.; von Rottkay, E.; Roth, F.; Renz, T.; Hausmann, J.; Kranz, J.; Rackwitz, J.; Nöth, U.; Rudert, M. A prospective randomized comparison of the minimally invasive direct anterior and the transgluteal approach for primary total hip arthroplasty. BMC Musculoskelet. Disord. 2018, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yang, G.; Zhao, H.; Zha, J.; Lu, S.; Xu, Y. Effect of SuperPath minimally invasive incision total hip arthroplasty on femoral head necrosis and the quality of life. J. Hebei Med. Univ. 2016, 37, 1416–1419. (In Chinese) [Google Scholar]
- Restrepo, C.; Parvizi, J.; Pour, A.E.; Hozack, W.J. Prospective randomized study of two surgical approaches for total hip ar-throplasty. J. Arthroplast. 2010, 25, 671–679.e1. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, M.; Budde, S.; von Lewinski, G.; Windhagen, H.; Keller, M.C.; Seehaus, F.; Hurschler, C.; Floerkemeier, T. No effect of conventional vs. minimally invasive surgical approach on clinical outcome and migration of a short stem total hip prosthesis at 2-year follow-up: A randomized controlled study. Clin. Biomech. 2018, 51, 105–112. [Google Scholar] [CrossRef]
- Taunton, M.J.; Mason, J.B.; Odum, S.M.; Springer, B.D. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: A prospective, randomized clinical trial. J. Arthroplast. 2014, 29, 169–172. [Google Scholar] [CrossRef]
- Taunton, M.J.; Trousdale, R.T.; Sierra, R.J.; Kaufman, K.; Pagnano, M.W. John Charnley Award: Randomized Clinical Trial of Direct Anterior and Miniposterior Approach THA: Which Provides Better Functional Recovery? Clin. Orthop. Relat. Res. 2018, 476, 216–229. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, W. SuperPATH approach total hip replacement for elderly patients with femoral neck fracture: Impact of hip function. Clin. Med. 2021, 41, 27–29. (In Chinese) [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Wang, L.; Yao, X.; Pan, Z.; Jiang, Q. Comparison of supercapsular percutaneously assisted approach total hip versus conventional posterior approach for total hip arthroplasty: A prospective, randomized controlled trial. J. Orthop. Surg. Res. 2017, 12, 138. [Google Scholar] [CrossRef]
- Yan, T.; Tian, S.; Wang, Y.; Yang, X.; Li, T.; Liu, J.; Pan, P.; Wang, R.; Wang, D.; Sun, K. Comparison of early effectiveness between SuperPATH approach and Hardinge approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017, 31, 17–24. (In Chinese) [Google Scholar]
- Yang, C.; Zhu, Q.; Han, Y.; Zhu, J.; Wang, H.; Cong, R.; Zhang, D. Minimally-invasive total hip arthroplasty will improve early post-operative outcomes: A prospective, randomized, controlled trial. Ir. J. Med. Sci. 2010, 179, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, J.; Sun, Z.; Zhang, Z. Comparison of effectiveness between SuperPATH approach and posterolateral approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 14–19. (In Chinese) [Google Scholar] [PubMed]
- Zhang, Z.; Lin, J.; Xia, B. Clinical research on joint function and life quality through SuperPath approach in total hip arthroplasty. China J. Integr. Trad. Chin. West. Med. 2019, 25, 709–714. (In Chinese) [Google Scholar]
- Zhao, H.Y.; Kang, P.D.; Xia, Y.Y.; Shi, X.J.; Nie, Y.; Pei, F.X. Comparison of Early Functional Recovery After Total Hip Arthroplasty Using a Direct Anterior or Posterolateral Approach: A Randomized Controlled Trial. J. Arthroplast. 2017, 32, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S. Minimally invasive SuperPATH approach for hip replacement in elderly patients. Analysis of clinical efficacy in patients with bone neck fractures. Mod. Diagn. Treat. 2021, 32, 3593. (In Chinese) [Google Scholar]
- De Anta-Díaz, B.; Serralta-Gomis, J.; Lizaur-Utrilla, A.; Benavidez, E.; López-Prats, F.A. No differences between direct anterior and lateral approach for primary total hip arthroplasty related to muscle damage or functional outcome. Int. Orthop. 2016, 40, 2025–2030. [Google Scholar] [CrossRef]
- Dienstknecht, T.; Lüring, C.; Tingart, M.; Grifka, J.; Sendtner, E. A minimally invasive approach for total hip arthroplasty does not diminish early post-operative outcome in obese patients: A prospective, randomised trial. Int. Orthop. 2013, 37, 1013–1018. [Google Scholar] [CrossRef]
- Khan, R.J.; Maor, D.; Hofmann, M.; Haebich, S. A comparison of a less invasive piriformis-sparing approach versus the standard posterior approach to the hip: A randomised controlled trial. J. Bone Joint Surg. Br. 2012, 94, 43–50. [Google Scholar] [CrossRef]
- Landgraeber, S.; Quitmann, H.; Güth, S.; Haversath, M.; Kowalczyk, W.; Kecskeméthy, A.; Heep, H.; Jaäger, M. A prospective randomized peri- and post-operative comparison of the minimally invasive anterolateral approach versus the lateral approach. Orthop. Rev. 2013, 5, e19. [Google Scholar] [CrossRef][Green Version]
- Li, L. SuperPATH minimally invasive total hip replacement surgery treatment. Analysis of clinical efficacy of aseptic necrosis of femoral head. Chin. J. Mod. Drug Appl. 2020, 14, 84–86. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Wang, Q.; Rong, K. Comparison of total hip arthroplasty with minimally invasive SuperPath approach vs. conventional posterolateral approach in elderly patients: A one-year follow-up randomized controlled research. Asian J. Surg. 2021, 44, 531–536. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Clayson, P.E.; Troussel, S.; Fraser, B.P.; Docquier, P.L. Anterolateral minimally invasive total hip arthroplasty: A prospective randomized controlled study with a follow-up of 1 year. J. Arthroplast. 2011, 26, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Mjaaland, K.E.; Kivle, K.; Svenningsen, S.; Pripp, A.H.; Nordsletten, L. Comparison of markers for muscle damage, inflammation, and pain using minimally invasive direct anterior versus direct lateral approach in total hip arthroplasty: A prospective, randomized, controlled trial. J. Orthop. Res. 2015, 33, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Nistor, D.V.; Caterev, S.; Bolboacă, S.D.; Cosma, D.; Lucaciu, D.O.G.; Todor, A. Transitioning to the direct anterior approach in total hip arthroplasty. Is it a true muscle sparing approach when performed by a low volume hip replacement surgeon? Int. Orthop. 2017, 41, 2245–2252. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, H.; Meng, W.; Luo, Z.; Wang, D.; Pei, F.; Zhou, Z. Randomized controlled trial of comparison between the SuperPATH and posterolateral approaches in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1500–1506. (In Chinese) [Google Scholar] [CrossRef]
- Rykov, K.; Reininga, I.H.F.; Sietsma, M.S.; Knobben, B.A.S.; Ten Have, B.L.E.F. Posterolateral vs Direct Anterior Approach in Total Hip Arthroplasty (POLADA Trial): A Randomized Controlled Trial to Assess Differences in Serum Markers. J. Arthroplast. 2017, 32, 3652–3658.e1. [Google Scholar] [CrossRef]
- Varela-Egocheaga, J.R.; Suárez-Suárez, M.A.; Fernández-Villán, M.; González-Sastre, V.; Varela-Gómez, J.R.; Murcia-Mazón, A. Minimally invasive hip surgery: The approach did not make the difference. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 47–52. [Google Scholar] [CrossRef]
- Xiao, C.; Gao, Z.; Zhang, S.; Long, N.; Yao, K.; Cai, P.; He, F.; Liu, L.; Jiang, F. Comparative prospective randomized study of minimally invasive transpiriformis approach versus conventional posterolateral approach in total hip arthroplasty as measured by biology markers. Int. Orthop. 2021, 45, 1707–1717. [Google Scholar] [CrossRef]
| RCT | Year of Publication, Origin | Patients, N | Gender, Male, N | Approach | THA with Bone Cement, N | Patient Position on Table | Mean Age, Years, SD | Mean BMI, kg/m2, SD | HHS Preoperatively, Points | Osteoarthritis, N | Femoral Neck Fracture, N | Dysplasia, N | ANFH, N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Anta-Diaz et al. [58] | 2016, Spain | 49 | 26 | MI DAA | 8 | NR | 64.80 ± 10.10 | 26.60 ± 3.90 | 44.40 ± 13.60 | 49 | 0 | 0 | 0 |
| 50 | 26 | CA L | 6 | NR | 63.50 ± 12.50 | 26.90 ± 3.10 | 42.90 ± 15.20 | 50 | 0 | 0 | 0 | ||
| Dienstknecht et al. [59] * | 2013, Germany | 42 | 14 | MI MH | 2 | Lat | 61.00 ± 13.00 | 26.10 ± 3.00 | 48.00 ± 15.00 | 42 | 0 | 0 | 0 |
| 36 | 12 | CA L | 1 | NR | 62.00 ± 13.00 | 24.30 ± 3.60 | 46.00 ± 16.00 | 36 | 0 | 0 | 0 | ||
| 41 | 24 | MI MH | 3 | Lat | 61.00 ± 11.00 | 34.30 ± 4.40 | 44.00 ± 15.00 | 41 | 0 | 0 | 0 | ||
| 15 | 10 | CA L | 0 | NR | 61.00 ± 10.00 | 34.60 ± 4.10 | 46.00 ± 16.00 | 15 | 0 | 0 | 0 | ||
| Khan et al. [60] | 2012, Australia | 44 | 24 | MI P | 44 | Lat | 72.30 ± 1.00 | 28.50 ± 0.70 | NR | 42 | 0 | 0 | 2 |
| 45 | 19 | CA P | 45 | Lat | 72.80 ± 1.10 | 28.90 ± 0.60 | NR | 43 | 0 | 0 | 2 | ||
| Landgraeber et al. [61] | 2013, Germany | 36 | 12 | MI AL | 36 | Lat | 70.26 ± 4.05 | 27.03 ± 2.82 | NR | 36 | 0 | 0 | 0 |
| 40 | 14 | CA L | 40 | Supine | 71.03 ± 5.38 | 26.75 ± 3.83 | NR | 40 | 0 | 0 | 0 | ||
| Li [62] | 2020, China | 30 | 16 | MI S | NR | Lat | 70.35 ± 4.26 | NR | 25.41 ± 2.41 | NR | NR | NR | NR |
| 30 | 18 | CA PL | NR | Lat | 70.12 ± 4.78 | NR | 26.35 ± 2.47 | NR | NR | NR | NR | ||
| Li et al. [63] | 2021, China | 49 | 27 | MI S | NR | Lat | 75.53 ± 7.34 | 22.99 ± 2.87 | NR | 0 | 15 | 0 | 34 |
| 47 | 24 | CA PL | NR | Lat | 77.21 ± 7.84 | 22.70 ± 3.00 | NR | 0 | 16 | 0 | 31 | ||
| Martin et al. [64] | 2011, Belgium | 42 | 12 | MI AL | 42 | Lat | 66.70 ± 10.10 | 30.60 ± 6.10 | 37.40 ± 15.50 | 37 | 0 | 0 | 5 |
| 41 | 14 | CA L | 41 | NR | 63.10 ± 10.20 | 29.40 ± 5.50 | 40.20 ± 12.90 | 37 | 0 | 0 | 4 | ||
| Mjaaland et al. [65] | 2015, Norway | 83 | 25 | MI DAA | 83 | Supine | 67.20 ± 8.60 | 27.70 ± 3.60 | 53.60 ± 13.70 | 83 | 0 | 0 | 0 |
| 80 | 30 | CA L | 80 | Lat | 65.60 ± 8.60 | 27.60 ± 3.90 | 56.00 ± 11.20 | 80 | 0 | 0 | 0 | ||
| Nistor et al. [66] | 2017, Romania | 35 | 26 | MI DAA | 0 | Supine | 67.00 ± 4.75 | 27.45 ± 3.76 | NR | 35 | 0 | 0 | 0 |
| 35 | 16 | CA L | 0 | Supine | 64.00 ± 3.25 | 28.63 ± 3.12 | NR | 35 | 0 | 0 | 0 | ||
| Ouyang et al. [67] | 2018, China | 12 | 8 | MI S | NR | Lat | 54.00 ± 6.50 | 23.10 ± 2.30 | 45.67 ± 5.93 | 5 | 0 | 0 | 7 |
| 12 | 9 | CA PL | NR | Lat | 55.00 ± 5.00 | 23.90 ± 3.40 | 46.92 ± 8.94 | 6 | 0 | 0 | 6 | ||
| Rykov et al. [68] | 2017, Netherlands | 23 | 8 | MI DAA | 23 | Supine | 62.80 ± 6.10 | 29.00 ± 5.60 | 52.00 ± 6.67 | 23 | 0 | 0 | 0 |
| 23 | 11 | CA PL | 23 | Lat | 60.20 ± 8.10 | 29.30 ± 4.80 | 51.00 ± 8.95 | 23 | 0 | 0 | 0 | ||
| Varela-Egocheaga et al. [69] | 2013, Spain | 25 | 12 | MI L | 0 | NR | 64.80 ± 10.50 | 28.27 ± 3.67 | 52.70 ± 12.92 | 21 | 0 | 0 | 4 |
| 25 | 12 | CA L | 0 | NR | 63.80 ± 9.70 | 27.78 ± 3.24 | 51.30 ± 14.94 | 22 | 0 | 0 | 3 | ||
| Xiao et al. [70] | 2021, China | 49 | 16 | MI P | 0 | Lat | 71.06 ± 10.87 | 26.73 ± 4.18 | NR | 0 | 49 | 0 | 0 |
| 57 | 26 | CA PL | 0 | Lat | 73.93 ± 10.02 | 26.39 ± 4.64 | NR | 0 | 57 | 0 | 0 |
| Study | Bias Arising from the Randomization Process | Bias Due to Deviation from Intended Interventions | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| De Anta-Diaz et al. [58] | - | + | + | + | + | ? |
| Dienstknecht et al. [59] | - | + | + | + | + | ? |
| Khan et al. [60] | + | + | + | + | + | + |
| Landgraeber et al. [61] | + | ? | + | + | ? | ? |
| Li [62] | + | ? | - | - | + | - |
| Li et al. [63] | + | + | - | + | + | ? |
| Martin et al. [64] | ? | ? | + | + | ? | ? |
| Mjaaland et al. [65] | + | + | + | + | + | + |
| Nistor et al. [66] | - | + | + | + | + | ? |
| Ouyang et al. [67] | + | + | + | + | + | + |
| Rykov et al. [68] | + | + | - | + | + | ? |
| Varela-Egocheaga et al. [69] | - | - | + | + | + | - |
| Xiao et al. [70] | ? | + | + | + | + | + |
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Quality of Evidence |
|---|---|---|---|---|---|---|---|
| 1. CK 1 day postoperatively | |||||||
| 6 | RCT | Moderate | No serious inconsistency | No serious indirectness | No serious imprecision | - | Moderate |
| 2. CK 2 days postoperatively | |||||||
| 3 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 3. CK 3 days postoperatively | |||||||
| 5 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 4. CK 4 days postoperatively | |||||||
| 3 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 5. CRP 1 day postoperatively | |||||||
| 5 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 6. CRP 2 days postoperatively | |||||||
| 6 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 7. CRP 3 days postoperatively | |||||||
| 4 | RCT | Moderate | No serious inconsistency | No serious indirectness | No serious imprecision | - | Moderate |
| 8. CRP 4 days postoperatively | |||||||
| 2 | RCT | Moderate | No serious inconsistency | No serious indirectness | No serious imprecision | - | Moderate |
| 9. Hb 1 day postoperatively | |||||||
| 7 | RCT | Moderate | Serious | No serious indirectness | No serious imprecision | - | Low |
| 10. Hb 2 days postoperatively | |||||||
| 5 | RCT | Moderate | No serious inconsistency | No serious indirectness | No serious imprecision | - | Moderate |
| 11. Hb 3 days postoperatively | |||||||
| 3 | RCT | Low | No serious inconsistency | No serious indirectness | No serious imprecision | - | High |
| Number of RCTs | Egger p-Value | Begg p-Value | |
|---|---|---|---|
| 1. CK 1 day postoperatively | 6 | 0.75 | 1.00 |
| 2. CK 2 days postoperatively | 3 | 0.76 | 1.00 |
| 3. CK 3 days postoperatively | 5 | 0.37 | 1.00 |
| 4. CK 4 days postoperatively | 3 | 0.70 | 1.00 |
| 5. CRP 1 day postoperatively | 5 | 0.76 | 0.81 |
| 6. CRP 2 days postoperatively | 6 | 0.44 | 0.26 |
| 7. CRP 3 days postoperatively | 4 | 0.46 | 0.73 |
| 8. CRP 4 days postoperatively | 2 | - | - |
| 9. Hb 1 day postoperatively | 7 | 0.04 * | 0.23 |
| 10. Hb 2 days postoperatively | 5 | 0.26 | 0.22 |
| 11. Hb 3 days postoperatively | 3 | 0.42 | 1.00 |
| Postoperative Serum Biomarkers | MI THA | CA THA |
|---|---|---|
| 1. CK 1 day postoperatively (in U/L) | 543.61 | 597.12 |
| 2. CK 2 days postoperatively (in U/L) | 649.69 | 661.28 |
| 3. CK 3 days postoperatively (in U/L) | 686.92 | 732.59 |
| 4. CK 4 days postoperatively (in U/L) | 587.48 | 499.72 |
| 5. CRP 1 day postoperatively (in mg/L) | 29.33 | 34.61 |
| 6. CRP 2 days postoperatively (in mg/L) | 65.62 | 56.01 |
| 7. CRP 3 days postoperatively (in mg/L) | 60.11 | 74.79 |
| 8. CRP 4 days postoperatively (in mg/L) | 23.63 | 26.32 |
| 9. Hb 1 day postoperatively (in g/dL) | 11.01 | 11.01 |
| 10. Hb 2 days postoperatively (in g/dL) | 10.73 | 10.72 |
| 11. Hb 3 days postoperatively (in g/dL) | 10.34 | 10.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadanov, N.; Marinova-Kichikova, P.; Hable, R.; Dimitrov, D.; Becker, R. Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prosthesis 2023, 5, 694-710. https://doi.org/10.3390/prosthesis5030049
Ramadanov N, Marinova-Kichikova P, Hable R, Dimitrov D, Becker R. Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prosthesis. 2023; 5(3):694-710. https://doi.org/10.3390/prosthesis5030049
Chicago/Turabian StyleRamadanov, Nikolai, Polina Marinova-Kichikova, Robert Hable, Dobromir Dimitrov, and Roland Becker. 2023. "Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Prosthesis 5, no. 3: 694-710. https://doi.org/10.3390/prosthesis5030049
APA StyleRamadanov, N., Marinova-Kichikova, P., Hable, R., Dimitrov, D., & Becker, R. (2023). Comparison of Postoperative Serum Biomarkers after Total Hip Arthroplasty through Minimally Invasive versus Conventional Approaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prosthesis, 5(3), 694-710. https://doi.org/10.3390/prosthesis5030049