Clinical, Radiological, and Aesthetic Outcomes after Placement of a Bioactive-Surfaced Implant with Immediate or Delayed Loading in the Anterior Maxilla: 1-Year Retrospective Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Preoperative Procedure
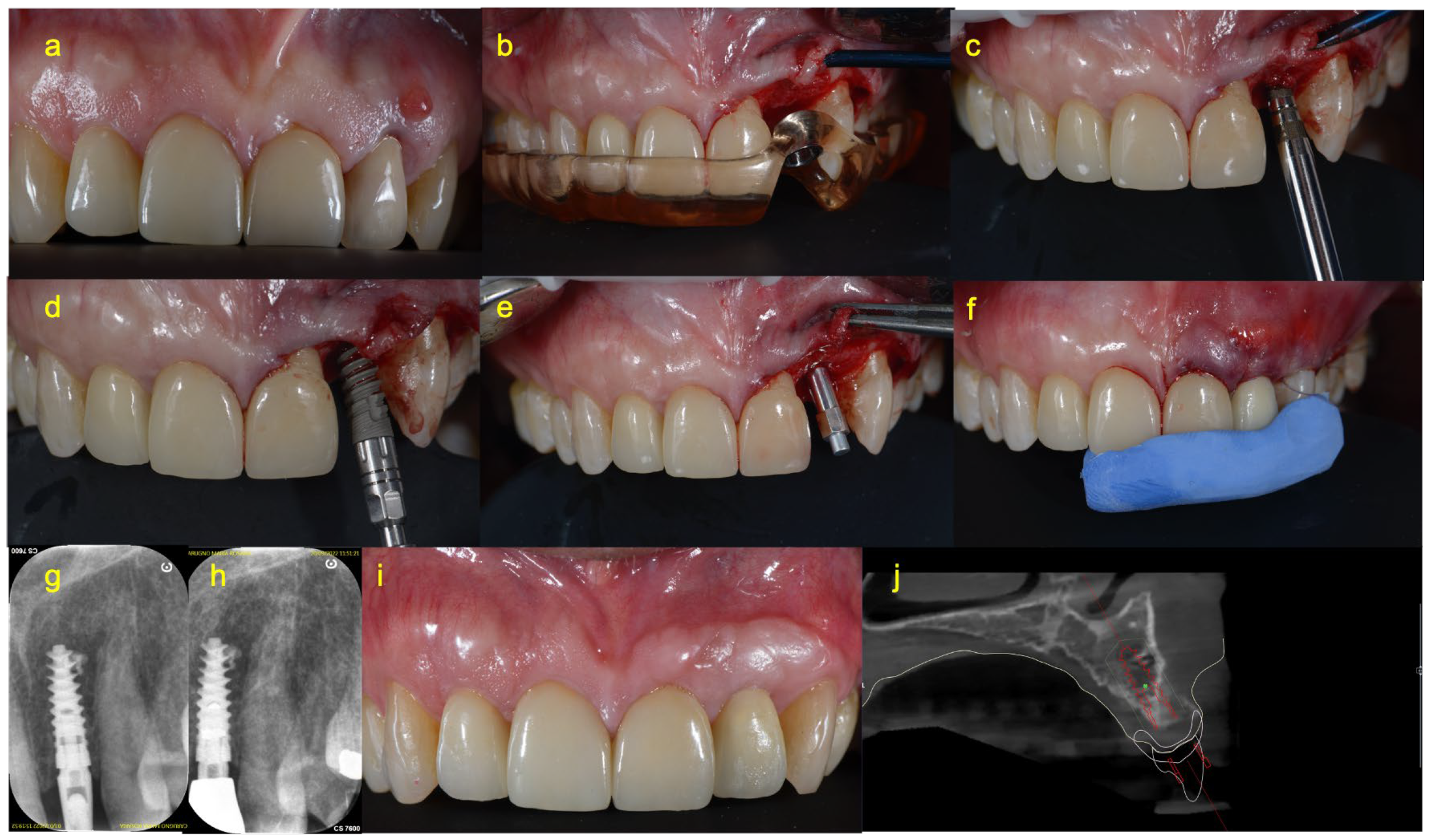
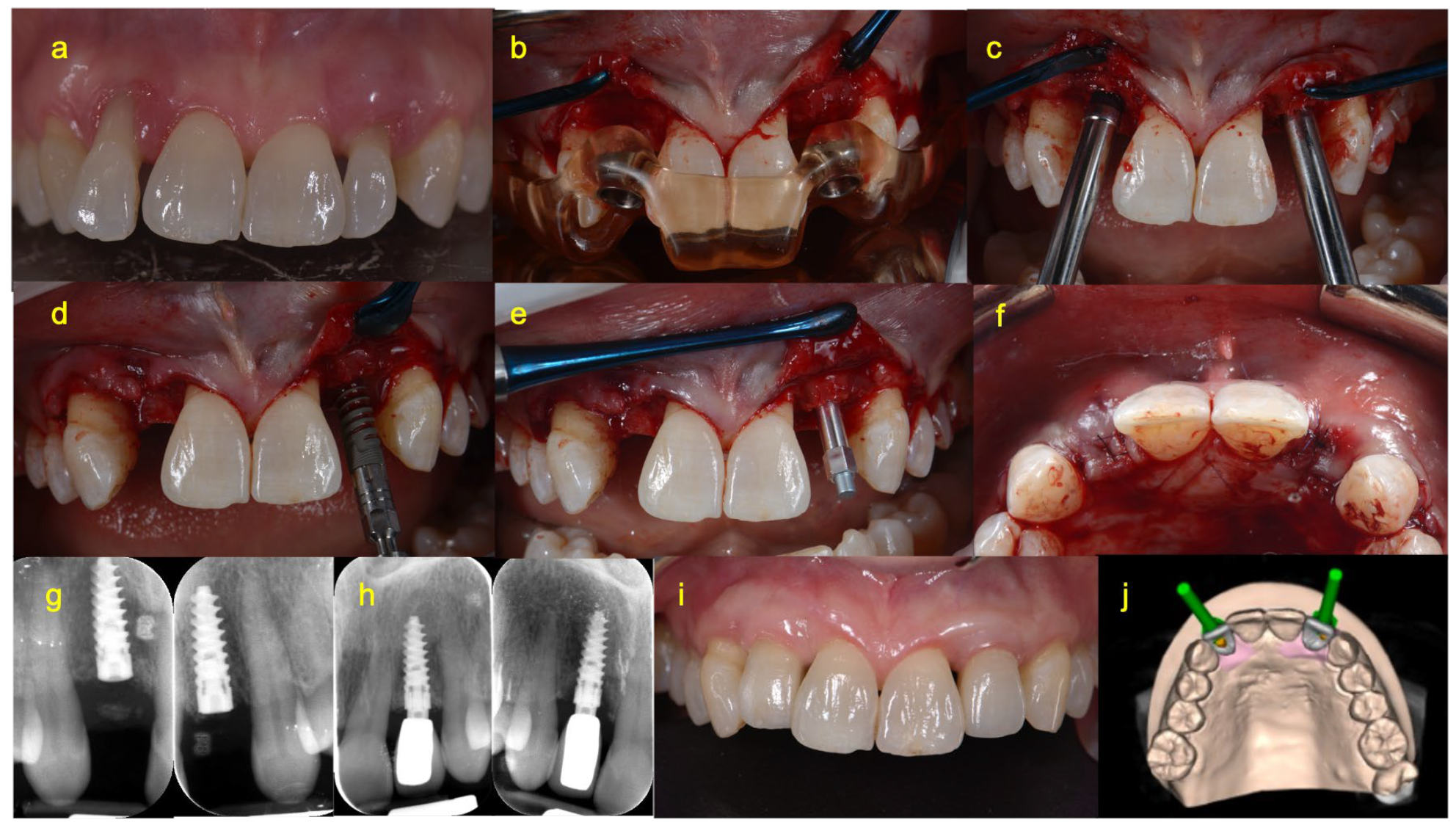
2.4. Surgical Procedure
2.5. Prosthetic Procedure of Immediate Loading Group (Group A)
2.6. Prosthetic Procedure of Delayed Loading Group (Group B)
2.7. Postoperative Management
2.8. Clinical and Radiographical Measurements
2.9. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amler, M.H. The time sequence of tissue regeneration in human extraction wounds. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol. 2000 2017, 73, 73–83. [Google Scholar] [CrossRef]
- Polder, B.J.; Van’t Hof, M.A.; Van der Linden, F.P.; Kuijpers-Jagtman, A.M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent. Oral Epidemiol. 2004, 32, 217–226. [Google Scholar] [CrossRef]
- Canullo, L.; Peñarrocha, D.; Peñarrocha, M.; Rocio, A.G.; Penarrocha-Diago, M. Piezoelectric vs. conventional drilling in implant site preparation: Pilot controlled randomized clinical trial with crossover design. Clin. Oral Implant. Res. 2014, 25, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Jorba-García, A.; González-Barnadas, A.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy assessment of dynamic computer-aided implant placement: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2479–2494. [Google Scholar] [CrossRef] [PubMed]
- Viña-Almunia, J.; Maestre-Ferrín, L.; Alegre-Domingo, T.; Peñarrocha-Diago, M. Survival of implants placed with the osteotome technique: An update. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e765–e768. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Wieland, M.; Sittig, C.; Brunette, D.M.; Textor, M.; Spencer, N.D. Measurement and evaluation of the chemical composition and topography of titanium implant surfaces. In Bone Engineering; Davies, J.E., Ed.; Em Squared: Toronto, ON, Canada, 2000; pp. 163–182. [Google Scholar]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Chambrone, L.; Shibli, J.A.; Mercúrio, C.E.; Cardoso, B.; Preshaw, P.M. Efficacy of standard (SLA) and modified sandblasted and acid-etched (SLA ctive) dental implants in promoting immediate and/or early occlusal loading protocols: A systematic review of prospective studies. Clin. Oral Implant. Res. 2015, 26, 359–370. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, S.-C. Bone cutting capacity and osseointegration of surface-treated orthodontic mini-implants. Korean J. Orthod. 2016, 46, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Miura, T.; Kou, I.; Muramatsu, T.; Furusawa, M.; Yoshinari, M. Influence of various superhydrophilic treatments of titanium on the initial attachment, proliferation, and differentiation of osteoblast-like cells. Dent. Mater. J. 2015, 34, 120–127. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Moses, O.; Bengazi, F.; Ferri, M.; Gianfreda, F.; Urbizo Velez, J.; Botticelli, D.; Canullo, L. Bioactivated Implant Surfaces Placed in Healed Sites or Extraction Sockets: A Preliminary Experimental Study in Dogs. Int. J. Oral Maxillofac. Implant. 2022, 37, 963–970. [Google Scholar] [CrossRef]
- Gianfreda, F.; Raffone, C.; Antonacci, D.; Mussano, F.; Genova, T.; Chinigò, G.; Canullo, L.; Bollero, P. Early Biological Response of an Ultra-Hydrophilic Implant Surface Activated by Salts and Dry Technology: An In-Vitro Study. Appl. Sci. 2021, 11, 6120. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Canullo, L.; Iacono, R.; Pires Godoy, E.; Punzo, A.; Cavicchia, A.; Gianfreda, F.; Bollero, P. Hybrid Funnel Technique: A Novel Approach for Implant Site Preparation: A Pilot Study. Dent. J. 2022, 10, 157. [Google Scholar] [CrossRef]
- Canullo, L.; Omori, Y.; Amari, Y.; Iannello, G.; Pesce, P. Five-year cohort prospective study on single implants in the esthetic area restored using one-abutment/one-time prosthetic approach. Clin. Implant. Dent. Relat. Res. 2018, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Pesce, P.; Tronchi, M.; Fiorellini, J.; Amari, Y.; Penarrocha, D. Marginal soft tissue stability around conical abutments inserted with the one abutment-one time protocol after 5 years of prosthetic loading. Clin. Implant. Dent. Relat. Res. 2018, 20, 976–982. [Google Scholar] [CrossRef]
- Canullo, L.; Troiano, G.; Sbricoli, L.; Guazzo, R.; Laino, L.; Caiazzo, A.; Pesce, P. The Use of Antibiotics in Implant Therapy: A Systematic Review and Meta-Analysis with Trial Sequential Analysis on Early Implant Failure. Int. J. Oral Maxillofac. Implant. 2020, 35, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Belser, U.C.; Grütter, L.; Vailati, F.; Bornstein, M.M.; Weber, H.P.; Buser, D. Outcome evaluation of early placed maxillary anterior single-tooth implants using objective esthetic criteria: A cross-sectional, retrospective study in 45 patients with a 2-to 4-year follow-up using pink and white esthetic scores. J. Periodontol. 2009, 80, 140–151. [Google Scholar] [CrossRef]
- Weigl, P.; Strangio, A. The impact of immediately placed and restored single-tooth implants on hard and soft tissues in the anterior maxilla. Eur. J. Oral Implantol. 2016, 9 (Suppl. S1), S89–S106. [Google Scholar]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontology 2000 2017, 73, 84–102. [Google Scholar] [CrossRef]
- Puisys, A.; Auzbikaviciute, V.; Vindasiute-Narbute, E.; Pranskunas, M.; Razukevicus, D.; Linkevicius, T. Immediate implant placement vs. early implant treatment in the esthetic area. A 1-year randomized clinical trial. Clin. Oral Implant. Res. 2022, 33, 634–655. [Google Scholar] [CrossRef]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2022, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef] [PubMed]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implant. 2007, 22, 755–760. [Google Scholar]
- Pesce, P.; Del Fabbro, M.; Menini, M.; De Giovanni, E.; Annunziata, M.; Khijmatgar, S.; Canullo, L. Effects of abutment materials on peri-implant soft tissue health and stability: A network meta-analysis. J. Prosthodont. Res. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Tommasato, G.; Patini, R.; Canullo, L. Influence of modified titanium abutment surface on peri-implant soft tissue behaviour: A systematic review of histological findings. Int. J. Oral Implantol. 2019, 12, 419–429. [Google Scholar]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Romero-Ruiz, M.M.; Gil, F.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a Novel Surface of Bioactive Implants on Osseointegration: AComparative and Histomorfometric Correlation and Implant Stability Study in Minipigs. Int. J. Mol. Sci. 2019, 20, 2307. [Google Scholar] [CrossRef]
- Clauser, T.; Lin, G.H.; Lee, E.; Del Fabbro, M.; Wang, H.L.; Testori, T. Risk of early implant failure in grafted and non-grafted sites: A systematic review and meta-analysis. Int. J. Oral Implantol. 2022, 15, 31–41. [Google Scholar]
| Demographic Parameters | Immediately Loaded Group n Implant = 7 | Delayed-Loaded Group n Implant = 6 | p Value |
|---|---|---|---|
| Age (mean ± SD) | 37 ± 24.86 | 58.60 ± 8.68 | p = 0.329 a |
| Sex (Female/male) | 5 females (83.33%)/1 male (16.67%) | 4 females (80%)/1 male (20%) | |
| Implant diameter (3.3/3.8 mm) | 3/4 | 2/4 | |
| Bone density (HU) (mean ± SD) | 410.00 ± 194.415 | 607.50 ± 140.83 | p = 0.051 a |
| Clinical Measurements | Immediately Loaded Group n Implant = 7 | Early-Loaded Group n Implant = 6 | p Value |
|---|---|---|---|
| ITV Ncm (mean ± SD) | 32.29 ± 9.01 | 28.50 ± 3.27 | p = 0.445 a |
| ISQ T0 (mean ± SD) | 72.71 ± 2.81 | 67.92 ± 8.43 | p = 0.234 a |
| MBL mm (mean ± SD) | 0.29 ± 0.29 | 0.33 ± 0.25 | p = 0.836 a |
| WES (mean ± SD) | 8.57 ± 0.79 | 9.17 ± 1.33 | p = 0.366 a |
| PES (mean ± SD) | 8.71 ± 1.89 | 8.33 ± 1.36 | p = 0.455 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacono, R.; Mayer, Y.; Marenzi, G.; Ferreira, B.V.; Pires, G.E.; Migliorati, M.; Bagnasco, F. Clinical, Radiological, and Aesthetic Outcomes after Placement of a Bioactive-Surfaced Implant with Immediate or Delayed Loading in the Anterior Maxilla: 1-Year Retrospective Follow-Up Study. Prosthesis 2023, 5, 610-621. https://doi.org/10.3390/prosthesis5030043
Iacono R, Mayer Y, Marenzi G, Ferreira BV, Pires GE, Migliorati M, Bagnasco F. Clinical, Radiological, and Aesthetic Outcomes after Placement of a Bioactive-Surfaced Implant with Immediate or Delayed Loading in the Anterior Maxilla: 1-Year Retrospective Follow-Up Study. Prosthesis. 2023; 5(3):610-621. https://doi.org/10.3390/prosthesis5030043
Chicago/Turabian StyleIacono, Roberta, Yaniv Mayer, Gaetano Marenzi, Balan Vitor Ferreira, Godoy Eduardo Pires, Marco Migliorati, and Francesco Bagnasco. 2023. "Clinical, Radiological, and Aesthetic Outcomes after Placement of a Bioactive-Surfaced Implant with Immediate or Delayed Loading in the Anterior Maxilla: 1-Year Retrospective Follow-Up Study" Prosthesis 5, no. 3: 610-621. https://doi.org/10.3390/prosthesis5030043
APA StyleIacono, R., Mayer, Y., Marenzi, G., Ferreira, B. V., Pires, G. E., Migliorati, M., & Bagnasco, F. (2023). Clinical, Radiological, and Aesthetic Outcomes after Placement of a Bioactive-Surfaced Implant with Immediate or Delayed Loading in the Anterior Maxilla: 1-Year Retrospective Follow-Up Study. Prosthesis, 5(3), 610-621. https://doi.org/10.3390/prosthesis5030043







