Using Rule-Based Decision Trees to Digitize Legislation
Abstract
1. Introduction
2. Digitizing Legislation
3. Decision Trees for Digitizing Legislation
Overview of Rule Based Decision Tree Algorithms
4. IVDR Legislation as a Case Study Example of RBDT-1C
4.1. Building the RBDT-1C
4.2. Classification Results from the IVDR Decision Tree, Build Using the RBDT-1C Algorithm
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, A. Innovation from differentiation: Pollution control departments and innovation in the printed circuit industry. IEEE Trans. Eng. Manag. 1995, 42, 270–277. [Google Scholar] [CrossRef]
- Wagner, M.; Bachor, V.; Ngai, E.W.T. Engineering and technology management for sustainable business development: Introductory remarks on the role of technology and regulation. J. Eng. Technol. Manag. 2014, 34, 1–8. [Google Scholar] [CrossRef]
- Gerstenfeld, A. Government regulation effects on the direction of innovation: A focus on performance standards. IEEE Trans. Eng. Manag. 1977, EM-24, 82–86. [Google Scholar] [CrossRef]
- de Jesus Pacheco, D.A.; Caten, C.S.T.; Jung, C.F.; Navas, H.V.G.; Cruz-Machado, V.A. Eco-innovation determinants in manufacturing SMEs from emerging markets: Systematic literature review and challenges. J. Eng. Technol. Manag. 2018, 48, 44–63. [Google Scholar] [CrossRef]
- Arnould, A.; Hendricusdottir, R.; Bergmann, J. The complexity of medical device regulations has increased, as assessed through data-driven techniques. Prosthesis 2021, 3, 314–330. [Google Scholar] [CrossRef]
- Ettlie, J.E.; Bridges, W.P. Environmental uncertainty and organizational technology policy. IEEE Trans. Eng. Manag. 1982, EM-29, 2–10. [Google Scholar] [CrossRef]
- Sanchez, C.M.; McKinley, W. Environmental regulatory influence and product innovation: The contingency effects of organizational characteristics. J. Eng. Technol. Manag. 1998, 15, 257–278. [Google Scholar] [CrossRef]
- Çanakoğlu, E.; Erzurumlu, S.S.; Erzurumlu, Y.O. How data-driven entrepreneur analyzes imperfect information for business opportunity evaluation. IEEE Trans. Eng. Manag. 2018, 65, 604–617. [Google Scholar] [CrossRef]
- Vries, M.J.D. Translating customer requirements into technical specifications. In Handbook of the Philosophy of Science, Philosophy of Technology and Engineering Sciences; North-Holland: Amsterdam, The Netherlands, 2009; pp. 489–512. [Google Scholar]
- Tiersma, P.M. The legal lexicon. In Legal Language; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Otto, P.N.; Anton, A.I. Addressing legal requirements in requirements engineering. In Proceedings of the 15th IEEE International Requirements Engineering Conference (RE 2007), Delhi, India, 15–19 October 2007; pp. 5–14. [Google Scholar] [CrossRef]
- Sergot, M.J.; Sadri, F.; Kowalski, R.A.; Kriwaczek, F.; Hammond, P.; Cory, H.T. The British nationality act as a logic program. Commun. ACM 1986, 29, 370–386. [Google Scholar] [CrossRef]
- Kowalski, R.A. Legislation as logic programs. In Informatics and the Foundations of Legal Reasoning, Logic Programming in Action Lecture Notes in Computer Science; Springer: Dordrecht, The Netherlands, 1995; pp. 235–256. [Google Scholar]
- Leith, P. Fundamental errors in legal logic programming. Comput. J. 1986, 29, 545–552. [Google Scholar] [CrossRef]
- Massacci, F.; Prest, M.; Zannone, N. Using a security requirements engineering methodology in practice: The compliance with the Italian data protection legislation. Comput. Stand. Interfaces 2005, 27, 445–455. [Google Scholar] [CrossRef][Green Version]
- Zhang, N.; Bodorik, P.; Jutla, D.N. Compliance of privacy policies with legal regulations—Compliance of privacy policies with Canadian PIPEDA. In Proceedings of the Second International Conference on e-Business, Barcelona, Spain, 28–31 July 2007. [Google Scholar]
- Bergmann, J.; Hendricusdottir, R.; Lee, R. Regulatory navigation: A digital tool to understand medical device classification pathways. Compr. Biotechnol. 2019, 5, 167–172. [Google Scholar]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Imam, I.F.; Michalski, R.S. Learning decision trees from decision rules: A method and initial results from a comparative study. J. Intell. Inf. Syst. 1993, 2, 279–304. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Traore, I.; Nakkabi, Y. Creating decision trees from rules using RBDT-1. Comput. Intell. 2014, 32, 216–239. [Google Scholar] [CrossRef]
- Kotsiantis, S.B. Decision trees: A recent overview. Artif. Intell. Rev. 2011, 39, 261–283. [Google Scholar] [CrossRef]
- Michalski, R.S.; Imam, I.F. Learning problem-oriented decision structures from decision rules: The AQDT-2 system. In Lecture Notes in Computer Science Methodologies for Intelligent Systems; Springer: Berlin/Heidelberg, Germany, 1994; pp. 416–426. [Google Scholar]
- Trevino, M. 8 Key Changes to Understand in the New European MDR and IVDR. Med. Device Online, Sepetmber 2018. Available online: https://www.meddeviceonline.com/doc/key-changes-to-understand-in-the-new-european-mdr-and-ivdr-0001 (accessed on 13 January 2020).
- Ceross, A.; Bergmann, J. Evaluating the presence of software-as-a-medical-device in the australian therapeutic goods register. Prosthesis 2021, 3, 221–228. [Google Scholar] [CrossRef]
- Obelis, Notified Bodies: IVDR. Obelis, 05 April 2019. Available online: https://www.obelis.net/notified-bodies-in-vitro-diagnostic-medical-devices/ (accessed on 13 January 2020).
- Notified Bodies—Post Brexit. BSIF, 01 January 1967. Available online: https://www.bsif.co.uk/notified-bodies-post-brexit/ (accessed on 27 April 2020).
- European Commission. Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/E. 2017. [Google Scholar]
- Lietz, P. Research into questionnaire design: A summary of the literature. Int. J. Mark. Res. 2010, 52, 249–272. [Google Scholar] [CrossRef]
- Holbrook, A.; Cho, Y.I.; Johnson, T. The impact of question and respondent characteristics on comprehension and mapping difficulties. Public Opin. Q. 2006, 70, 565–595. [Google Scholar] [CrossRef]
- Foddy, W. The limitations of human memory. In Constructing Questions for Interviews and Questionnaires: Theory and Practice in Social Research, Cambridge; Cambridge University Press: Cambridge, UK, 1993; pp. 90–100. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, W.; Kim, S.; Kim, D.; Kim, S.; So, C.H.; Kang, J. BioBERT: A pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 2019, 36, 1234–1240. [Google Scholar] [CrossRef]
- MDCG 2019-11 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745—MDR and Regulation (EU) 2017/746—IVDR. 2019.
- British Standards Institution. IVDR Classification and Conformity Assessment. 2017.
- British Standards Institute. Classification of IVDs Infographic. 2020. [Google Scholar]
- Australian Government Department of Health. IVD Classification Examples. Therapeutic Goods Administration (TGA), 06-December 2015. Available online: https://www.tga.gov.au/book-page/ivd-classification-examples#r14eg (accessed on 16 February 2020).
- Center for Devices; Radiological Health. Nucleic Acid Based Tests. U.S. Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/vitro-diagnostics/nucleic-acid-based-tests (accessed on 19 February 2020).
- Center for Devices; Radiological Health. List of Cleared or Approved Companion Diagnostic Devices. U.S. Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools (accessed on 1 March 2020).
- Daniels, G.L.; Fletcher, A.; Garratty, G.; Henry, S.; Jorgensen, J.; Judd, W.J.; Levene, C.; Lomas-Francis, C.; Moulds, J.J.; Moulds, J.M.; et al. Blood group terminology 2004: From the International Society of Blood Transfusion committee on terminology for red cell surface antigens. Vox Sang. 2004, 87, 304–316. [Google Scholar] [CrossRef]
- Medical Device Coordination Group. MDCG 2020-16: Guidance on Classification Rules for In Vitro Diagnostic Medical Devices under Regulation (EU) 2017/746. 2020. [Google Scholar]
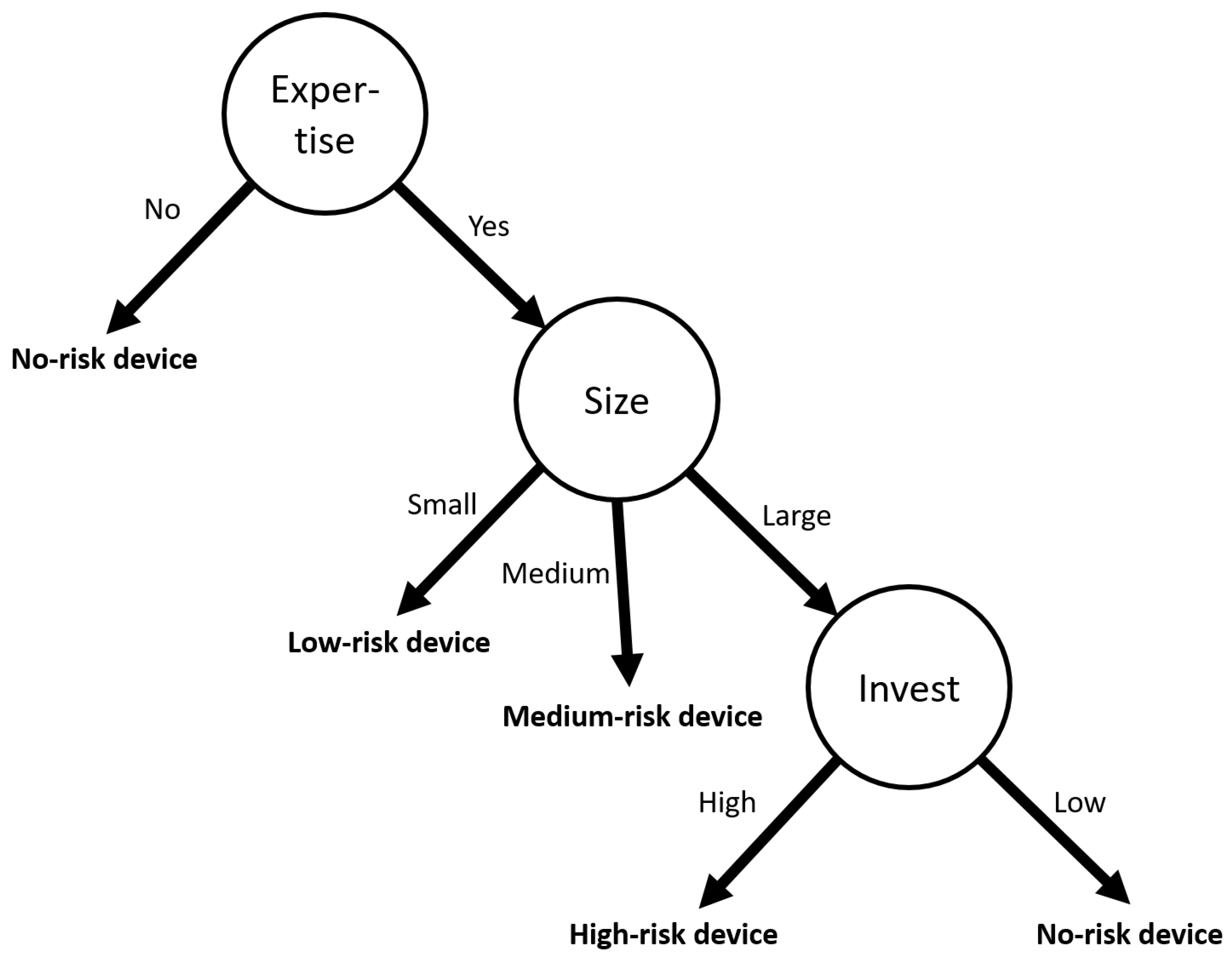

| Company | Expertise | Size | Investment | Device Risk |
|---|---|---|---|---|
| 1 | No | Small | High | None |
| 2 | Yes | Small | High | Low |
| 3 | No | Large | High | None |
| 4 | No | Medium | High | None |
| 5 | Yes | Medium | Low | Medium |
| 6 | No | Medium | Low | None |
| 7 | Yes | Large | Low | None |
| 8 | Yes | Large | High | High |
| 9 | No | Large | High | None |
| 10 | Yes | Small | High | Low |
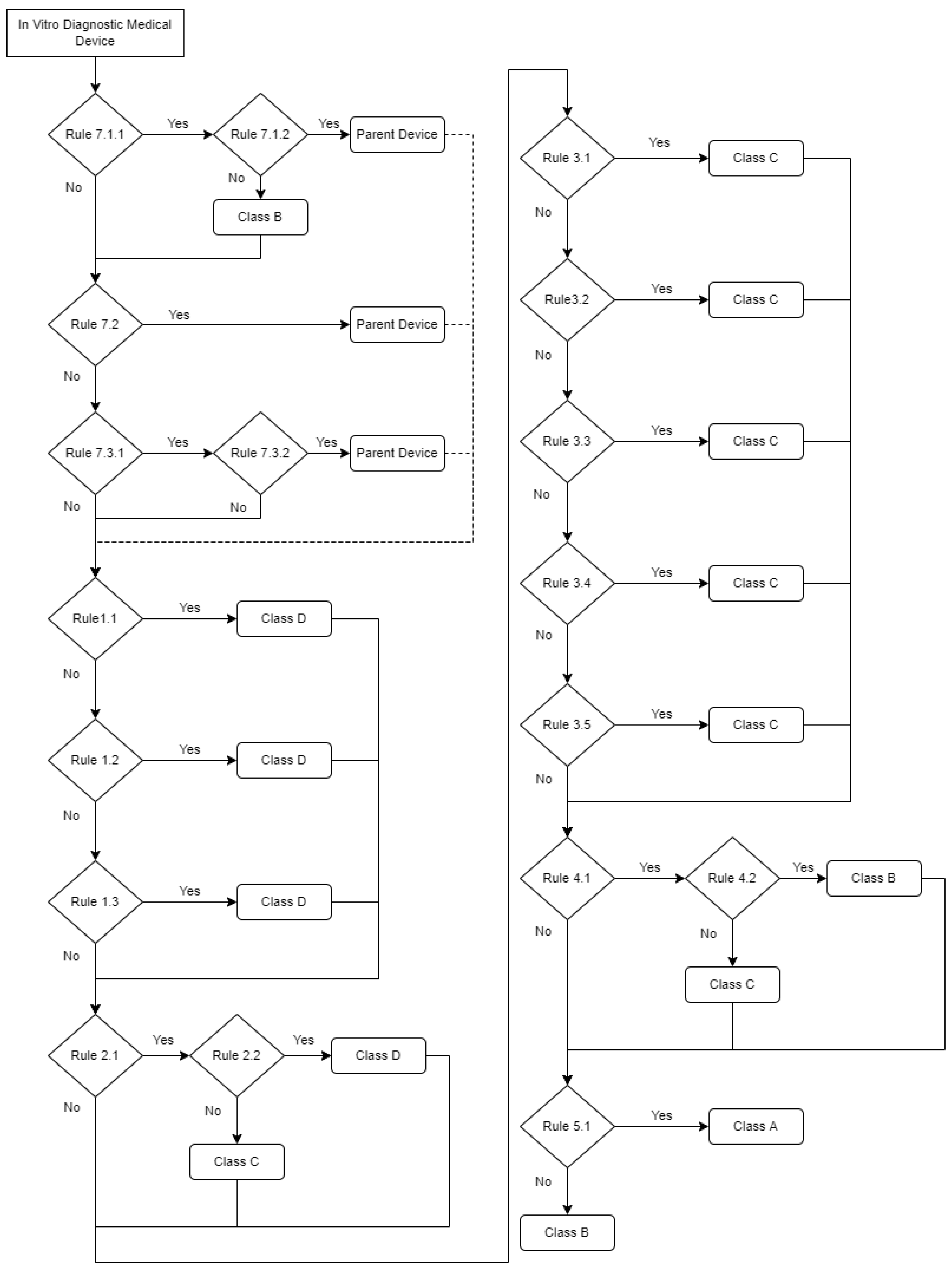
| Rule | IVDR Classification Rule | Independent Medical Device | Personal Risk | Public Health Risk | Device Risk |
|---|---|---|---|---|---|
| 7.1.1 | Rule 7 | Yes | Moderate/Low | Low | Class B |
| 7.1.2 | Implementing Rules 1.6 | No | - | - | Parent device classification |
| 7.2 | Implementing Rules 1.5 | No | - | - | Parent device classification |
| 7.3.1 | Implementing Rules 1.4 | No | - | - | Parent device classification |
| 7.3.2 | Implementing Rules 1.4 | Yes | - | - | Device classified in its own right |
| 1.1 | Rule 1 | Yes | High | High | Class D |
| 1.2 | Rule 1 | Yes | High | High | Class D |
| 1.3 | Rule 1 | Yes | High | High | Class D |
| 2.1 | Rule 2 | Yes | High | Moderate/Low | Class C |
| 2.2 | Rule 2 | Yes | High | High | Class D |
| 3.1 | Rule 3 | Yes | High | Moderate/Low | Class C |
| 3.2 | Rule 3 | Yes | High | Moderate/Low | Class C |
| 3.3 | Rule 3 | Yes | High | Moderate/Low | Class C |
| 3.4 | Rule 3 | Yes | High | Moderate/Low | Class C |
| 3.5 | Rule 3 | Yes | High | Moderate/Low | Class C |
| 4.1 | Rule 4 | Yes | High | Moderate/Low | Class C |
| 4.2 | Rule 4 | Yes | Moderate/Low | Low | Class B |
| 5.1 | Rule 5 | Yes | Low | Low | Class A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingay, H.R.F.; Hendricusdottir, R.; Ceross, A.; Bergmann, J.H.M. Using Rule-Based Decision Trees to Digitize Legislation. Prosthesis 2022, 4, 113-124. https://doi.org/10.3390/prosthesis4010012
Mingay HRF, Hendricusdottir R, Ceross A, Bergmann JHM. Using Rule-Based Decision Trees to Digitize Legislation. Prosthesis. 2022; 4(1):113-124. https://doi.org/10.3390/prosthesis4010012
Chicago/Turabian StyleMingay, Henry R. F., Rita Hendricusdottir, Aaron Ceross, and Jeroen H. M. Bergmann. 2022. "Using Rule-Based Decision Trees to Digitize Legislation" Prosthesis 4, no. 1: 113-124. https://doi.org/10.3390/prosthesis4010012
APA StyleMingay, H. R. F., Hendricusdottir, R., Ceross, A., & Bergmann, J. H. M. (2022). Using Rule-Based Decision Trees to Digitize Legislation. Prosthesis, 4(1), 113-124. https://doi.org/10.3390/prosthesis4010012







