Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrophoretic Deposition
2.3. Chemical Treatment
2.4. EPD Parameters
2.5. Microstructural Examinations
2.6. Electrochemical Measurements
2.7. In Vitro Bioactivity Study
3. Results
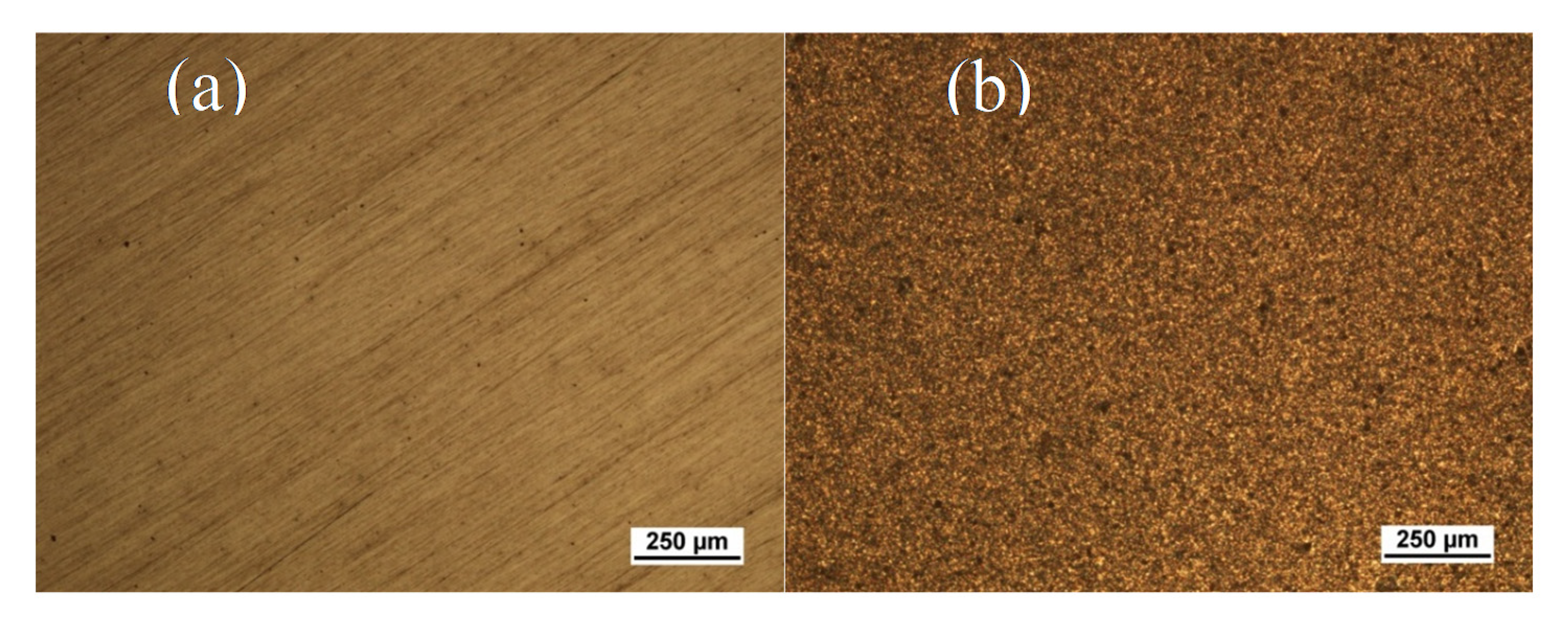
3.1. Microstructural Examination of Mg after Pre-Treatment
3.2. SEM Analysis of Zein/BG Coatings
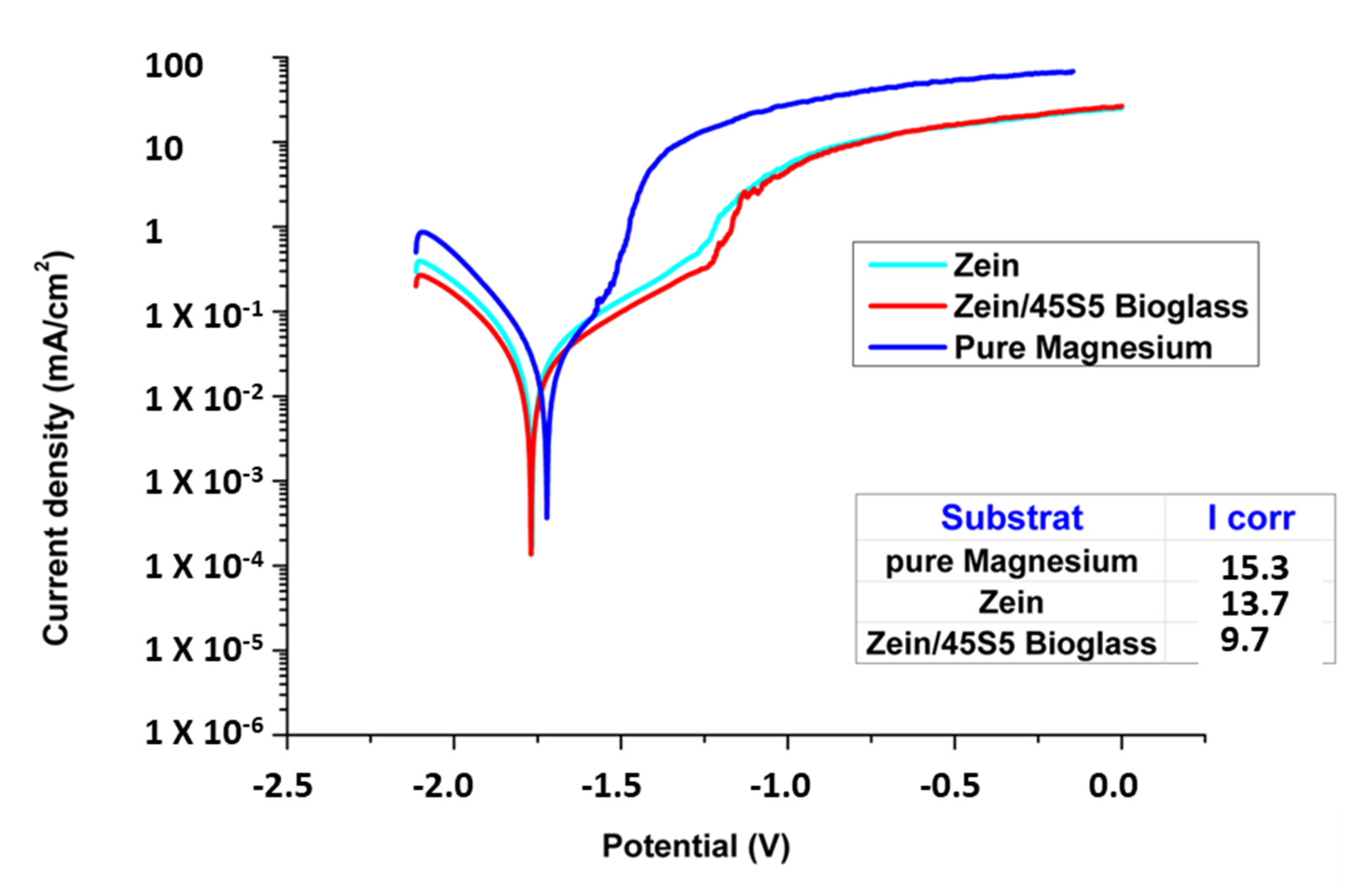
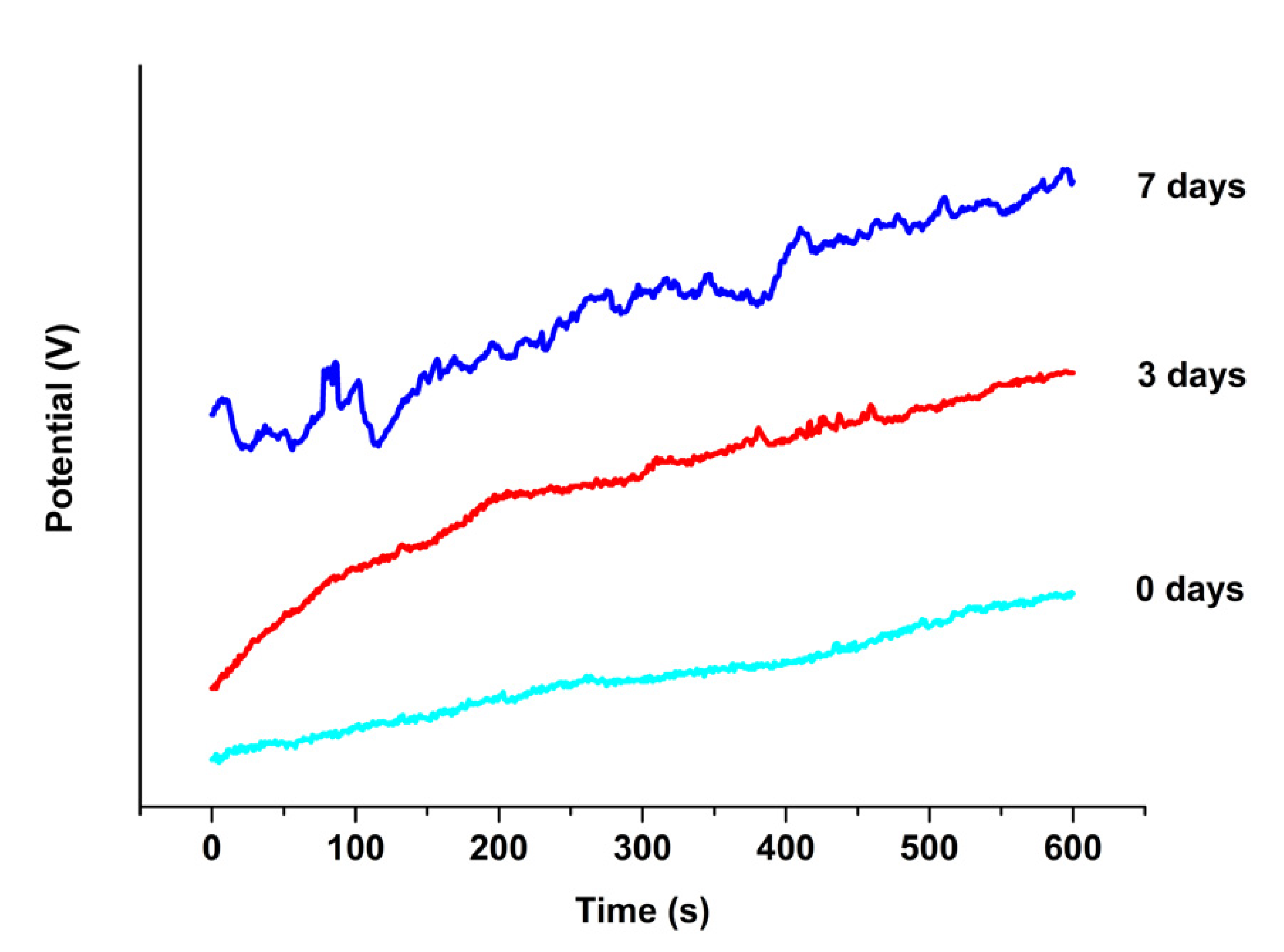
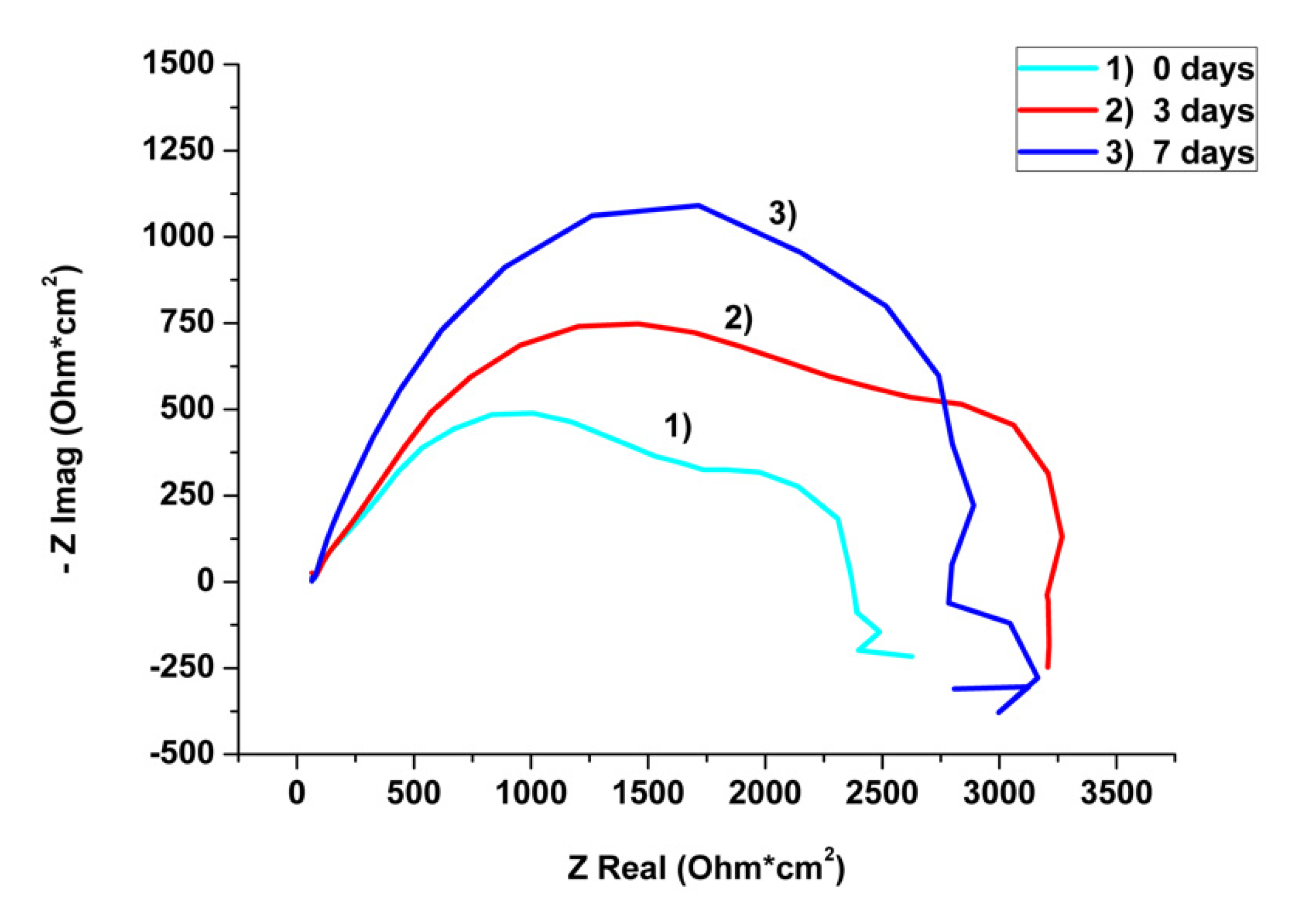
3.3. Electrochemical Investigations
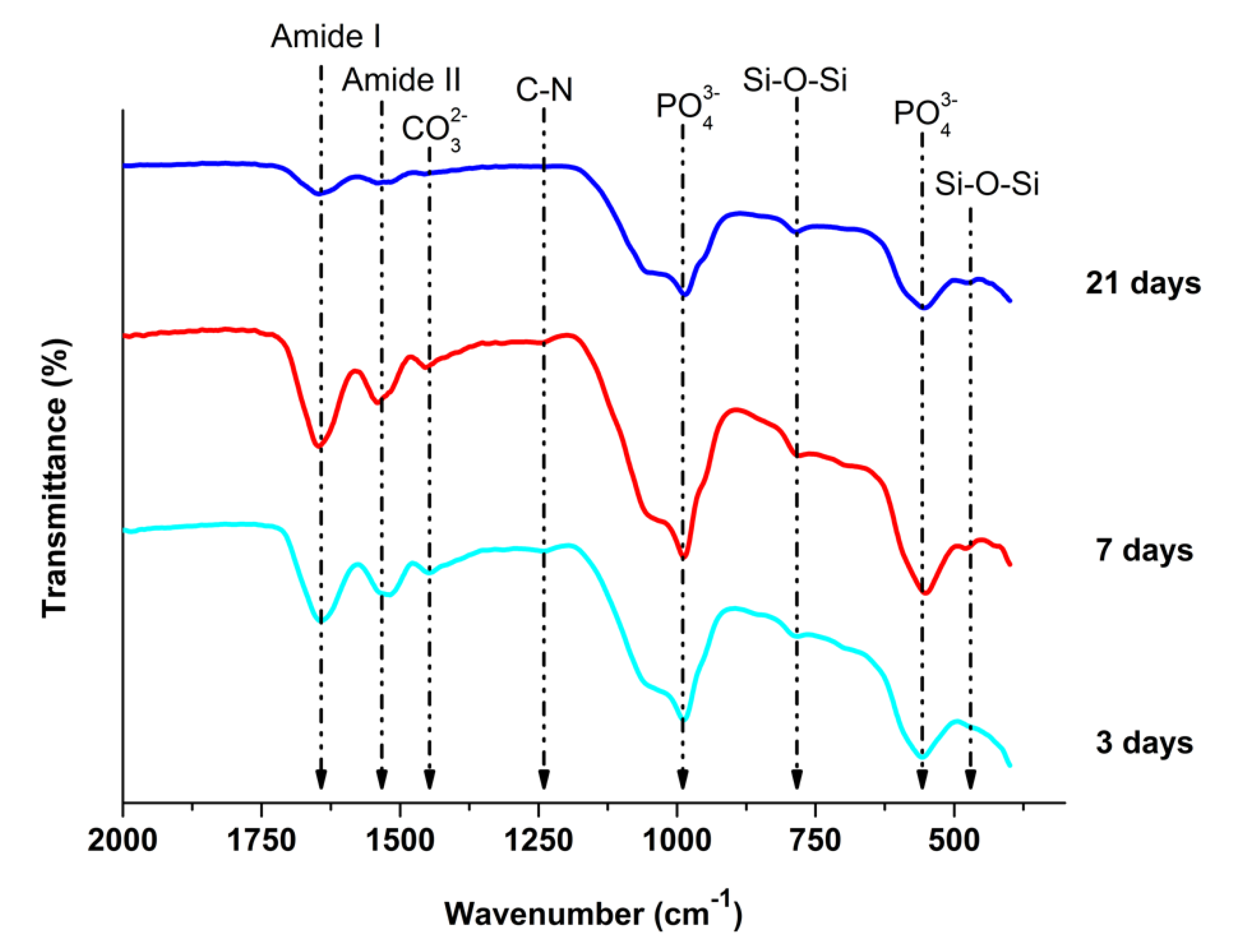
3.4. In Vitro Bioactivity
4. Discussion
4.1. EPD Kinetics and Suspension Stability
4.2. EPD of Zein/BG on Mg
4.3. Corrosion Study
4.4. In Vitro Bioactivity
5. Conclusions
- The zein/BG composite coatings were deposited on pre-treated pure magnesium substrate via EPD.
- The optimum deposition parameters were deduced from a series of experiments on SS. The initial experiments inferred that the deposition voltage of 14.5 V, BG concentration of 5 g/L, pH of 3, deposition time of 5 min, and an electrode distance of 10 mm are optimum for the deposition of zein/BG composite coatings on the Mg substrate.
- SEM images showed that a homogeneous film of zein was embedded with BG particles.
- Electrochemical tests confirmed that the coating provides effective protection against corrosion.
- Zein/BG coatings developed hydroxyapatite crystals on the surface of the coatings after immersion in SBF.
- FTIR analysis showed the dissolution of zein and BG upon immersion in SBF.
- The electrochemical tests after immersion in SBF revealed that the zein/BG slows down the degradation rate of Mg compared to the available literature, which was the main aim of the present study.
Funding
Conflicts of Interest
References
- Prateeksha Kaul, P.J. Bioimplants Market by Type (Cardiovascular Bioimplants, Dental Bioimplants, Orthopedic Bioimplants, Spinal Bioimplants, and Ophthalmology Bioimplants), Material (Metallic Biomaterials, Ceramic Biomaterials, Polymers Biomaterials, and Natural Biomaterials)—Global Opportunity Analysis and Industry Forecast, 2017–2023. Available online: https://www.alliedmarketresearch.com/bioimplants-market (accessed on 1 August 2020).
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Elahinia, M. 9—Biodegradable magnesium alloys. In Metals for Biomedical Devices; Woodhead Publ. Ser., Biomater.; Niinomi, S.E., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 265–289. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Hou, Z.-T.; Ye, X.; Xu, Z.-B.; Bai, X.-L.; Shang, P. The effect of selected alloying element additions on properties of Mg-based alloy as bioimplants: A literature review. Front. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rehman, M.A.U. Improvement in the surface properties of stainless steel via zein/hydroxyapatite composite coatings for biomedical applications. Surf. Interfaces 2020, 100589. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghomi, M.; Padil, V.V.T.; Shalchy, F.; Ashrafizadeh, M.; Askarinejad, S.; Mokhtari, B. Biofabricated Nanostructures and Their Composites in Regenerative Medicine. ACS Appl. Nano Mater. 2020, 3, 6210–6238. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Meyer, N.; Rivera, L.R.; Ellis, T.; Qi, J.; Ryan, M.P.; Boccaccini, A.R. Bioactive and antibacterial coatings based on zein/bioactive glass composites by electrophoretic deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef]
- Filho, O.P.; La Torre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Lu, H.H.; El-Amin, S.F.; Scott, K.D.; Laurencin, C.T. Three-dimensional, bioactive, biodegradable, polymer–bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. Part. A An. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 64, 465–474. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yasir, M.; Ur Rehman, M.A. Fabrication and Characterization of Zein/Hydroxyapatite Composite Coatings for Biomedical Applications. Surfaces 2020, 3, 237–250. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Ramos Rivera, L.; Dippel, J.; Boccaccini, A.R. Formation of Zein/Bioactive Glass Layers Using Electrophoretic Deposition Technique. ECS Trans. 2018, 82, 73–80. [Google Scholar] [CrossRef]
- Kaya, S.; Boccaccini, A.R. Electrophoretic deposition of zein coatings. J. Coat. Technol. Res. 2017, 14, 683–689. [Google Scholar] [CrossRef]
- Wang, C.Y.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L.N. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Ther. 2020, 3, 2000024. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of Chitosan-based Composite Coatings for Biomedical Applications: A Review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Dickerson, J.H. Electrophoretic Deposition: Fundamentals and Applications; ACS Publications: Washington, DC, USA, 2013. [Google Scholar]
- Zhao, H.; Cai, S.; Ding, Z.; Zhang, M.; Li, Y.; Xu, G. A simple method for the preparation of magnesium phosphate conversion coatings on a AZ31 magnesium alloy with improved corrosion resistance. RSC Adv. 2015, 5, 24586–24590. [Google Scholar] [CrossRef]
- Höhlinger, M.; Heise, S.; Wagener, V.; Boccaccini, A.R.; Virtanen, S. Developing surface pre-treatments for electrophoretic deposition of biofunctional chitosan-bioactive glass coatings on a WE43 magnesium alloy. Appl. Surf. Sci. 2017, 405, 441–448. [Google Scholar] [CrossRef]
- Ahmed, Y.; Nawaz, A.; Singh Virk, R.; Wadood, A.; Rehman, M.A.U. Fabrication and characterization of zein/bioactive glass (BG) deposited on pre-treated magnesium via electrophoretic deposition. Int. J. Ceram. Eng. Sci. 2020. [Google Scholar] [CrossRef]
- Hertel, M. Elektrochemische Charakterisierung von Stents mit Hilfe des Adaptierten Mini-Cell-Systems (MCS). Ph.D. Thesis, Freie Universität, Berlin, Germany, 2012. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Hum, J.; Lepry, W.C.; Miri, A.K.; Nazhat, S.N.; Boccaccini, A.R. Fabrication and characterization of zein–bioactive glass scaffolds. BioinspiredBiomim. Nanobiomater. 2015, 4, 73–78. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Heise, S.; Höhlinger, M.; Hernández, Y.T.; Palacio, J.J.P.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition and characterization of chitosan/bioactive glass composite coatings on Mg alloy substrates. Electrochim. Acta 2017, 232, 456–464. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic deposition of nano-zirconia coating on AZ91D magnesium alloy for bio-corrosion control purposes. Surf. Coat. Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Mehdipour, M.; Afshar, A.; Mohebali, M. Electrophoretic deposition of bioactive glass coating on 316L stainless steel and electrochemical behavior study. Appl. Surf. Sci. 2012, 258, 9832–9839. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic deposition of PEEK/bioactive glass composite coatings on stainless steel for orthopedic applications: An optimization for in vitro bioactivity and adhesion strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhu, Y.-J.; Chen, F.; Lu, B.-Q.; Wu, J. Nanosheet-assembled hierarchical nanostructures of hydroxyapatite: Surfactant-free microwave-hydrothermal rapid synthesis, protein/DNA adsorption and pH-controlled release. CrystEngComm 2013, 15, 206–212. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part. A 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J. In vitro reactivity of Cu doped 45S5 Bioglass® derived scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 5659–5674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Zou, Y.H.; Han, E.H. In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid. Materials 2017, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Dehghanghadikolaei, A.; Advincula, R.; Dean, D.; Luo, A.; Elahinia, M. Ceramic coating for delayed degradation of Mg-1.2Zn-0.5Ca-0.5Mn bone fixation and instrumentation. Thin Solid Films 2019, 687, 137456. [Google Scholar] [CrossRef]
- Steiner Petrovič, D.; Mandrino, D.; Šarler, B.; Horky, J.; Ojdanic, A.; Zehetbauer, M.J.; Orlov, D. Surface Analysis of Biodegradable Mg-Alloys after Immersion in Simulated Body Fluid. Materials 2020, 13, 1740. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ur Rehman, M.A. Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants. Prosthesis 2020, 2, 211-224. https://doi.org/10.3390/prosthesis2030018
Ur Rehman MA. Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants. Prosthesis. 2020; 2(3):211-224. https://doi.org/10.3390/prosthesis2030018
Chicago/Turabian StyleUr Rehman, Muhammad Atiq. 2020. "Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants" Prosthesis 2, no. 3: 211-224. https://doi.org/10.3390/prosthesis2030018
APA StyleUr Rehman, M. A. (2020). Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants. Prosthesis, 2(3), 211-224. https://doi.org/10.3390/prosthesis2030018




