Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin
Abstract
:1. Introduction
2. Results
2.1. Surface Roughness
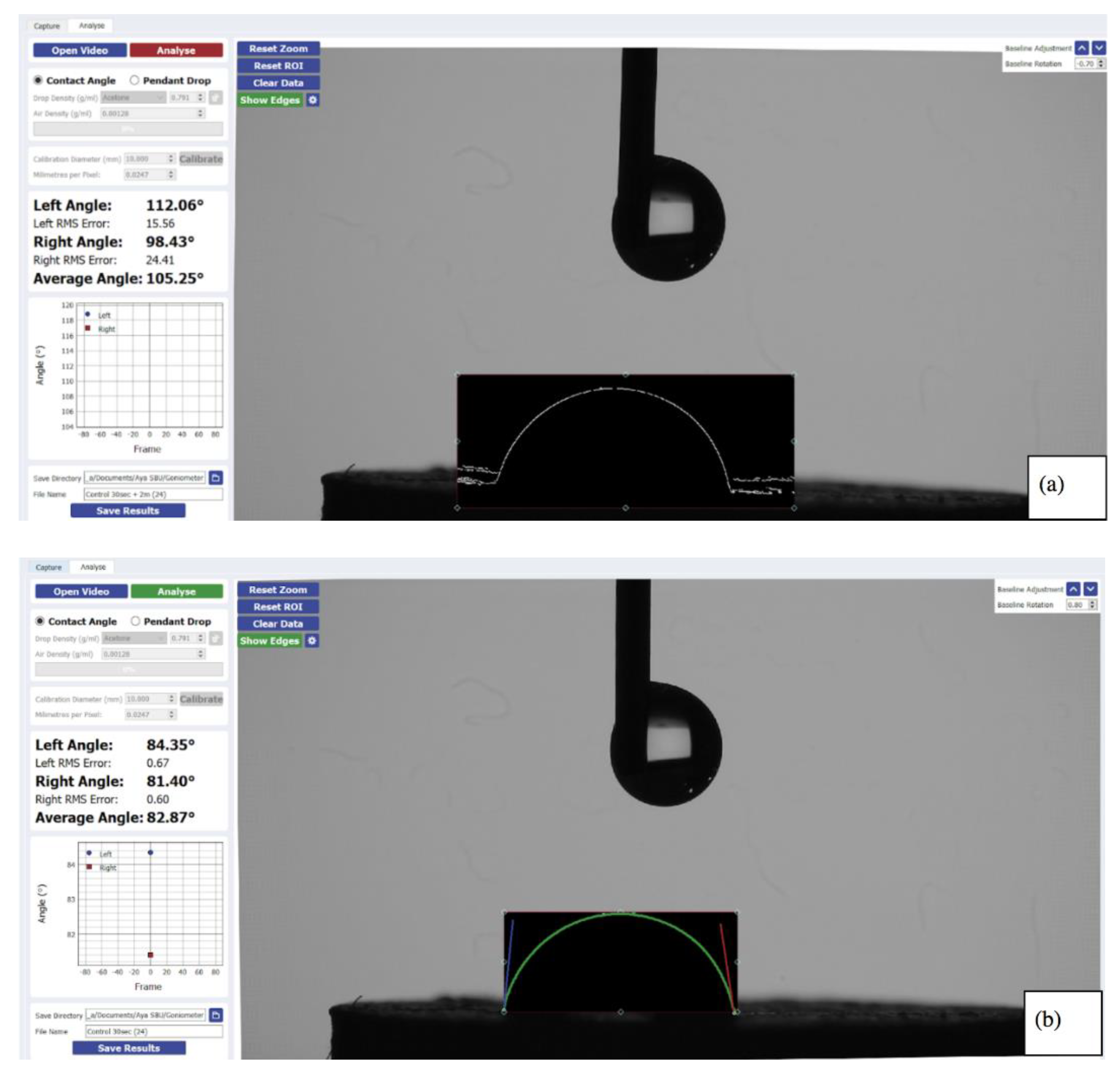
2.2. Hydrophobicity
3. Discussion
- (a)
- Changing the time or temperature will affect the polymerization level, which influences the product characteristics [21,22]. Increasing the post-curing time, by exposing the samples to the same laser used in polymerization for an additional two hours (120 min instead of only 60 min), would enhance the mechanical characteristics and alter the color [22]. It would also improve the accuracy (trueness and precision) [19]. For those reasons and others, the dentist might invest in the material quality by increasing the post curing time or temperature.
- (b)
- The sandblasting process results from an impact of aluminum oxide particles against the intaglio denture surface. There are different uses in dentistry for the sandblasting process, such as in bonding and resisting shear forces [20]. It is important when denture repairs or addition of new resin material are needed. The sandblasting process can create irregularities on the surface. This possibly increases the roughness of the polymer surfaces. A rougher surface increases the chances of biofilm colonization on different dental restorative materials [12]. The sandblasting process can also aim to assure removal of unwanted defects and smooth rough irregularities [23].
4. Materials and Methods
4.1. 3D-Printed Samples
4.2. Experimental Groups
4.3. Surface Roughness Measurement
4.4. Hydrophobicity Measurement
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiland-Bräuer, N. Friends or Foes-Microbial Interactions in Nature. Biology 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerubhotla, R.; Varanasi, J.L.; Das, D. Chapter 12—Biofilm Formation within Microbial Fuel Cells. In Progress and Recent Trends in Microbial Fuel Cells; Kundu, P.P., Dutta, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–242. [Google Scholar] [CrossRef]
- Almaguer-Flores, A. Biofilms in the oral environment. In Bio-Tribocorrosion in Biomaterials and Medical Implants; Yan, Y., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 169–186. [Google Scholar] [CrossRef]
- Monteiro, D.R.; de Souza Batista, V.E.; Caldeirão, A.C.M.; Jacinto, R.C.; Pessan, J.P. Oral prosthetic microbiology: Aspects related to the oral microbiome, surface properties, and strategies for controlling biofilms. Biofouling 2021, 37, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Wady, A.F.; Machado, A.L.; Zucolotto, V.; Zamperini, C.A.; Berni, E.; Vergani, C.E. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J. Appl. Microbiol. 2012, 112, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Jiang, F.; Yeh, C.-K.; Sun, Y. Controlling fungal biofilms with functional drug delivery denture biomaterials. Colloids Surf. B Biointerfaces 2016, 140, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- van Oss, C.J. Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. J. Mol. Recognit. 2003, 16, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Koodaryan, R.; Hafezeqoran, A. Effect of surface treatment methods on the shear bond strength of auto-polymerized resin to thermoplastic denture base polymer. J. Adv. Prosthodont. 2016, 8, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D printed complete removable dental prostheses: A narrative review. BMC Oral Health 2020, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef]
- Lee, B.I.; You, S.G.; You, S.M.; Kim, D.Y.; Kim, J.H. Evaluating the accuracy (trueness and precision) of interim crowns manufactured using digital light processing according to post-curing time: An In Vitro study. J. Adv. Prosthodont. 2021, 13, 89–99. [Google Scholar] [CrossRef]
- Nishigawa, G.; Maruo, Y.; Irie, M.; Maeda, N.; Yoshihara, K.; Nagaoka, N.; Matsumoto, T.; Minagi, S. Various Effects of Sandblasting of Dental Restorative Materials. PLoS ONE 2016, 11, e0147077. [Google Scholar] [CrossRef]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef]
- Kim, D.; Shim, J.S.; Lee, D.; Shin, S.H.; Nam, N.E.; Park, K.H.; Shim, J.S.; Kim, J.E. Effects of Post-Curing Time on the Mechanical and Color Properties of Three-Dimensional Printed Crown and Bridge Materials. Polymers 2020, 12, 2762. [Google Scholar] [CrossRef]
- Somers, N.; Lasgorceix, M. Surface Treatment of Bioceramics. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 701–715. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Cochis, A.; Varoni, E.; Azzimonti, B.; Carrassi, A. Biofilm Formation on Implants and Prosthetic Dental Materials. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 991–1027. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Panagoda, G.J.; Ellepola, A.N.B.; Samaranayake, L.P. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses 2001, 44, 29–35. [Google Scholar] [CrossRef]
- Ellepola, A.N.B.; Samaranayake, L.P. The effect of limited exposure to antimycotics on the relative cell-surface hydrophobicity and the adhesion of oral Candida albicans to buccal epithelial cells. Arch. Oral Biol. 1998, 43, 879–887. [Google Scholar] [CrossRef]
- Beaussart, A.; Alsteens, D.; El-Kirat-Chatel, S.; Lipke, P.N.; Kucharíková, S.; Van Dijck, P.; Dufrene, Y.F. Single-Molecule Imaging and Functional Analysis of Als Adhesins and Mannans during Candida albicans Morphogenesis. ACS Nano 2012, 6, 10950–10964. [Google Scholar] [CrossRef] [Green Version]
- Lyons, K.M.; Cannon, R.D.; Beumer, J.; Bakr, M.M.; Love, R.M. The Role of Biofilms and Material Surface Characteristics in Microbial Adhesion to Maxillary Obturator Materials: A Literature Review. Cleft Palate-Craniofacial J. 2020, 57, 487–498. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; van Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef]
- Everaert, E.P.; Mahieu, H.F.; Wong Chung, R.P.; Verkerke, G.J.; van der Mei, H.C.; Busscher, H.J. A new method for in vivo evaluation of biofilms on surface-modified silicone rubber voice prostheses. Eur. Arch. Otorhinolaryngol. 1997, 254, 261–263. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Kogo, T.; Hirai, N.; Ogawa, A.; Kanematsu, H.; Takahara, J.; Awazu, A.; Fujita, N.; Haruzono, Y.; Ichida, S.; et al. In-situ detection based on the biofilm hydrophilicity for environmental biofilm formation. Sci. Rep. 2019, 9, 8070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdziennicka, A.; Krawczyk, J.; Jańczuk, B. Wettability and Adhesion Work Prediction in the Polymer–Aqueous Solution of Surface Active Agent Systems. Colloids Interfaces 2018, 2, 21. [Google Scholar] [CrossRef]
- Miyamae, T.; Nozoye, H. Morphology and chemical structure of poly(methyl methacrylate) surfaces and interfaces: Restructuring behavior induced by the deposition of SiO2. Surf. Sci. 2003, 532–535, 1045–1050. [Google Scholar] [CrossRef]
- Webb, J.S.; Mei, H.C.V.d.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Read, S.J.; Robson, G.D.; Handley, P.S. Plasticizers Increase Adhesion of the Deteriogenic Fungus Aureobasidium pullulans to Polyvinyl Chloride. Appl. Environ. Microbiol. 1999, 65, 3575–3581. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.R.; Adams, M.R.; Zhdan, P.A.; Chamberlain, A.H. The role of surface physicochemical properties in determining the distribution of the autochthonous microflora in mineral water bottles. J. Appl. Microbiol. 1999, 86, 917–927. [Google Scholar] [CrossRef]
- Chandra, J.; Patel, J.D.; Li, J.; Zhou, G.; Mukherjee, P.K.; McCormick, T.S.; Anderson, J.M.; Ghannoum, M.A. Modification of surface properties of biomaterials influences the ability of Candida albicans to form biofilms. Appl. Environ. Microbiol. 2005, 71, 8795–8801. [Google Scholar] [CrossRef] [Green Version]
- Gingichashvili, S.; Feuerstein, O.; Steinberg, D. Topography and Expansion Patterns at the Biofilm-Agar Interface in Bacillus subtilis Biofilms. Microorganisms 2020, 9, 84. [Google Scholar] [CrossRef]
- Segonds-Pichon, A. Introduction to Sample Size Estimation. Introduction to Power Calculation 2015–2019. Available online: https://www.bioinformatics.babraham.ac.uk/training/Sample_Size_Estimation_and_Experimental_Design/Sample%20Size%20estimation%20Course%20manual.pdf (accessed on 24 November 2021).
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials-Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
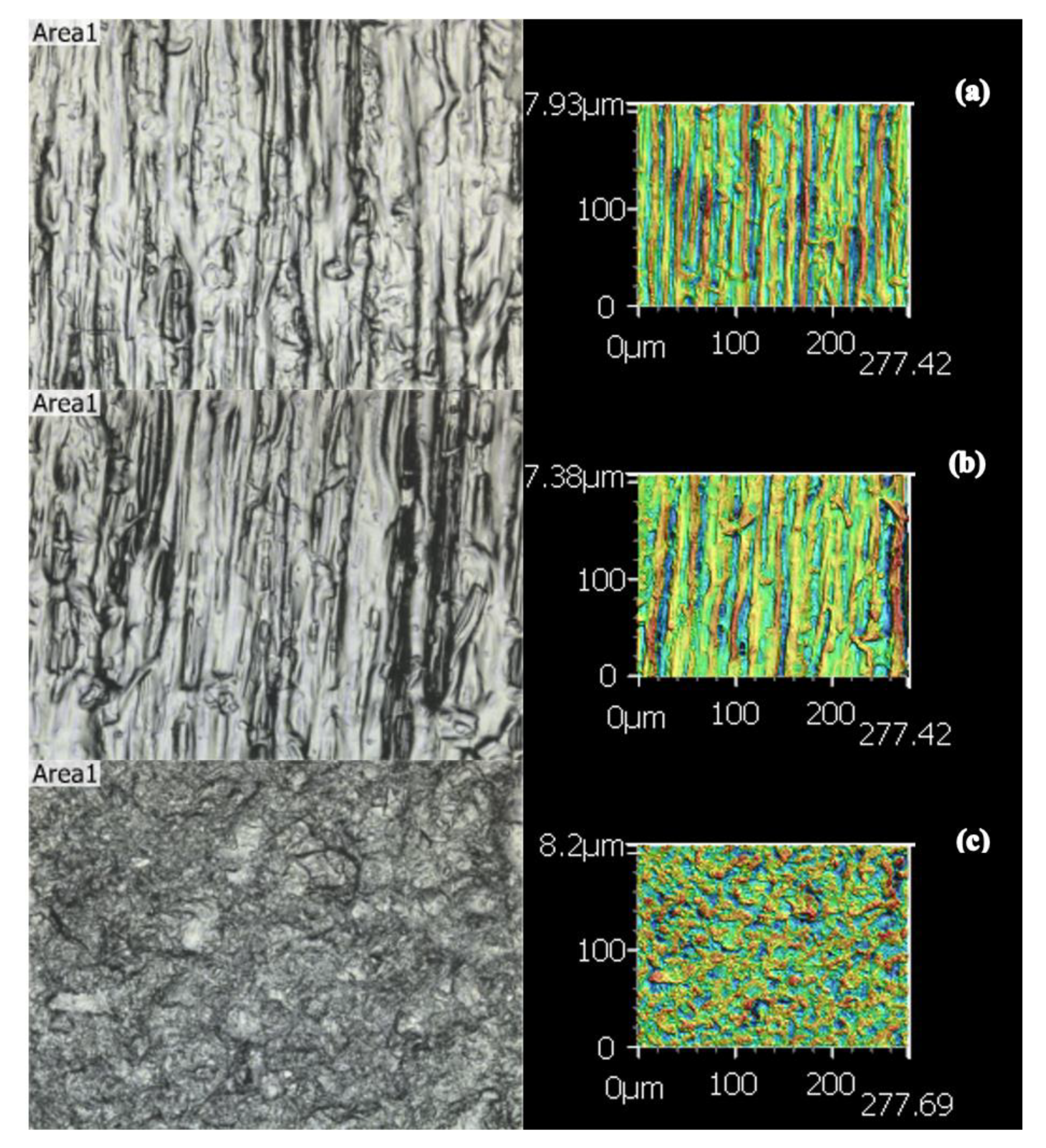
| Group’s Roughness Means ± STDEV | Sa (μm) | Sz (μm) | Str | Spc (μm) | Sdr |
|---|---|---|---|---|---|
| Control (A) | 4.64 ± 0.48 | 99.97 ± 15.1 | 0.18 ± 0.14 | 4.43 × 108 ± 4.2 × 107 | 4.65 × 105 ± 9.89 × 104 |
| Extended Cure (B) | 4.65 ± 0.83 | 102.52 ± 23.2 | 0.31 ± 0.23 | 4.58 × 108 ± 5.13 × 107 | 5.00 × 105 ± 1.27 × 105 |
| Sandblasted (C) | 3.43 ± 0.78 | 46.06 ± 10.62 | 0.84 ± 0.21 | 3.64 × 108 ± 1.1 × 108 | 3.83 × 105 ± 1.5 × 105 |
| ANOVA p Value | <0.001 * | <0.001 * | <0.001 * | 0.027 * | 0.123 |
| Tukey Post hoc Subset (paired groups) | (Control, ExCure) and (Sandblast) | (Control, ExCure) and (Sandblast) | (Control, ExCure) and (Sandblast) | (Control, ExCure) (Control, Sandblast) | All in one subset |
| Group’s Contact Angle | First Reading (Mean ± STDEV) (o) | Comparison between the 3 Groups 1st Reading | Second Reading (Mean ± STDEV) (°) | Comparison between 1st and 2nd Readings, T-test (p Value) |
|---|---|---|---|---|
| Control | 81.91 ± 3.90 | ANOVA p value < 0.001 *. Tukey post hoc subset: (Control, ExCure) and (Sandblast) | 78.02 ± 4.47 | <0.001 * |
| Extended cure | 80.25 ± 7.14 | 72.65 ± 8.19 | <0.001 * | |
| Sandblasted | 60.14 ± 10.51 | 51.33 ± 10.57 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbah, A.; Romanos, G.; Delgado-Ruiz, R. Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin. Prosthesis 2022, 4, 80-90. https://doi.org/10.3390/prosthesis4010009
Sabbah A, Romanos G, Delgado-Ruiz R. Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin. Prosthesis. 2022; 4(1):80-90. https://doi.org/10.3390/prosthesis4010009
Chicago/Turabian StyleSabbah, Aya, Georgios Romanos, and Rafael Delgado-Ruiz. 2022. "Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin" Prosthesis 4, no. 1: 80-90. https://doi.org/10.3390/prosthesis4010009
APA StyleSabbah, A., Romanos, G., & Delgado-Ruiz, R. (2022). Extended Post-Curing Light Exposure and Sandblasting Effects on Surface Hydrophobicity of 3D-Printed Denture Base Resin. Prosthesis, 4(1), 80-90. https://doi.org/10.3390/prosthesis4010009








