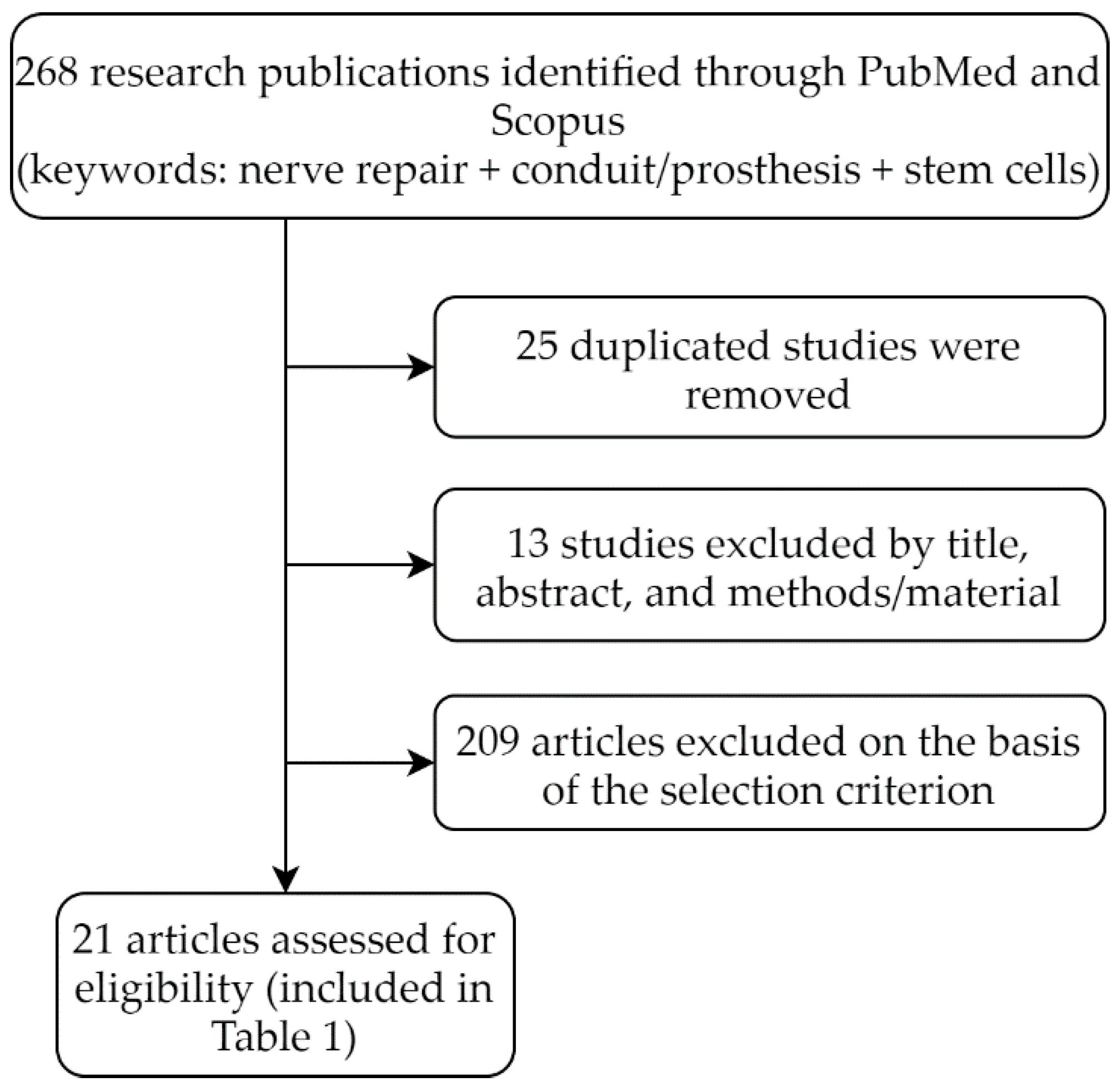
A Systematic Review of the Effectiveness of Cell-Based Therapy in Repairing Peripheral Nerve Gap Defects
Abstract
1. Introduction
- (a)
- Studies that employed a nerve crush injury model, gapless repair model, or in vitro model.
- (b)
- Studies that used non-synthetic or biological prostheses derived from nerves, muscles, or intestines.
- (c)
- Studies where the segmental defect was 10 mm or less (the threshold for the length of a nerve gap was taken as 10 mm because most of the studies used a peripheral nerve injury model in rodents, and due to higher nerve regeneration capacity in smaller animals, the true efficacy of a nerve repair cannot be revealed over shorter nerve defects).
- (d)
- Studies where nerve growth factors were applied either endo- or exogenously.
2. Scope of the Manuscript
3. Effectiveness of Cell Therapy
- Count of (i) regenerated axons, (ii) blood vessels, and (iii) FG-labeled motoneurons;
- Measurement of (iv) axonal diameter, (v) myelin thickness, (vi) muscle weight ratio, (vii) compound muscle action potential, (viii) sciatic function index, (ix) nerve conduction velocity, (x) gap length, and (xi) follow-up time.
4. Discussion and Perspective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Archibald, S.J.; Shefner, J.; Krarup, C.; Madison, R.D. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J. Soc. Neurosci. 1995, 15, 4109–4123. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, C.; Day, A.G.E.; Murray-Dunning, C.; Thanabalasundaram, L.; Cowan, J.; Stevanato, L.; Phillips, J.B. An allogeneic “off the shelf” therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells. Sci. Rep. 2018, 8, 2951. [Google Scholar] [CrossRef] [PubMed]
- Ladak, A.; Olson, J.; Tredgeta, E.E.; Gordon, T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp. Neurol. 2011, 228, 242–252. [Google Scholar] [CrossRef]
- Evans, G.R.D.; Brandt, K.; Katz, S. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23, 841–848. [Google Scholar] [CrossRef]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sanen, K.; Martens, W.; Georgiou, M.; Ameloot, M.; Lambrichts, I.; Phillips, J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: Potential for peripheral nerve repair? J. Tissue Eng. Regen. Med. 2017, 11, 3362–3372. [Google Scholar] [CrossRef]
- Allbright, K.O.; Bliley, J.M.; Havis, E.; Kim, D.-Y.; Dibernardo, G.A.; Grybowski, D.; Waldner, M.; James, I.B.; Sivak, W.N.; Rubin, J.P.; et al. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve 2018, 58, 251–260. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, L.; Yang, Y.; Quan, X.; Huang, L.; Liu, Z.; Luo, Z. Enhanced in vivo survival of Schwann cells by a synthetic oxygen carrier promotes sciatic nerve regeneration and functional recovery. J. Tissue Eng. Regen. Med. 2018, 12, e177–e189. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Hernández, J.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Schwann cells and mesenchymal stem cells in laminin-or fbronectin-aligned matrices and regeneration across a critical size defect of 15 mm in the rat sciatic nerve. J. Neurosurg. Spine 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Marchesi, C.; Pluderi, M.; Colleoni, F.; Belicchi, M.; Meregalli, M.; Farini, A.; Porretti, L. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia 2007, 55, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Ansselin, A.D.; Fink, T.; Davey, D.F. Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathol. Appl. Neurobiol. 1997, 23, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Sinis, N.; Schaller, H.E.; Schulte-Eversum, C.; Schlosshauer, B.; Doser, M.; Dietz, K.; Haerle, M. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J. Neurosurg. 2005, 103, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Hayashi, T.; Kitada, M.; Kohama, M.; Matsue, D.; Teramoto, N.; Iida, H. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp. Neurol. 2010, 223, 537–547. [Google Scholar] [CrossRef]
- Cheng, B.; Chen, Z. Fabricating autologous tissue to engineer artificial nerve. Microsurgery 2002, 22, 133–137. [Google Scholar] [CrossRef]
- Brown, R.E.; Erdmann, D.; Lyons, S.F.; Suchy, H. The Use of Cultured Schwann Cells in Nerve Repair in a Rabbit Hind-Limb Model. J. Reconstr. Microsurg. 1996, 12, 149–152. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Kakinoki, R.; Ikeguchi, R.; Ohta, S.; Noguchi, T.; Oda, H.; Matsuda, S. Bridging a 30 mm defect in the canine ulnar nerve using vessel-containing conduits with implantation of bone marrow stromal cells. Microsurgery 2016, 36, 316–324. [Google Scholar] [CrossRef]
- Muheremu, A.; Chen, L.; Wang, X.; Wei, Y.; Gong, K.; Ao, Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci. Rep. 2017, 7, 44002. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, Y.; Zhao, Y.; Xiao, Z.; Cao, Z.; Han, S.; Li, X.; Huan, Y.; Pan, J.; Dai, J. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J. Tissue Eng. Regen. Med. 2018, 12, 1285–1296. [Google Scholar] [CrossRef]
- Hu, N.; Wu, H.; Xue, C.; Gong, Y.; Wu, J.; Xiao, Z. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 2013, 34, 100–111. [Google Scholar] [CrossRef]
- Ding, F.; Wu, J.; Yang, Y. Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic- co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng. Part. A. 2010, 16, 3779–3790. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Hu, N.; Gu, Y.; Yang, Y.; Liu, Y.; Liu, J.; Gu, X. Joint use of a chitosan/PLGA scaffold and MSCs to bridge an extra large gap in dog sciatic nerve. Neurorehabilit. Neural Repair 2012, 26, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ren, H.; Zhu, H.; Gu, X.; Guo, Q.; Zhou, Y.; Yang, Y. Bone marrow mesenchymal stem cell-derived acellular matrix-coated chitosan/silk scaffolds for neural tissue regeneration. J. Mater. Chem. B 2017, 5, 1246–1257. [Google Scholar] [CrossRef]
- Sahar, M.S.U.; Barton, M.; Tansley, G.D. Bridging larger gaps in peripheral nerves using neural prosthetics and physical therapeutic agents. Neural Regen. Res. 2019, 14, 1109. [Google Scholar] [CrossRef]
- Babu, P.; Behl, A.; Chakravarty, B.; Bhandari, P.S. Entubulation techniques in peripheral nerve repair. Indian J. Neurotrauma 2008, 5, 15–20. [Google Scholar] [CrossRef]
- Mantovani, C.; Terenghi, G.; Shawcross, S.G. Isolation of Adult Stem Cells and Their Differentiation to Schwann Cells; Humana Press: Totowa, NJ, USA, 2012; pp. 47–57. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef]
- Kolar, M.K.; Kingham, P.J. Regenerative effects of adipose-tissue-derived stem cells for treatment of peripheral nerve injuries. Biochem. Soc. Trans. 2014, 42, 697–701. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Frattini, F.; Marques, S.A.; de Almeida, F.M.; de Moura Campos, L.C.; Langone, F.; Martinez, A.M.B. Transplantation of bone-marrow-derived cells into a nerve guide resulted in transdifferentiation into Schwann cells and effective regeneration of transected mouse sciatic nerve. Micron 2010, 41, 783–790. [Google Scholar] [CrossRef]
- Ide, C. Peripheral nerve regeneration. Neurosci. Res. 1996, 25, 101–121. [Google Scholar] [CrossRef]
- Levi, A.; Guenard, V.; Aebischer, P.; Bunge, R.P. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J. Neurosci. 1994, 14, 1309–1319. [Google Scholar] [CrossRef]
- Gulati, A.K. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J. Neurosurg. 1988, 68, 117–123. [Google Scholar] [CrossRef]
- Wrobel, S.; Morano, M.; Meyer, C.; Shahar, A.; Ziv, O.; Haastert-Talini, K.; Grothe, C. Development of cell-enhanced chitosan scaffolds to overcome long gaps after peripheral nerve injury. Cytotherapy 2014, 16, S102. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-K.; Lee, J.-H. Peripheral nerve regeneration using a three dimensionally cultured schwann cell conduit. J. Craniofacial Surg. 2007, 18, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.H.A.; Haycock, J.W. Read: Next Generation Nerve Guides: Materials, Fabrication, Growth Factors, and Cell Delivery. Tissue Eng. Part. B Rev. 2012, 18, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.L.; Radtke, C. Co-culturing human adipose derived stem cells and schwann cells on spider silk—A new approach as prerequisite for enhanced nerve regeneration. Int. J. Mol. Sci. 2019, 20, 71. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-B.; Cheng, Y.X.; Feng, Y.K.; Pang, C.J.; Li, Q.; Wang, Y. Adipose-derived stem cells promote peripheral nerve repair. Arch. Med. Sci. 2011, 7, 592–596. [Google Scholar] [CrossRef]
- Peng, J.; Feng, X.Y.; Cui, B.L.; Law, F.; Jiang, X.W.; Yang, L.Y.; Huang, T.H. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res. Bull. 2011, 84, 235–243. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Sun, X.; Xu, X.L.; Zhao, Q.; Peng, J.; Wang, Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen. Res. 2015, 10, 651. [Google Scholar] [CrossRef]
- Xiao, B.; Rao, F.; Guo, Z.Y.; Sun, X.; Wang, Y.G.; Liu, S.Y.; Peng, J. Extracellular matrix from human umbilical cord-derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration. Neural Regen. Res. 2016, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Su, C.F.; Chang, L.H.; Kao, C.Y.; Lee, D.C.; Cho, K.H.; Kuo, L.W.; Chiu, M. Application of amniotic fluid stem cells in repairing sciatic nerve injury in minipigs. Brain Res. 2018, 1678, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Laroni, A.; Freedman, M.S. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011, 649–656. [Google Scholar] [CrossRef]
- António, J.B.O.G.S.; RuI, L.G.R.; Nuno, J.C.S.; Jeffrey, M.G. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem. Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Liu, J.; Zhang, L.; Zhang, J.; Tang, P. Repairing sciatic nerve injury with an EPO-loaded nerve conduit and sandwiched-in strategy of transplanting mesenchymal stem cells. Biomaterials 2017, 142, 90–100. [Google Scholar] [CrossRef]
- You, H.; Wei, L.; Liu, Y.; Oudega, M.; Jiao, S.S.; Feng, S.N.; Li, B.C. Olfactory ensheathing cells enhance Schwann cell-mediated anatomical and functional repair after sciatic nerve injury in adult rats. Exp. Neurol. 2011, 229, 158–167. [Google Scholar] [CrossRef]
- Lopatina, T.; Kalinina, N.; Karagyaur, M.; Stambolsky, D.; Rubina, K.; Revischin, A.; Tkachuk, V. Adipose-Derived Stem Cells Stimulate Regeneration of Peripheral Nerves: BDNF Secreted by These Cells Promotes Nerve Healing and Axon Growth De Novo. PLoS ONE 2011, 6, e17899. [Google Scholar] [CrossRef]
- Boecker, A.H.; Bozkurt, A.; Kim, B.S.; Altinova, H.; Tank, J.; Deumens, R.; Tolba, R.; Weis, J.; Brook, G.A.; Pallua, N.; et al. Cell-enrichment with olfactory ensheathing cells has limited local extra beneficial effects on nerve regeneration supported by the nerve guide Perimaix. J. Tissue Eng. Regen. Med. 2018, 12, 2125–2137. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef]
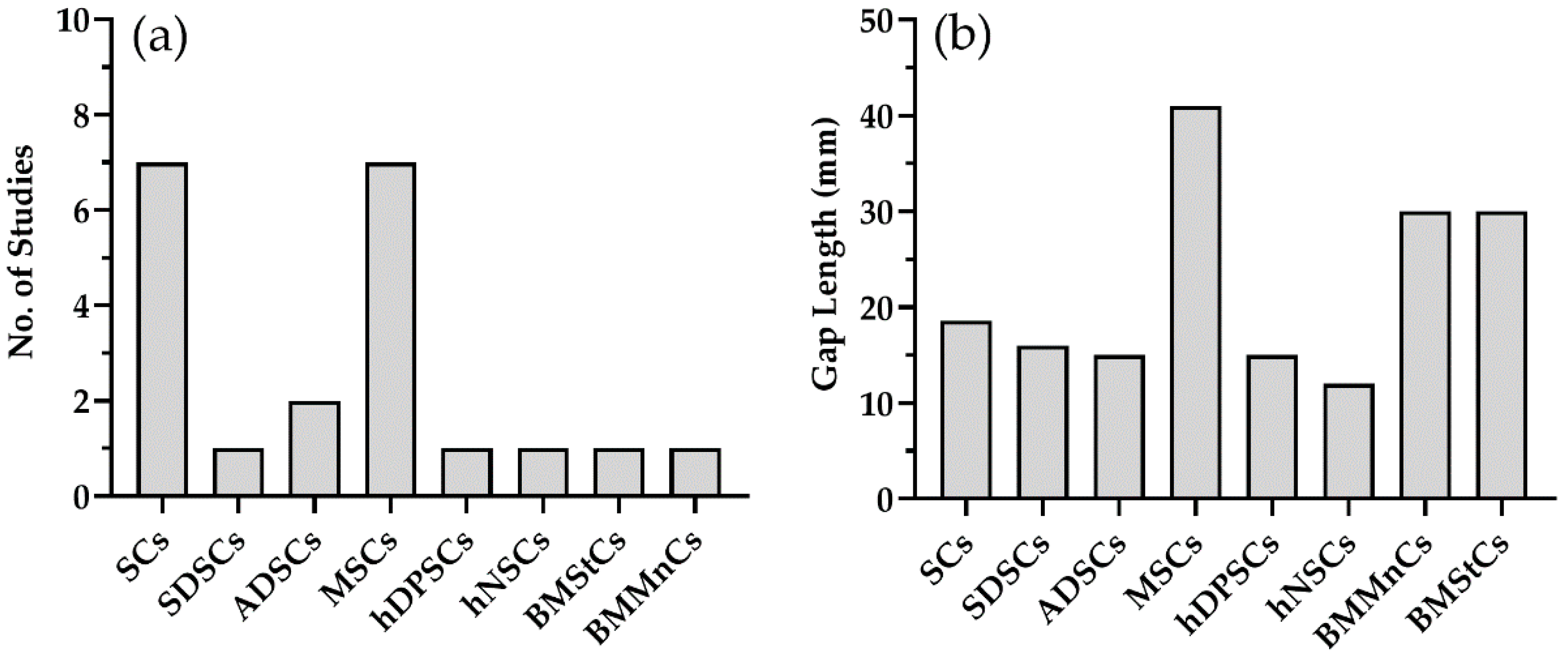
| Study No. | Cell Type | Mode of Cell Delivery | Gap (mm) | Animal Model | Assessment of Nerve Regeneration | Research Findings | ||
|---|---|---|---|---|---|---|---|---|
| Subject | Groups | Histological Assessments | Functional Assessments | |||||
| 1 * | Human neural stem cells | Collagen prosthesis with an inner lining of engineered neural tissues (d-neural stem cells + collagen). | 12 | Rat sciatic nerve | G1: Autograft G2: Treatment G3: Prosthesis alone | Two months No. of Axons G1: 5000, G2: 4300, G3: 5200 Axonal diameter G1: 0.9, G2: 1.2, G3: 0 Myelin thickness G1: 0.63, G2: 0.57, G3: 0 No. of blood vessels G1: 18, G2: 23, G3: 0 Gastrocnemius weight (% of control) G1: 42, G2: 25, G3: 17 | Two months CMAP (gastrocnemius muscle) G1: 2.5, G2: 7.5, G3: 3.7 | Enhanced growth of neurites and vasculature along with reinnervation of the target muscle [3]. |
| 2 | MSCs | Injection of d-MSCs into the lumen of the collagen prosthesis | 12 | Rat sciatic nerve | G1: Reverse autograft G2: Prosthesis with ud-MSCs G3: Prosthesis with d-MSCs G4: Prosthesis with SCs G5: Prosthesis alone | Three months No. of FG-labeled motoneurons G1: 1310, G2: 430, G3: 605, G4: 800, G5: 300 | Three months CMAP G1: 2.2, G2: 1.3, G3: 1.2, G4: 1.2, G5: 1.15, Control (healthy): 2.5 | Sufficiently supported limited axonal regeneration that was comparable to implant repair using SCs [4]. |
| 3 | SCs | Injection of SCs in collagen gel into the lumen of the PLLA prosthesis | 12 | Rat sciatic nerve | G1: Reverse autograft G2: Prosthesis with 1 × 104 SC/mL G3: Prosthesis with 1 × 106 SC/mL G4: Prosthesis with gel only G5: Silicone prosthesis | Four months No. of Axons G1: 23,000, G2: 25,000, G3: 17,000, G4: 27,000, G5: 30,000 Gastrocnemius muscle weight G1: 1.5, G2: 1.25, G3: 1.1, G4: 1.2, G5: 1.1 | Four months Sciatic function index score G1: 86.90 G2: 84.74 G3: 89.92 G4: 83.74 G5: 74.43 | Regeneration in all groups with no significant difference between groups [5]. |
| 4 | d-ADSCs | Sheet developed from ADSCs in collagen gel was placed inside Neura-Wrap™ | 15 | Rat sciatic nerve | G1: Autograft G2: Treatment G3: NeuraWrap alone | Two months Myelin thickness G1 and G3: Lower in G3 compared to the G1; G1 and G2: No difference | Not tested | Axonal regeneration in the distal stump was 3.5 times greater in G2 than G3 [6]. |
| 5 | d- human dental pulp stem cells | Sheets of stem cells in the Neura-Wrap™ prosthesis | 15 | Rat sciatic nerve | G1: Allograft G2: Treatment G3: Prosthesis alone | Two months No. of Axons G1: ≈10,000, G2: ≈1000, G3: ≈800 No. of blood vessels G1: 260, G2: 250, G3: 100 | Not tested | Positive effect on the regeneration of nerve tissue in vivo [7]. |
| 6 | ADSCs | ADSCs mixed in poloxamer and injected into the lumen of the PCL prosthesis | 15 | Rat sciatic nerve | G1: Reverse autograft G2: Treatment G3: Prosthesis with hydrogel G4: Prosthesis with ADSCs G5: Prosthesis only | One and a half months Axonal growth G2: 8.23, G3: 7.1, G4: 6, G5: 5.9 Gastrocnemius weight ratio G1–G6: 0.29 ± 0.06 | Not tested | ADSCs in poloxamer hydrogel could proliferate better and demonstrated the longest axonal regrowth [8]. |
| 7 | SCs | PFTBA hydrogel mixed with SCs and injected into the lumen of a collagen-chitosan prosthesis | 15 | Rat sciatic nerve | G1: Autograft G2: Treatment G3: SCs only G4: Hydrogel only G5: Prosthesis alone | Three months No. of Axons G1: 1.4, G2: 1.3, G3: 1.0, G4: 1.1, G5: 0.6 (×104) Axonal diameter G1: 3.8, G2: 3.85, G3: 2.5, G4: 2.55, G5: 1.7 FG-labeled motoneurons G1: 530, G2: 505, G3: 415, G4: 410, G5: 340 | Three months SFI score G1: −37, G2: −40, G3: −51, G4: −53, G5: −68 NCV G1: 23, G2: 22, G3: 18, G4: 19, G5: 15 | Prosthesis containing SCs in PFTBA hydrogel helped with SCs proliferation and the formation of vascular networks [9]. |
| 8 | SCs | MSCs or SCs in collagen-fibronectin-based gels and placed inside the chitosan prosthesis | 15 | Rat sciatic nerve | G1: Autograft G2: Collagen- fibronectin and MSCs G3: Collagen- fibronectin and SCs G4: Fibronectin prosthesis alone | Four months No. of Axons G1: 14,409, G2: 6192, G3: 7656, G4: 3105 | Four months CMAP of gastrocnemius G1: 60, G2: 34, G3: 54, G4: 35 | SC-seeded chitosan prosthesis is a potential alternative to the autograft for repairing long-gap peripheral nerve injuries [10]. |
| 9 | SDSCs | SDSCs labeled with GFP placed inside PLA-TMC or collagen prosthesis | 16 | Rat sciatic nerve | G1: PLA-TMC prosthesis + SdSCs G2: PLA-TMC prosthesis + PBS G3: Collagen prosthesis + SdSCs G4: Collagen prosthesis + PBS | Three months No. of Axons G1: 780, G3: 1125 Axonal diameter G1: 2.8, G3: 3.3, Contralateral nerve: 6.7 Density of myelinated axons G1: 3661, G2: 2361, G3: 3835, G4: 2779 | Three months SFI score G1: −75, G2: −82, G3: −69.4, G4: −76 NCV G1: 14.71, G3: 16.51, Control: 22.6 | Collagen prosthesis yielded significantly better results than the PLA-TMC prosthesis [11]. |
| 10 | SCs | Injection of SCs into the lumen of a collage prosthesis | 18 | Rat sciatic nerve | G1: Prosthesis with >0.5 × 106 SCs G2: Prosthesis <0.5 × 106 SCs G3: Prosthesis with PBS | Six months No. of myelinated axons G1: 13,000, G2: 4800, G3: 0, Normal nerve: 6000 | Twelve months SFI G1: −70, G2: −80, G3: 0 NCV operated side G1: 29, G2: 28, G3: 0 | Increasing the number of cells in the guides had a greater beneficial effect [12]. |
| 11 | SCs | Injection of SCs in matrigel into the lumen of a TMC/CL prosthesis | 20 | Rat median nerve | G1: Reverse autograft G2: Treatment G3: Prosthesis alone | Nine months No. of Axons G1: 3453, G2: 4402, G3: 0, Normal: 2361 Myelin thickness G1: 1.086, G2: 0.954, G3: 0, Normal: 1.631 | Nine months CMAP G1, G2: No difference G3: 0 | Functional recovery comparable to autograft repair [13]. |
| 12 | MSCs | Induction of d-MSCs into PTFE prosthesis | 20 | Monkey median nerve | G1: Treatment G2: Prosthesis alone | Twelve months NF-positive area/total nerve area G1: 51.5, G2: 33.3 | Twelve months CMAP G1: 7.5, G2: 2.1 NCV G1: 13.5, G2: 10.2, Control: 18 | Transplantation of stem cells was helpful for nerve regeneration [14]. |
| 13 | SCs | Injection of SCs into the lumen of a polyglactin prosthesis | 20 | Rabbit sciatic nerve | G1: Treatment G2: Autograft | Two months No. of Axons G1: 900, G2: 1102 | Not tested | Axonal regeneration was observed, even in the distal nerve stump [15]. |
| 14 | SCs | SCs mixed in gelatin and pipetted into the lumen of the PGA prosthesis | 30 | Rabbit peroneal nerve | G1: Treatment G2: Prosthesis with gelatine only | Four months Scoring of myelinations (3—good, 2—fair, 1—poor, P—proximal, M—middle, D—distal) G1: 2.7 P, 1.5 M, 1.0 D G2: 3.0 P, 1.7 M, 1.4 D | Four months NCV (% of the NCV of the CUiS) G1: 94.7, G2: 96.5 | Local initial effect on nerve regeneration; a gap of 30 mm was not enough to observe a significant difference [16]. |
| 15 * | ud-BMStCs | Injection of autologous ud-BMSCs into the lumen of a PLCL prosthesis | 30 | Dog ulnar nerve | G1: Reverse autograft G2: Prosthesis with ud-BMSCs | Six months No. of Axons G1: 7032, G2: 7165 Axonal diameter G1: 1.73, G2: 2.09 | Six months CMAP G1: 25.3, G2: 10.9 NCV G1: 23.5, G2: 31.6 | A viable option for the treatment of peripheral nerve injuries [17]. |
| 16 * | BMMnCs | Injection of autologous BmMnCs into the lumen of a chitosan prosthesis | 30 | Goat peroneal nerve | G1: Autograft G2: Prosthesis with BmMnCs G3: Prosthesis with Basal Medium Eagle | Twelve months Axonal diameter G1: 3.60, G2: 3.67, G3: 0, Normal: 6.12 Myelin thickness G1: 0.97, G2: 0.88, G3: 0, Normal: 1.32 | Twelve months NCV G1: 51, G2: 37, G3: 0 | BmMnCs not only helped in bridging longer defects but also induced functional recovery [18]. |
| 17 * | MSCs | Injection of MSCs into the lumen of a collagen prosthesis | 35 | Dog sciatic nerve | G1: Autograft G2: Treatment G3: Prosthesis alone | Nine months Axonal diameter G1: 7.7, G2: 3.6, G3: 2.5 Thickness of neo-fibers G1: 0.75, G2: 0.33, G3: 0.08 Gastrocnemius weight (%) G1: 48, G2: 69, G3: 84, G4: 90 | Nine months CMAP ratio G1: 77, G2: 68, G3: 46 | Axonal regeneration and improved functional recovery [19]. |
| 18 * | MSCs | Injection of an autologous bone marrow MSC suspension in a chitosan/PLGA prosthesis | 50 | Monkey median nerve | G1: Autograft G2: Treatment G3: Prosthesis alone | Twelve months Axons diameter G1: 5.7, G2: 4.7, G3: 4, Normal: 7.1 Myelin thickness G1: 1.1, G2: 0.8, G3: 0.6, Normal: 1.7 FG-labeled motoneurons G1: 15,800, G2: 15,700, G3: 15,050, Normal: 17,500 | Twelve months CMAP G1: 6.8, G2: 3.9, G3: 3.8, Normal: 12.1 NCV G1: 30, G2: 19, G3: 13, Normal: 87 | Repair similar to autograft repair and better than the repair done using a prosthesis alone [20]. |
| 19 * | MSCs | Injection of an autologous bone marrow MSC suspension in a chitosan/PLGA prosthesis | 50 | Dog sciatic nerve | G1: Reverse autograft G2: Prosthesis alone G3: Treatment | Six months Myelin thickness G1: 1.3, G2: 1.1, G3: 1.4, Normal: 2.4 Density of myelinated fibers G1: 6, G2: 4.1, G3: 5.8, Normal: 11.2 Gastrocnemius muscle weight ratio G1: 0.92, G2: 0.79, G3: 0.86, G4: 0.6 | Six months CMAPG1: 9, G2: 6, G3: 9.6, Normal: 15.8 NCVG1: 41, G2: 23, G3: 39, Normal: 101 | Nerve regeneration and functional recovery comparable to autograft repair [21]. |
| 20 * | MSCs | Injection of an autologous MSC suspension in a chitosan/PLGA prosthesis | 60 | Dog sciatic nerve | G1: Autograft G2: Prosthesis with MSCs G3: Prosthesis alone | Twelve months Axonal diameter G1: 5.2, G2: 4.1, G3: 4.0, CUiS: 8.1 Myelin thickness G1: 1.3, G2: 1.1, G3: 0.8, CUiS: 1.7 Muscle weight ratio Gastronomes G1: 0.82, G2: 0.78, G3: 0.76, G4: 0.25 FG-labeled motoneurons G1: 18,300, G2: 17,500, G3: 15,000, CUiS: 21,000 | Twelve months CMAP G1: 10, G2: 6.2, G3: 5.5, CUiN: 14 NCV G1: 36, G2: 25, G3: 21, CUiN: 90 | Repair outcomes were similar to the autograft repair and better than those of prosthesis alone [22]. |
| 21 * | MSCs | Injection of bone-marrow-derived mesenchymal stem cells into the lumen of a chitosan/silk prosthesis | 60 | Dog sciatic nerve | G1: Reverse autograft G2: Prosthesis with BMSCs | Twelve months Axonal diameter G1: 6.2, G2: 6.1, CUiS: 10.1 Myelin thickness G1: 1.3, G2: 1.2, CUiS: 2.2 FG-labeled motoneurons G1: 14,000, G2: 13,000, CUiS: 21,000 Muscle weight ratio (%) Gastronomes G1: 67, G2: 43, G3: 25 | Twelve months CMAP G1: 11, G2: 9, CUiS: 23 NCV G1: 34, G2: 33, CUiS: 86 | Repair outcomes of the treatment group were comparable to autograft repair [23]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahar, M.S.U.; Barton, M.; Tansley, G. A Systematic Review of the Effectiveness of Cell-Based Therapy in Repairing Peripheral Nerve Gap Defects. Prosthesis 2020, 2, 153-167. https://doi.org/10.3390/prosthesis2030014
Sahar MSU, Barton M, Tansley G. A Systematic Review of the Effectiveness of Cell-Based Therapy in Repairing Peripheral Nerve Gap Defects. Prosthesis. 2020; 2(3):153-167. https://doi.org/10.3390/prosthesis2030014
Chicago/Turabian StyleSahar, Muhammad Sana Ullah, Matthew Barton, and Geoffrey Tansley. 2020. "A Systematic Review of the Effectiveness of Cell-Based Therapy in Repairing Peripheral Nerve Gap Defects" Prosthesis 2, no. 3: 153-167. https://doi.org/10.3390/prosthesis2030014
APA StyleSahar, M. S. U., Barton, M., & Tansley, G. (2020). A Systematic Review of the Effectiveness of Cell-Based Therapy in Repairing Peripheral Nerve Gap Defects. Prosthesis, 2(3), 153-167. https://doi.org/10.3390/prosthesis2030014






