Research Progress on Methane Emission Reduction Strategies for Dairy Cows
Abstract
1. Introduction
2. Mechanism of CH4 Production in the Gastrointestinal Tract of Dairy Cows
2.1. Relationship Between Rumen Microorganisms and CH4 Production in Dairy Cows
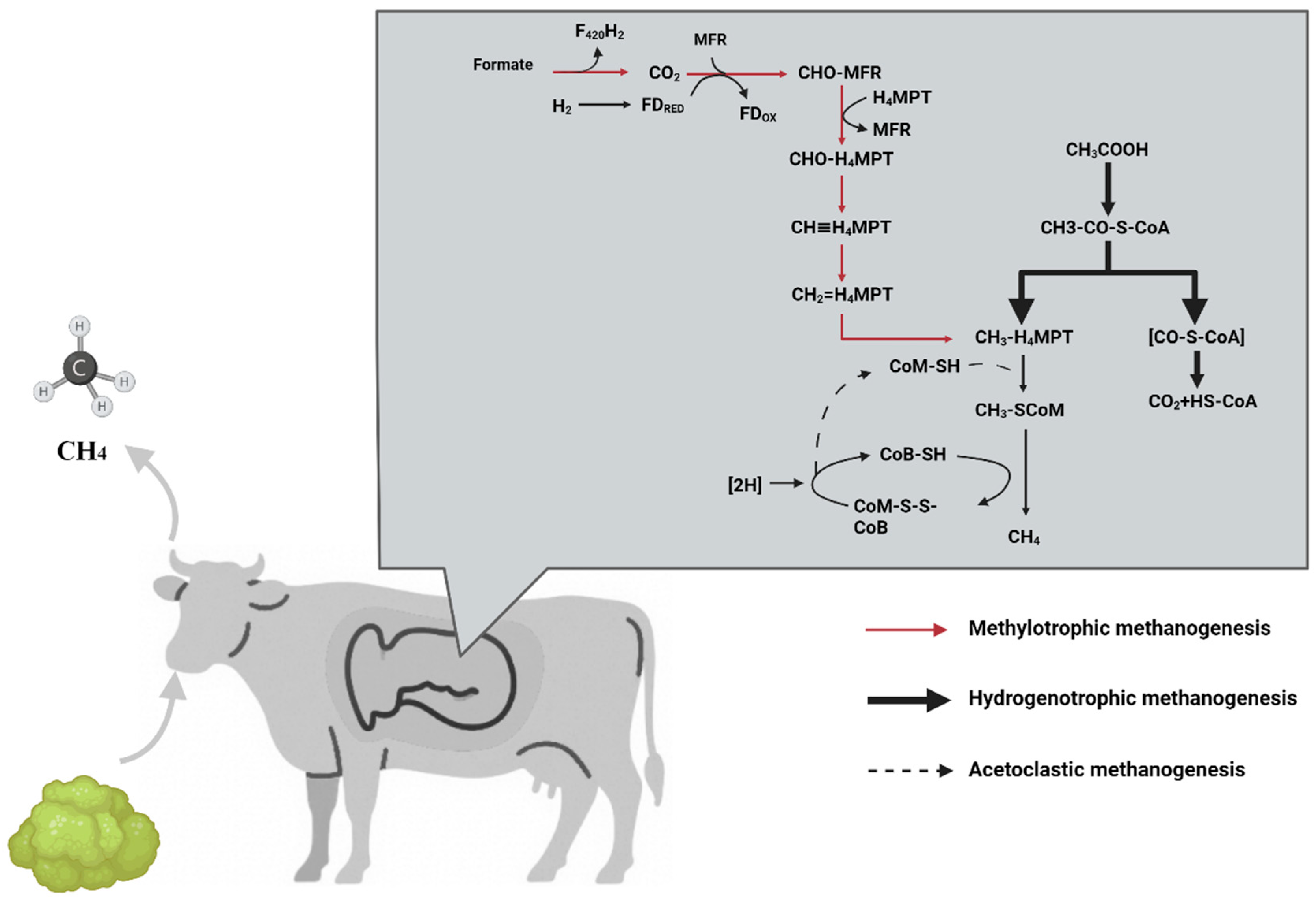
2.2. Process of CH4 Production in the Rumen of Dairy Cows
3. Factors Affecting CH4 Production in Dairy Cows
3.1. Genetic Factors
3.2. Diet Quality
3.3. Growth Stage and Lactation Stage
3.4. Environment
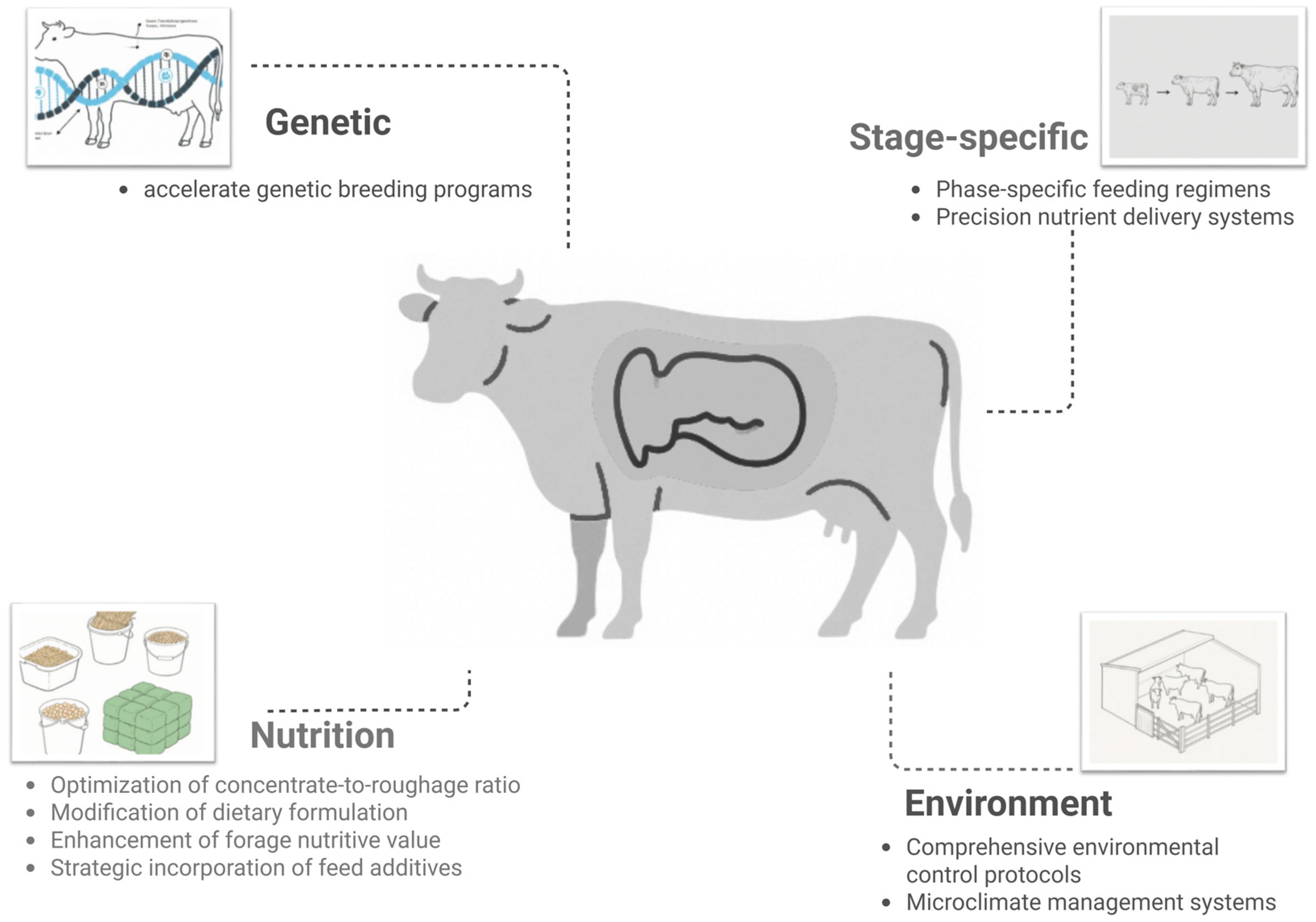
4. CH4 Emission Reduction Strategies for Dairy Cows
4.1. Accelerating Genetic Breeding
4.2. Improving Diet Composition
4.2.1. Adjusting the Ratio of Concentrate to Roughage
4.2.2. Changing Diet Type
4.2.3. Improving the Quality of Forage Grass
4.2.4. Adding Feed Additives
Nitrate
3-Nitroxypropanol
Organic Acid
Secondary Metabolites of Plants
Probiotics
Algae
Melatonin
Grease
4.3. Optimizing Feeding Management
4.3.1. Environmental Control
4.3.2. Precision Feeding
4.4. Improving Stool Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, I.H.; Manzoor, M.A.; Jinhui, W.; Li, X.; Hameed, M.K.; Rehaman, A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Comprehensive review: Effects of climate change and greenhouse gases emission relevance to environmental stress on horticultural crops and management. J. Environ. Manag. 2024, 351, 119978. [Google Scholar] [CrossRef]
- Akinbi, G.O.; Ngatia, L.W.; Grace, J.M.; Fu, R.; Tan, C.; Olaborode, S.O.; Abichou, T.; Taylor, R.W. Organic matter composition and thermal stability influence greenhouse gases production in subtropical peatland under different vegetation types. Heliyon 2022, 8, e11547. [Google Scholar] [CrossRef]
- Skytt, T.; Nielsen, S.N.; Jonsson, B. Global warming potential and absolute global temperature change potential from carbon dioxide and methane fluxes as indicators of regional sustainability—A case study of Jämtland, Sweden. Ecol. Indic. 2020, 110, 105831. [Google Scholar] [CrossRef]
- Bansal, K.; Tripathi, A.K. An explainable MHSA enabled deep architecture with dual-scale convolutions for methane source classification using remote sensing. Environ. Model. Softw. 2024, 181, 106178. [Google Scholar] [CrossRef]
- FAO. Methane Emissions in Livestock and Rice Systems—Sources, Quantification, Mitigation and Metrics; FAO: Rome, Italy, 2023. [Google Scholar]
- Graeme, A.; Christopher, M. Methanogen genomics to discover targets for methane mitigation technologies and options for alternative H2 utilisation in the rumen. Aust. J. Exp. Agric. 2008, 48, 28–37. [Google Scholar] [CrossRef]
- Crowley, S.B.; Purfield, D.C.; Conroy, S.B.; Kelly, D.N.; Evans, R.D.; Ryan, C.V.; Berry, D.P. Associations between a range of enteric methane emission traits and performance traits in indoor-fed growing cattle. J. Anim. Sci. 2024, 102, skae346. [Google Scholar] [CrossRef]
- FAO. Pathways Towards Lower Emissions—A Global Assessment of the Greenhouse Gas Emissions and Mitigation Options from Livestock Agrifood Systems; FAO: Rome, Italy, 2023. [Google Scholar]
- Kumari, S.; Fagodiya, R.K.; Hiloidhari, M.; Dahiya, R.P.; Kumar, A. Methane production and estimation from livestock husbandry: A mechanistic understanding and emerging mitigation options. Sci. Total Environ. 2020, 709, 136135. [Google Scholar] [CrossRef]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Wei, S.; Wang, W.; Lin, X. Windrow composting mitigated CH4 emissions: Characterization of methanogenic and methanotrophic communities in manure management. FEMS Microbiol. Ecol. 2014, 90, 575–586. [Google Scholar] [CrossRef]
- Cunha, C.S.; Veloso, C.M.; Marcondes, M.I.; Mantovani, H.C.; Tomich, T.R.; Pereira, L.G.R.; Ferreira, M.F.L.; Dill-McFarland, K.A.; Suen, G. Assessing the impact of rumen microbial communities on methane emissions and production traits in holstein cows in a tropical climate. Syst. Appl. Microbiol. 2017, 40, 492–499. [Google Scholar] [CrossRef]
- Rebecca, D.; Johan, D.; Li, S.; Horacio, G.; Bettina, M.; Anna, S.; Jan, B. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef]
- Pitta, D.; Indugu, N.; Narayan, K.; Hennessy, M. Symposium review: Understanding the role of the rumen microbiome in enteric methane mitigation and productivity in dairy cows. J. Dairy Sci. 2022, 105, 8569–8585. [Google Scholar] [CrossRef]
- Plaizier Jan, C.; Allan, K.; Khafipour, K. 400 relationship between fecal microbiota and enteral methane emissions of dairy cows. J. Anim. Sci. 2019, 97, 158. [Google Scholar] [CrossRef]
- Ripoll, E.; López, I.; Borzacconi, L. Hydrogenotrophic activity: A tool to evaluate the kinetics of methanogens. J. Environ. Manag. 2020, 270, 110937. [Google Scholar] [CrossRef]
- Chang, H.; Du, B.; He, K.; Yin, Q.; Wu, G. Mechanistic understanding of acclimation and energy metabolism of acetoclastic methanogens under different substrate to microorganism ratios. Environ. Res. 2024, 252, 118911. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, G.; Li, M.M. Using nutritional strategies to mitigate ruminal methane emissions from ruminants. Front. Agric. Sci. Eng. 2023, 10, 390–402. [Google Scholar] [CrossRef]
- Dhakal, R.; Neves, A.L.A.; Sapkota, R.; Khanal, P.; Ellegaard-Jensen, L.; Winding, A.; Hansen, H.H. Investigating dose-dependent effects of chemical compounds targeting rumen fermentation pathways using an in-vitro rumen fermentation system. BMC Microbiol. 2025, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- ManzanillaPech, C.I.V.; Difford, G.F.; Sahana, G.; Romé, H.; Løvendahl, P.; Lassen, J. Genome-wide association study for methane emission traits in Danish Holstein cattle. J. Dairy Sci. 2021, 105, 1357–1368. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Difford, G.F.; Bell, M.J.; Bayat, A.R.; Huhtanen, P.; Kuhla, B.; Lassen, J.; Peiren, N.; Pszczola, M.; Sorg, D.; et al. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals 2019, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Zetouni, L.; Kargo, M.; Norberg, E.; Lassen, J. Genetic correlations between methane production and fertility, health, and body type traits in Danish Holstein cows. J. Dairy Sci. 2018, 101, 2273–2280. [Google Scholar] [CrossRef]
- Breider, I.S.; Wall, E.; Garnsworthy, P.C. Short communication: Heritability of methane production and genetic correlations with milk yield and body weight in Holstein-Friesian dairy cows. J. Dairy Sci. 2019, 102, 7277–7281. [Google Scholar] [CrossRef]
- Lassen, J.; Difford, G.F. Review: Genetic and genomic selection as a methane mitigation strategy in dairy cattle. Animal 2020, 14, s473–s483. [Google Scholar] [CrossRef] [PubMed]
- Difford, G.F.; Plichta, D.R.; Løvendahl, P.; Lassen, J.; Noel, S.J.; Højberg, O.; Wright, A.D.G.; Zhu, Z.; Kristensen, L.; Nielsen, H.B.; et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018, 14, e1007580. [Google Scholar] [CrossRef]
- Dong, L.; Jia, P.; Li, B.; Wang, B.; Yang, C.; Liu, Z.; Diao, Q. Quantification and prediction of enteric methane emissions from Chinese lactating Holstein dairy cows fed diets with different dietary neutral detergent fiber/non-fibrous carbohydrate (NDF/NFC) ratios. J. Integr. Agric. 2022, 21, 797–811. [Google Scholar] [CrossRef]
- Min, B.R.; Yutaka, U.; Ismael, H.; Abdo, H.; Chaudhary, S.; Hilaire, M.; Kanyi, V. Malted barley as a potential feed supplementation for the reduction of enteric methane emissions, rumen digestibility, and microbiome community changes in laboratory conditions. Animals 2025, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Ormston, S.; Yan, T.; Chen, X.; Gordon, A.W.; Theodoridou, K.; Huws, S.; Stergiadis, S. Impact of dietary forage proportion and crossbreeding on feed efficiency and methane emissions in lactating dairy cows. Anim. Nutr. 2025, 20, 419–429. [Google Scholar] [CrossRef]
- Hammond, K.J.; Jones, A.K.; Humphries, D.J.; Crompton, L.A.; Reynolds, C.K. Effects of diet forage source and neutral detergent fiber content on milk production of dairy cattle and methane emissions determined using GreenFeed and respiration chamber techniques. J. Dairy Sci. 2016, 99, 7904–7917. [Google Scholar] [CrossRef]
- Åby, B.A.; Randby, Å.T.; Bonesmo, H.; Aass, L. Impact of grass silage quality on greenhouse gas emissions from dairy and beef production. Grass Forage Sci. 2019, 74, 525–534. [Google Scholar] [CrossRef]
- Grandl, F.; Amelchanka, S.L.; Furger, M.; Clauss, M.; Zeitz, J.O.; Kreuzer, M.; Schwarm, A. Biological implications of longevity in dairy cows: 2. Changes in methane emissions and efficiency with age. J. Dairy Sci. 2016, 99, 3472–3485. [Google Scholar] [CrossRef]
- Dall-Orsoletta, A.C.; Leurent-Colette, S.; Launay, F.; Ribeiro-Filho, H.M.N.; Delaby, L. A quantitative description of the effect of breed, first calving age and feeding strategy on dairy systems enteric methane emission. Livest. Sci. 2019, 224, 87–95. [Google Scholar] [CrossRef]
- Fresco, S.; Boichard, D.; Lefebvre, R.; Barbey, S.; Gaborit, M.; Fritz, S.; Martin, P. Short communication: Correlation of methane production, intensity, and yield with residual feed intake throughout lactation in Holstein cows. Animal 2024, 18, 101110. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.; Bielak, A.; Doyle, E.; Kuhla, B. Variations in methane yield and microbial community profiles in the rumen of dairy cows as they pass through stages of first lactation. J. Dairy Sci. 2018, 101, 5102–5114. [Google Scholar] [CrossRef]
- Kumar, M.D.; Bhakat, C.; Dutta, T.K. Impact of environmental factors on physiological adaptability, thermo-tolerance indices, and productivity in jersey crossbred cows. Int. J. Biometeorol. 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Souza, V.C.; Baumgard, M.E.L.; Santos, L.H.; Mueller, J.E.P.; Rhoads, N.D.; Kebreab, E. Modeling the effects of heat stress in animal performance and enteric methane emissions in lactating dairy cows. J. Dairy Sci. 2023, 106, 4725–4737. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Mahmood, M.; Lerch, F.; Traintinger, F.P.; Petri, R.M.; Münnich, M.; Zebeli, Q. Physicochemical stressors and mixed alkaloid supplementation modulate ruminal microbiota and fermentation in vitro. Anaerobe 2020, 65, 102263. [Google Scholar] [CrossRef]
- Chen, L.; Thorup, V.M.; Østergaard, S. Modeling the effects of heat stress on production and enteric methane emission in high-yielding dairy herds. J. Dairy Sci. 2025, 108, 3956–3964. [Google Scholar] [CrossRef]
- Duarte Andrea, C.; Holman Devin, B.; Alexander Trevor, W.; Kerstin, K.; Gerhard, B.; Chaves Alexandre, V. Incubation temperature, but not pequi oil supplementation, affects methane production, and the ruminal microbiota in a rumen simulation technique (rusitec) system. Front. Microbiol. 2017, 8, 1076. [Google Scholar] [CrossRef]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global warming and dairy cattle: How to control and reduce methane emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef]
- Schmithausen, A.J.; Schiefler, I.; Trimborn, M.; Gerlach, K.; Südekum, K.; Pries, M.; Büscher, W. Quantification of methane and ammonia emissions in a naturally ventilated barn by using defined criteria to calculate emission rates. Animals 2018, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, P.F.; Ferraz, G.A.; Ferreira, J.C.; Aguiar, J.V.; Santana, L.S.; Norton, T. Assessment of ammonia emissions and greenhouse gases in dairy cattle facilities: A bibliometric analysis. Animals 2024, 14, 1721. [Google Scholar] [CrossRef]
- González-Recio, O.; López-Paredes, J.; Ouatahar, L.; Charfeddine, N.; Ugarte, E.; Alenda, R.; Jiménez-Montero, J.A. Mitigation of greenhouse gases in dairy cattle via genetic selection: 2. Incorporating methane emissions into the breeding goal. J. Dairy Sci. 2020, 103, 7210–7221. [Google Scholar] [CrossRef]
- Shi, R.; Wang, Y.; van Middelaar, C.E.; Ducro, B.; Oosting, S.J.; Hou, Y.; Wang, Y.; van der Linden, A. Balancing farm profit and greenhouse gas emissions along the dairy production chain through breeding indices. J. Clean. Prod. 2024, 451, 142099. [Google Scholar] [CrossRef]
- Pszczola, M.; Calus, M.P.L.; Strabel, T. Short communication: Genetic correlations between methane and milk production, conformation, and functional traits. J. Dairy Sci. 2019, 102, 5342–5346. [Google Scholar] [CrossRef]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 15. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Noel, S.J.; Lund, P.; Larsen, M.; Weisbjerg, M.R.; Børsting, C.F. Feeding up to 91% concentrate to holstein and jersey dairy cows: Effects on enteric methane emission, rumen fermentation and bacterial community, digestibility, production, and feeding behavior. J. Dairy Sci. 2022, 105, 9523–9541. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, M.H.; de Evan Rozada, T.; Noel, S.J.; Schönherz, A.; Hellwing, A.L.F.; Lund, P.; Weisbjerg, M.R. Phenotypic traits related to methane emissions from holstein dairy cows challenged by low or high forage proportion. J. Dairy Sci. 2024, 107, 10787–10810. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Akter, A.; Li, X.; Grey, E.; Wang, S.C.; Kebreab, E. Grape pomace supplementation reduced methane emissions and improved milk quality in lactating dairy cows. J. Dairy Sci. 2024, 108, 2468–2480. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Wattiaux, M.A.; Powell, J.M.; Broderick, G.A.; Arndt, C. Effect of forage-to-concentrate ratio in dairy cow diets on emission of methane, carbon dioxide, and ammonia, lactation performance, and manure excretion. J. Dairy Sci. 2011, 94, 3081–3093. [Google Scholar] [CrossRef]
- Culbertson, R.L.; Oviedo, F.A.G.; Uzun, P.; Seneviratne, N.; Fontoura, A.B.P.; Yau, B.K.; Judge, J.L.; Davis, A.N.; Reyes, D.C.; McFadden, J.W. Effects of dietary starch concentration on milk production, nutrient digestibility, and methane emissions in mid-lactation dairy cows. Agriculture 2025, 15, 211. [Google Scholar] [CrossRef]
- Gamonmas, D.; Sawitree, W.; Rittikeard, P.; Anusorn, C. The effects of fermented cassava pulp with yeast waste and different roughage-to-concentrate ratios on ruminal fermentation, nutrient digestibility, and milk production in lactating cows. Heliyon 2023, 9, e14585. [Google Scholar]
- Zhang, R.; Liu, J.; Jiang, L.; Mao, S. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows. Anim. Feed. Sci. Technol. 2020, 269, 114619. [Google Scholar] [CrossRef]
- Della Rosa, M.M.; Sandoval, E.; Reid, P.; Luo, D.; Pacheco, D.; Janssen, P.H.; Jonker, A. Substituting ryegrass-based pasture with graded levels of forage rape in the diet of lambs decreases methane emissions and increases propionate, succinate, and primary alcohols in the rumen. J. Anim. Sci. 2022, 100, skac223. [Google Scholar] [CrossRef]
- Gislon, G.; Colombini, S.; Borreani, G.; Crovetto, G.M.; Sandrucci, A.; Galassi, G.; Tabacco, E.; Rapetti, L. Milk production, methane emissions, nitrogen, and energy balance of cows fed diets based on different forage systems. J. Dairy Sci. 2020, 103, 8048–8061. [Google Scholar] [CrossRef]
- Keller, M.; Scheurer, A.; Reidy, B.; Liesegang, A.; Amelchanka, S.L.; Kreuzer, M.; Giller, K. Nitrogen and energy losses and methane emissions from beef cattle fed diets with gradual replacement of maize silage and concentrate with grass silage and corn-cob mix. Animal 2023, 17, 100722. [Google Scholar] [CrossRef]
- van Gastelen, S.; Jan, D.; Heck Jeroen, M.L.; Maik, K.; Arie, K.; de Mol, R.; Dennis, R.; Nicola, W.; André, B. Methane mitigation potential of 3-nitrooxypropanol in lactating cows is influenced by basal diet composition. J. Dairy Sci. 2022, 105, 4064–4082. [Google Scholar] [CrossRef] [PubMed]
- Weiby, K.V.; Årvik, L.; Eknæs, M.; Schwarm, A.; Steinshamn, H.; Beauchemin, K.A.; Lund, P.; Schei, I.; Nnem, I.D. Milk production and methane emissions from dairy cows fed silages from different grassland species and harvesting frequencies. J. Dairy Sci. 2024, 108, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Della Rosa, M.M.; Sandoval, E.; Luo, D.; Pacheco, D.; Jonker, A. Effect of feeding fresh forage plantain (Plantago lanceolata) or ryegrass-based pasture on methane emissions, total-tract digestibility, and rumen fermentation of nonlactating dairy cows. J. Dairy Sci. 2022, 105, 6628–6638. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Larsen, M.; Weisbjerg, M.R.; Hellwing, A.L.F.; Lund, P. Effect of nitrate supplementation on diurnal emission of enteric methane and nitrous oxide. JDS Commun. 2024, 5, 558–562. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Ungerfeld, E.M.; Zhang, X.M.; Long, D.L.; Mao, H.X.; Deng, J.P.; Bannink, A.; Tan, Z.L. Nitrate improves ammonia incorporation into rumen microbial protein in lactating dairy cows fed a low-protein diet. J. Dairy Sci. 2018, 101, 9789–9799. [Google Scholar] [CrossRef]
- Sheehy, M.R.; Fahey, A.G.; Aungier, S.P.M.; Carter, F.; Crowe, M.A.; Mulligan, F.J. A comparison of serum metabolic and production profiles of dairy cows that maintained or lost body condition 15 days before calving. J. Dairy Sci. 2017, 100, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Wenji, W.; Peter, L.; Mogens, L.; Riis, W.M. Effect of nitrate supplementation, dietary protein supply, and genetic yield index on performance, methane emission, and nitrogen efficiency in dairy cows. J. Dairy Sci. 2023, 106, 5433–5451. [Google Scholar] [CrossRef]
- Black John, L.; Davison Thomas, M.; Ilona, B. Methane emissions from ruminants in Australia: Mitigation potential and applicability of mitigation strategies. Animals 2021, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Beauchemin, K.A.; Dong, R. A review of 3-nitrooxypropanol for enteric methane mitigation from ruminant livestock. Animals 2021, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Sarwono, K.A.; Kondo, M.; Matsui, H.; Ridla, M.; Laconi, E.B.; Nahrowi. Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: A meta-analysis. Ital. J. Anim. Sci. 2018, 17, 650–656. [Google Scholar] [CrossRef]
- Maigaard, M.; Weisbjerg, M.R.; Ohlsson, C.; Walker, N.; Lund, P. Effects of different doses of 3-nitrooxypropanol combined with varying forage composition on feed intake, methane emission, and milk production in dairy cows. J. Dairy Sci. 2024, 108, 2489–2502. [Google Scholar] [CrossRef]
- Matthias, S.; von Soosten, D.; Liane, H.; Ulrich, M.; Annette, Z.; Sven, D. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef]
- Pitta, D.W.; Melgar, A.; Hristov, A.N.; Indugu, N.; Narayan, K.S.; Pappalardo, C.; Hennessy, M.L.; Vecchiarelli, B.; KaplanShabtai, V.; Kindermann, M.; et al. Temporal changes in total and metabolically active ruminal methanogens in dairy cows supplemented with 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 8721–8735. [Google Scholar] [CrossRef]
- Martinez-Fernandez, G.; Duval, S.; Kindermann, M.; Schirra, H.J.; Denman, S.E.; McSweeney, C.S. 3-NOP vs. Halogenated compound: Methane production, ruminal fermentation and microbial community response in forage fed cattle. Front. Microbiol. 2018, 9, 1582. [Google Scholar] [CrossRef]
- Kara, K.; Özkaya, S.; Erbaş, S.; Baytok, E. Effect of dietary formic acid on the in vitro ruminal fermentation parameters of barley-based concentrated mix feed of beef cattle. J. Appl. Anim. Res. 2018, 46, 178–183. [Google Scholar] [CrossRef]
- Partanen, K.; Jalava, T. Effects of some organic acids and salts on microbial fermentation in the digestive tract of piglets estimated using an in vitro gas production technique. Agric. Food Sci. 2008, 14, 311–324. [Google Scholar] [CrossRef]
- Thakur, S.; Dey, A.; Berwal, R.S.; Sihag, S.; Datta, T.K. Malic acid-heat- treatment of proteins reduces methane and nitrogen emissions with improvement in growth, feed efficiency and nutrient utilization in Murrah buffalo (Bubalus bubalis). J. Agric. Food Res. 2025, 19, 101647. [Google Scholar] [CrossRef]
- Yoo, D.; Oh, J.; Jeong, S.; Seo, J. Effects of citric acid and heat-treated soybean meal on rumen fermentation characteristics, methane emissions, and microbiota: An in vitro study. J. Anim. Sci. Technol. 2025, 67, 393–409. [Google Scholar] [CrossRef]
- Lazzari, G.; Münger, A.; Eggerschwiler, L.; Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A.; Schrade, S.; Zähner, M.; Zeyer, K.; Kreuzer, M.; et al. Effects of Acacia mearnsii added to silages differing in nutrient composition and condensed tannins on ruminal and manure-derived methane emissions of dairy cows. J. Dairy Sci. 2023, 106, 6816–6833. [Google Scholar] [CrossRef] [PubMed]
- Sari, N.F.; Kliem, K.E.; Whistance, L.; Smith, J.; Natalello, A.; Christodoulou, C.; Crompton, L.A.; Theodoridou, K.; Ray, P.; Rymer, C.; et al. Tannin variation in tree fodder from temperate climates and implications for methane emissions from enteric fermentation. Anim. Feed. Sci. Technol. 2025, 323, 116299. [Google Scholar] [CrossRef]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Cardozo, P.W.; Kamel, C. Effects of cinnamaldehyde and garlic oil on rumen microbial fermentation in a dual flow continuous culture. J. Dairy Sci. 2005, 88, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.S.H.; Chaudhry, A.S. The effects of garlic as a feed additive on ruminal fermentability and ruminant performance: A meta-analysis. J. Agric. Food Res. 2024, 18, 101531. [Google Scholar] [CrossRef]
- Ruchita, K.; Tassilo, B.; Ilma, T.; AliReza, B. Effect of a garlic and citrus extract supplement on performance, rumen fermentation, methane production, and rumen microbiome of dairy cows. J. Dairy Sci. 2023, 106, 4608–4621. [Google Scholar] [CrossRef]
- Klop, G.; van Laar-van Schuppen, S.; Pellikaan, W.F.; Hendriks, W.H.; Bannink, A.; Dijkstra, J. Changes in in vitro gas and methane production from rumen fluid from dairy cows during adaptation to feed additives in vivo. Animal 2016, 11, 591–599. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F. Diet supplementation with a mixture of essential oils: Effects on enteric methane emissions, apparent total-tract nutrient digestibility, nitrogen utilization, and lactational performance. J. Dairy Sci. 2025, 108, 3560–3572. [Google Scholar] [CrossRef]
- Danielsson, R.; Werner-Omazic, A.; Ramin, M.; Schnürer, A.; Griinari, M.; Dicksved, J.; Bertilsson, J. Effects on enteric methane production and bacterial and archaeal communities by the addition of cashew nut shell extract or glycerol—An in vitro evaluation. J. Dairy Sci. 2014, 97, 5729–5741. [Google Scholar] [CrossRef]
- Sarmikasoglou, E.; Sumadong, P.; Roesch, L.F.W.; Halima, S.; Arriola, K.; Yuting, Z.; Jeong, K.C.C.; Vyas, D.; Hikita, C.; Watanabe, T.; et al. Effects of cashew nut shell extract and monensin on in vitro ruminal fermentation, methane production, and ruminal bacterial community. J. Dairy Sci. 2024, 107, 840–856. [Google Scholar] [CrossRef]
- Bryszak, M.; Szumacher-Strabel, M.; Huang, H.; Pawlak, P.; Lechniak, D.; Kołodziejski, P.; Yanza, Y.R.; Patra, A.K.; Váradyová, Z.; Cieslak, A. Lupinus angustifolius seed meal supplemented to dairy cow diet improves fatty acid composition in milk and mitigates methane production. Anim. Feed. Sci. Technol. 2020, 267, 114590. [Google Scholar] [CrossRef]
- Carlos, K.J.; Rafael, J.; Stephanie, V.S.; Denisse, M.M.; Cristina, M.I.; Jacobo, A.; Alfredo, G.C.; Fernando, A.C.; Javier, S.F. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar]
- Alex, B.; Guillermo, E.; Miguel, E.; Katrin, S.; Arnaud, J. Modulation of milking performance, methane emissions, and rumen microbiome on dairy cows by dietary supplementation of a blend of essential oils. Animal 2023, 17, 100825. [Google Scholar] [CrossRef]
- Kim, S.H.; Mamuad, L.L.; Islam, M.; Lee, S.S. Reductive acetogens isolated from ruminants and their effect on in vitro methane mitigation and milk performance in Holstein cows. J. Anim. Sci. Technol. 2020, 62, 1–13. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Saunders, N.; Goodman, J.R.; Algherair, I.H.; Ambrose, J.D. Effects of live yeast on milk yield, feed efficiency, methane emissions and fertility of high-yielding dairy cows. Animals 2025, 19, 101379. [Google Scholar] [CrossRef]
- Ma, H.; Dong, A.; Xu, Y.; Wu, Q.; Lambo, M.T.; Zhang, Y.; Dou, X.; Li, Y. Regulatory effects of high concentrate diet synergistically fermented with cellulase and lactic acid bacteria: In vitro ruminal fermentation, methane production, and rumen microbiome. Anim. Feed. Sci. Technol. 2025, 319, 116194. [Google Scholar] [CrossRef]
- Park, J.; Kwak, M.J.; Kang, M.G.; Cho, D.Y.; Kim, J.N.; Choi, I.G.; Kim, Y. Metabolic-methane mitigation by combination of probiotic Escherichia coli strain Nissle 1917 and biochar in rumen fluid in vitro fermentation of dairy cow. J. Environ. Chem. Eng. 2024, 12, 113977. [Google Scholar] [CrossRef]
- Sandra, V.; Paulus, C.D.; Nikki, D.; Athanasios, F.; Matthias, H.; Hristov, A.N.; Kalscheur, K.F.; Ermias, K.; Nuzhdin, S.V.; Price, N.N.; et al. Key considerations for the use of seaweed to reduce enteric methane emissions from cattle. Front. Vet. Sci. 2020, 7, 597430. [Google Scholar]
- Eslam, A.; Kengo, S.; Takehiro, N. Micro- and macro-algae combination as a novel alternative ruminant feed with methane-mitigation potential. Animals 2023, 13, 796. [Google Scholar]
- Youyoung, C.; Ja, L.S.; Sang, K.H.; Sik, E.J.; Uk, J.S.; Le Luo, G.; Tansol, P.; Jakyeom, S.; Yookyung, L.; Dongryeoul, B.; et al. Red seaweed extracts reduce methane production by altering rumen fermentation and microbial composition in vitro. Front. Vet. Sci. 2022, 9, 985824. [Google Scholar]
- Thorsteinsson, M.; Chassé, É.; Curtasu, M.V.; Battelli, M.; Bruhn, A.; Hellwing, A.L.F.; Weisbjerg, M.R.; Nielsen, M.O. Potential of 2 northern European brown seaweeds (Fucus serratus and Fucus vesiculosus) as enteric methane inhibitors in dairy cows. J. Dairy Sci. 2024, 107, 10628–10640. [Google Scholar] [CrossRef]
- Angellotti, M.; Lindberg, M.; Ramin, M.; Krizsan, S.J.; Danielsson, R. Asparagopsis taxiformis supplementation to mitigate enteric methane emissions in dairy cows—Effects on performance and metabolism. J. Dairy Sci. 2025, 108, 2503–2516. [Google Scholar] [CrossRef]
- Mihaila Alisa, A.; Glasson Christopher, R.K.; Rebecca, L.; Stefan, M.; German, M.; Marie, M. New temperate seaweed targets for mitigation of ruminant methane emissions: An in vitro assessment. Appl. Phycol. 2022, 3, 274–284. [Google Scholar] [CrossRef]
- Min Byeng, R.; David, P.; David, B.; Heidi, W.; Catherine, L.; Kristin, H.; Alexia, A.; Simona, A. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim. Nutr. 2021, 7, 1371–1387. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Weisbjerg, M.R.; Lund, P.; Battelli, M.; Chassé, É.; Bruhn, A.; Nielsen, M.O. Effects of seasonal and interspecies differences in macroalgae procured from temperate seas on the northern hemisphere on in vitro methane mitigating properties and rumen degradability. Algal Res. 2023, 73, 103139. [Google Scholar] [CrossRef]
- Pandey, D.; Hansen, H.H.; Dhakal, R.; Aryal, N.; Rai, S.P.; Sapkota, R.; Nielsen, M.O.; Novoa-Garrido, M.; Khanal, P. Interspecies and seasonal variations in macroalgae from the Nordic region: Chemical composition and impacts on rumen fermentation and microbiome assembly. J. Clean. Prod. 2022, 363, 132456. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef]
- Sena, F.; Portugal, P.V.; Dentinho, M.T.; Paulos, K.; Costa, C.; Soares, D.M.; Oliveira, A.; Ramos, H.; Alves, S.P.; Santos-Silva, J.; et al. Effects of sunflower oil infusions of Asparagopsis taxiformis on in vitro ruminal methane production and biohydrogenation of polyunsaturated fatty acids. J. Dairy Sci. 2024, 107, 1472–1484. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Jacobs, J.L.; Kinley, R.D.; Roque, B.M.; Neachtain, A.S.O.; Chandra, S.; Williams, S.R.O. Twice daily feeding of canola oil steeped with Asparagopsis armata reduced methane emissions of lactating dairy cows. Anim. Feed. Sci. Technol. 2023, 297, 115579. [Google Scholar] [CrossRef]
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Wenkai, R.; Peng, W.; Jiameng, Y.; Gang, L.; Benhua, Z.; Tarique, H.; Can, P.; Jie, Y.; Tiejun, L.; Hong, W.; et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar]
- Yao, F.; Songyang, Y.; Tiankun, W.; Yongqiang, L.; Huigang, H.; Xuening, L.; Dongying, L.; Xiao, M.; Shengyu, G.; Yujun, Y.; et al. Correction: Effects of melatonin on rumen microorganisms and methane production in dairy cow: Results from in vitro and in vivo studies. Microbiome 2023, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Giagnoni, G.; Lund, P.; Johansen, M.; Weisbjerg, M.R. Effect of dietary fat source and concentration on feed intake, enteric methane and milk production in dairy cows. J. Dairy Sci. 2024, 108, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.S.A.; Jacobs, J.L.; Kinley, R.D.; Roque, B.M.; Neachtain, A.S.O.; Chandra, S.; Russo, V.M.; Williams, S.R.O. Effects of a range of effective inclusion levels of Asparagopsis armata steeped in oil on enteric methane emissions of dairy cows. Anim. Feed. Sci. Technol. 2024, 310, 115932. [Google Scholar] [CrossRef]
- van Gastelen, S.; David, Y.; Hajer, K.; Alexandra, B.; André, B. Effect of a blend of cinnamaldehyde, eugenol, and capsicum oleoresin on methane emission and lactation performance of Holstein-Friesian dairy cows. J. Dairy Sci. 2023, 107, 857–869. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Corn silage-based diet supplemented with increasing amounts of linseed oil: Effects on methane production, rumen fermentation, nutrient digestibility, nitrogen utilization, and milk production of dairy cows. J. Dairy Sci. 2021, 104, 5375–5390. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Li, S.; Wang, W. Rumen metagenome reveals the mechanism of mitigation methane emissions by unsaturated fatty acid while maintaining the performance of dairy cows. Anim. Nutr. 2024, 18, 296–308. [Google Scholar] [CrossRef]
- Kuipers, A.; Galama, P.; Spoelstra, S.F.; Wiering, C.J.; Groot Koerkamp, P.W.G. Invited review: Combined mitigation of methane and ammonia emissions from dairy barns through barn design, ventilation and air treatment systems. J. Dairy Sci. 2025, 108, 6565–6586. [Google Scholar] [CrossRef]
- Fischer, A.; Edouard, N.; Faverdin, P. Precision feed restriction improves feed and milk efficiencies and reduces methane emissions of less efficient lactating Holstein cows without impairing their performance. J. Dairy Sci. 2020, 103, 4408–4422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, R.; Li, L.; Zheng, G.; Wang, J.; Wang, G.; Bao, Z.; Yin, Z.; Li, G.; Yuan, J. A global meta-analysis of greenhouse gas emissions and carbon and nitrogen losses during livestock manure composting: Influencing factors and mitigation strategies. Sci. Total Environ. 2023, 885, 163900. [Google Scholar] [CrossRef]
- Guillermo, P.; Raúl, M.; Eduardo, A.; Agustín, D.P. Gaseous emissions from management of solid waste: A systematic review. Glob. Chang. Biol. 2015, 21, 1313–1327. [Google Scholar]
- Alkhrissat, T.; Matarneh, S. Impact of anaerobic co-digestion of ryegrass (RG) and cow manure (CM) on methane production and kinetic analysis. Results Eng. 2025, 25, 104028. [Google Scholar] [CrossRef]

| Secondary Metabolites of Plants | Source | Addition Amount | CH4 Emission Reduction (g/d) | Literature Sources |
|---|---|---|---|---|
| Tannin | White Finch, Mimosa, chestnut | 2% DM | 10.0~17.0% | [76] |
| Garlic and citrus extracts | Garlic, citrus | 44 g/d | 10.3% | [80] |
| Essential oil mixture | Flowers, leaves, and seeds of plants | 1 g/d | 12.4% | [87] |
| Cashew nut shell extract | Cashew nuts | 0.02% DM | 10.64% | [84] |
| Lupine seed powder | Lupine | 10% DM | 16.0~17.0% | [85] |
| Saponin | Ginseng, Panax notoginseng, Astragalus membranaceus | 1% DM | 12.0% | [86] |
| Classification | Name of Algae | Addition Amount | CH4 Production Reduction | Literature Source |
|---|---|---|---|---|
| Brown algae | Fucus serratus | 17% DM | 54.0% | [99] |
| Fucus vesiculosus | 20% DM | 62.6% | [100] | |
| Ascophyllum nodosum | 20% DM | 48.2% | [100] | |
| Dictyota | 17% OM | 92.2% | [101] | |
| Red algae | Asparagopsis taxiformis | 7.5% DM | 33.0% | [102] |
| 0.01% DM as bromaform | 47.0% | |||
| 0.015% DM as bromaform | 87.0% | |||
| 17% OM | 98.9% | [101] | ||
| Bonnemaisonia hamifera | 2% OM | 17.1% | [97] | |
| 6% OM | 95.4% | |||
| 10% OM | 98.8% | |||
| Asparagopsis armata | 134 g/d | 44.0% | [103] | |
| 0.5% OM | 26.4% | [104] | ||
| 1% OM | 67.2% | |||
| Euptilota formisissima | 10% OM | 50.5% | [97] | |
| Plocamium cirrhosum | 10% OM | 39.5% | [97] | |
| Green algae | Cladophora patentiramea | 17% OM | 69.7% | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, K.; Yuan, S.; Liu, J.; Guo, J.; Guo, Y. Research Progress on Methane Emission Reduction Strategies for Dairy Cows. Dairy 2025, 6, 48. https://doi.org/10.3390/dairy6050048
Wang Y, Chen K, Yuan S, Liu J, Guo J, Guo Y. Research Progress on Methane Emission Reduction Strategies for Dairy Cows. Dairy. 2025; 6(5):48. https://doi.org/10.3390/dairy6050048
Chicago/Turabian StyleWang, Yu, Kuan Chen, Shulin Yuan, Jianying Liu, Jianchao Guo, and Yongqing Guo. 2025. "Research Progress on Methane Emission Reduction Strategies for Dairy Cows" Dairy 6, no. 5: 48. https://doi.org/10.3390/dairy6050048
APA StyleWang, Y., Chen, K., Yuan, S., Liu, J., Guo, J., & Guo, Y. (2025). Research Progress on Methane Emission Reduction Strategies for Dairy Cows. Dairy, 6(5), 48. https://doi.org/10.3390/dairy6050048








