High Pressure Processing of Raw Ewe’s Cheese Promotes Microbiological Safety and Quality During Prolonged Storage
Abstract
1. Introduction
2. Materials and Methods
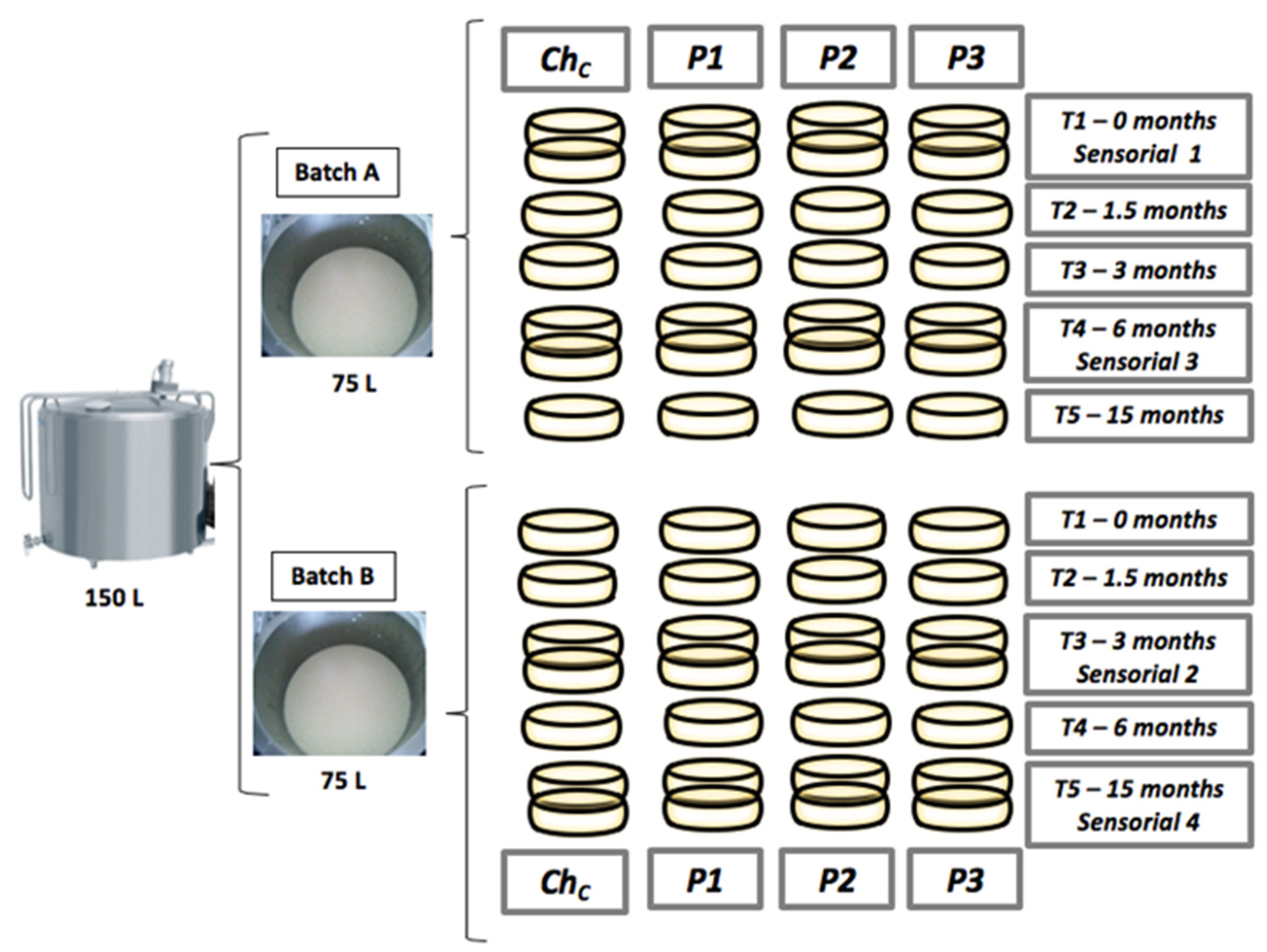
2.1. Cheese Manufacture
2.2. High-Pressure Processing
2.3. Sampling
2.4. Microbiological Analyses
2.5. Physicochemical Analyses
2.6. Colour
2.7. Statistical Analyses
3. Results and Discussion
3.1. Changes in Serra da Estrela Cheese Microbial Composition Induced by HPP
3.1.1. Lactic Acid Bacteria, Enterococcus, Total Aerobic Mesophilic, Anaerobic and Psychotropic Bacteria
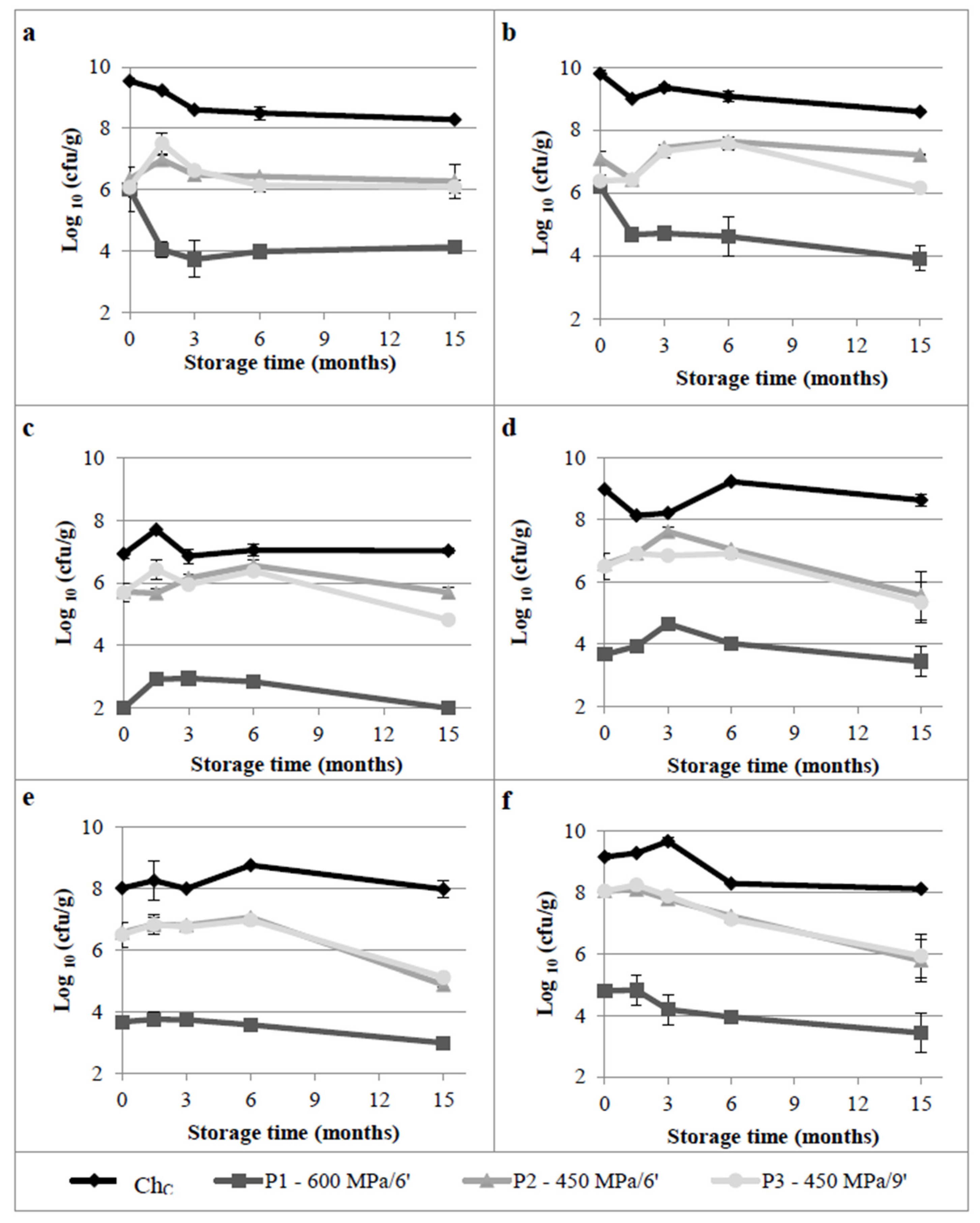
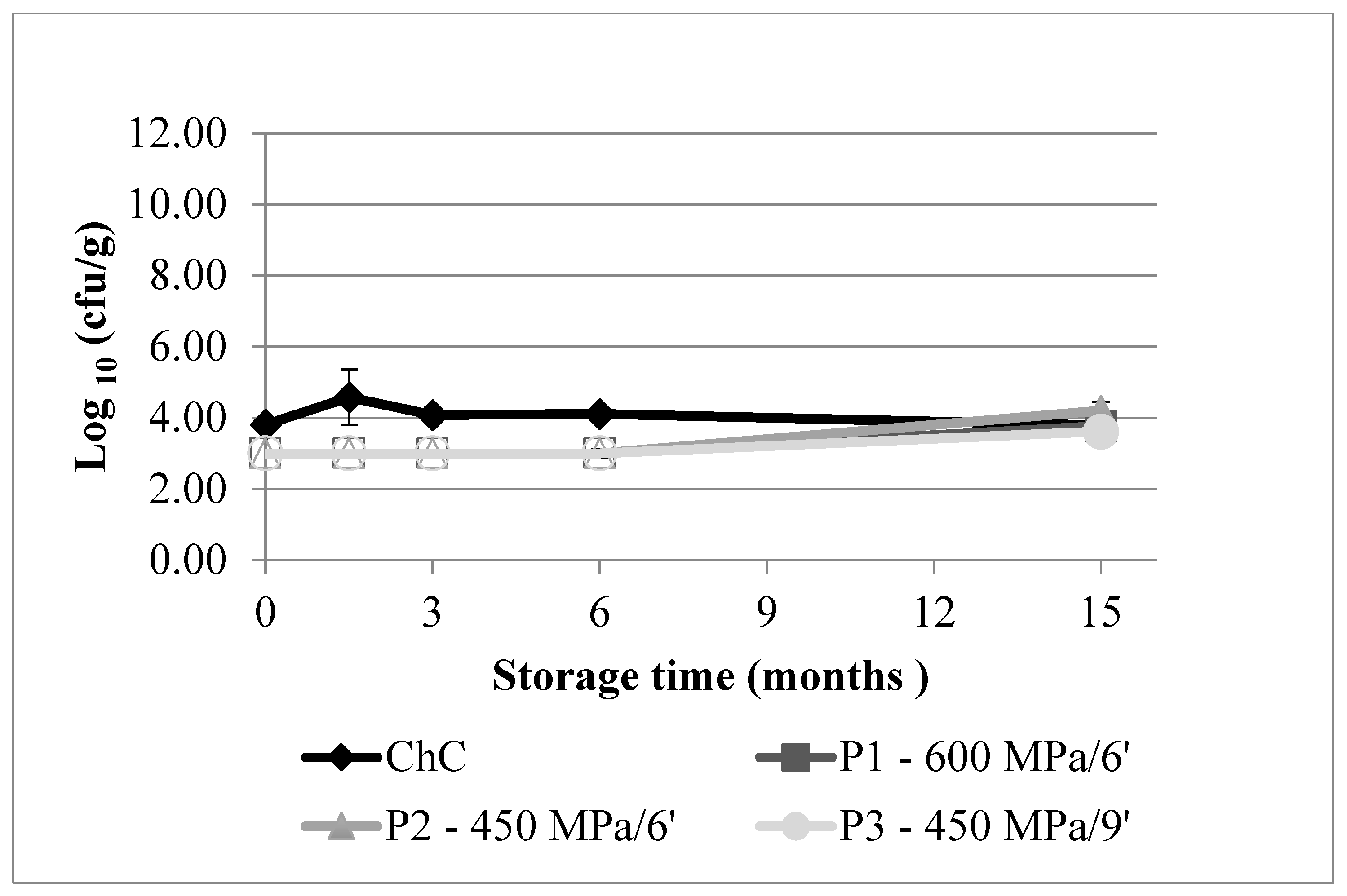
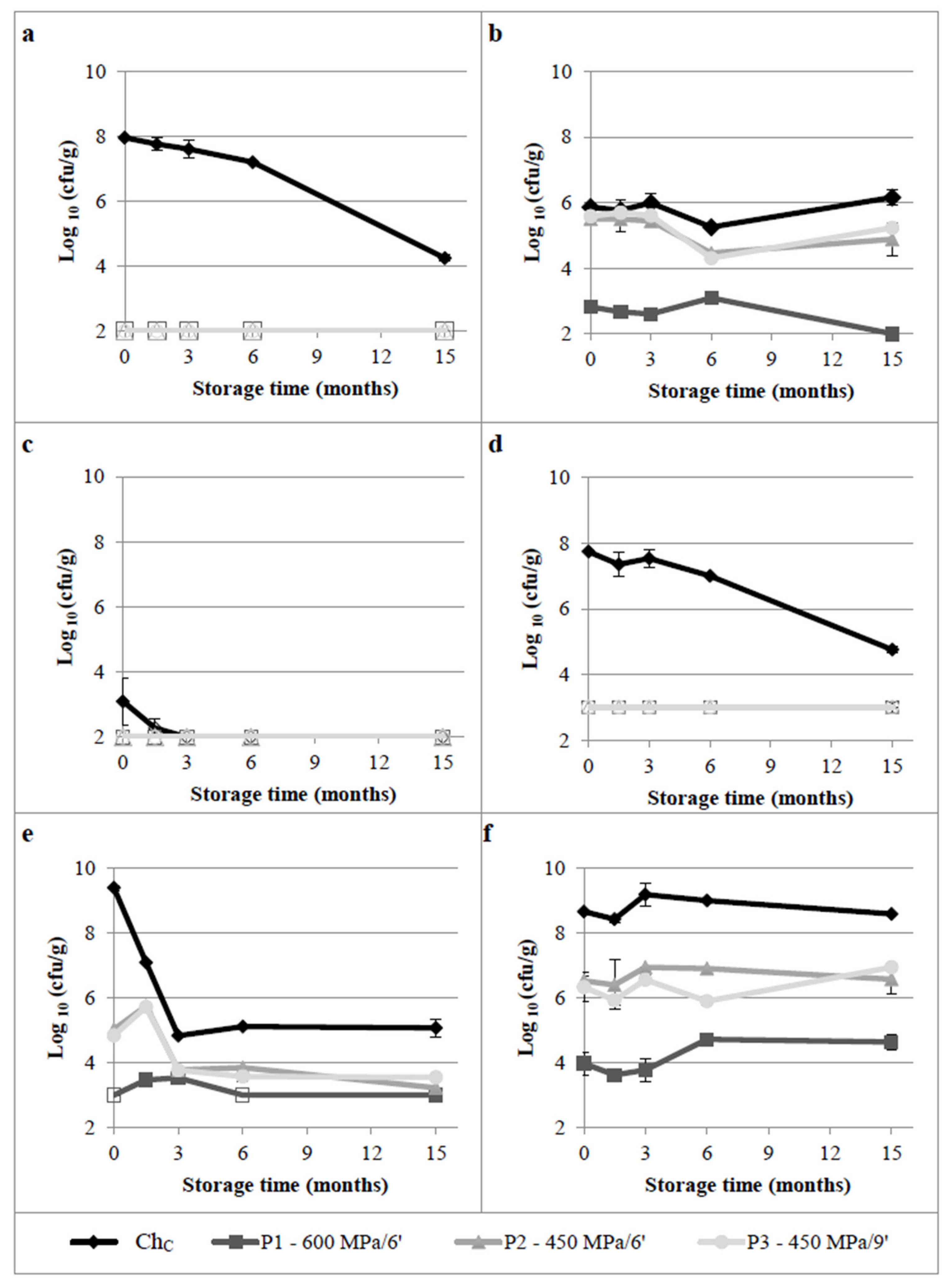
3.1.2. Contaminant Microbial Groups
3.1.3. Yeasts and Moulds
3.1.4. Overview of the Effect of HPP on Microbiota and Cheese Quality
3.2. Changes in Physicochemical Characteristics Induced by HPP
3.2.1. Moisture, Fat and Protein Contents
3.2.2. pH Values and Titratable Acidity
3.2.3. Water Activity
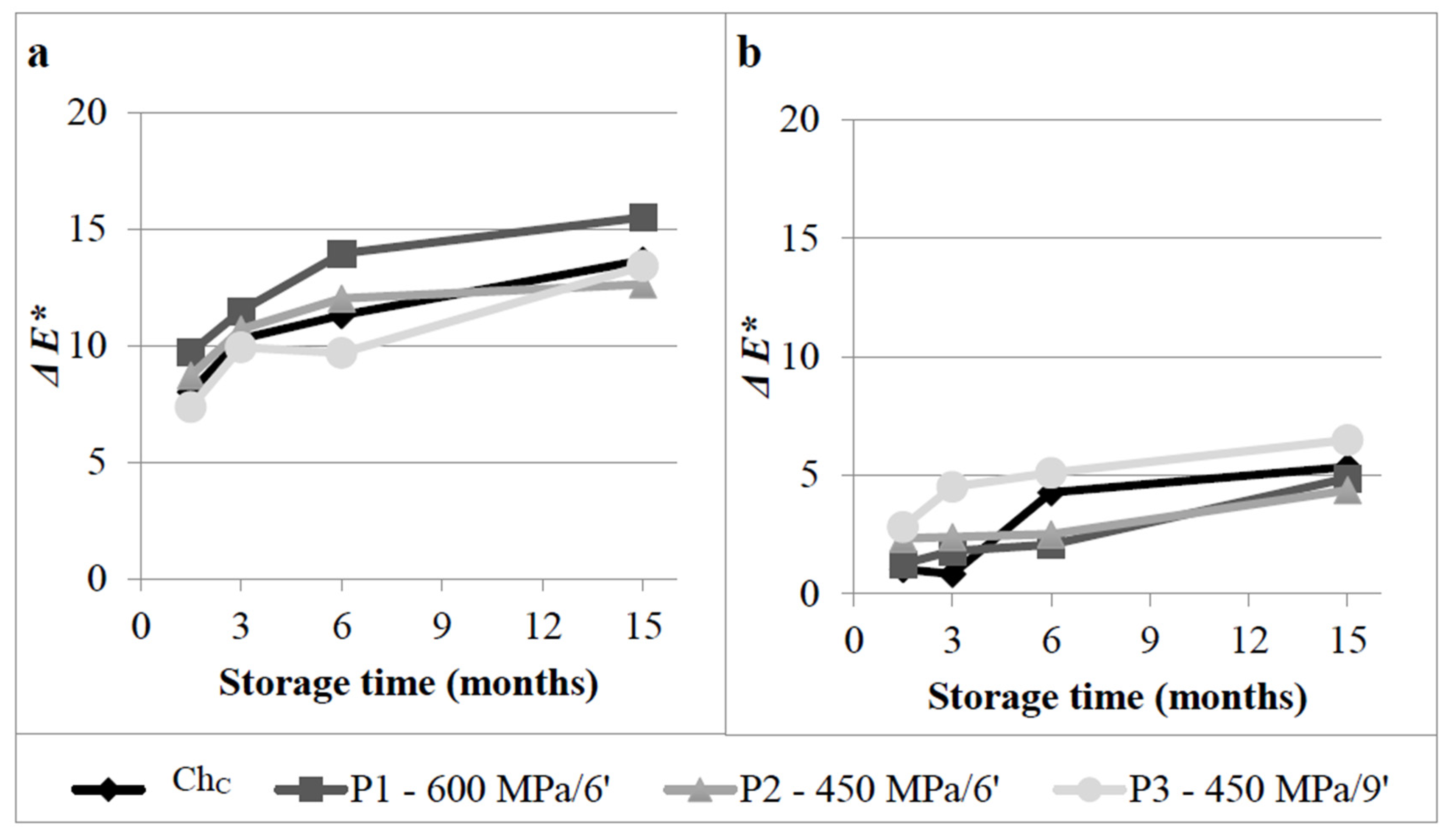
3.2.4. Colour
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ChC | P1—600 MPa/6′ | P2—450 MPa/6′ | P3—450 MPa/9′ | |||
|---|---|---|---|---|---|---|
| Months | STD | STD | STD | STD | ||
| Cheese Surface Colour | L* | 0 | 68.44 ± 0.57 | 67.60 ± 2.62 | 69.11 ± 0.75 | 69.61 ± 1.44 |
| 1.5 | 76.25 ± 1.11 | 77.00 ± 0.53 | 77.43 ± 1.18 | 76.60 ± 1.23 | ||
| 3 | 78.57 ± 1.10 | 78.78 ± 1.02 | 79.43 ± 1.23 | 79.18 ± 1.25 | ||
| 6 | 79.66 ± 1.10 | 81.34 ± 1.07 | 80.56 ± 0.52 | 78.87 ± 0.62 | ||
| 15 | 81.78 ± 1.23 | 81.59 ± 1.01 | 81.03 ± 1.15 | 82.32 ± 0.98 | ||
| a* | 0 | 0.00 ± 0.20 | −0.35 ± 0.48 | −0.76 ± 0.21 | −0.45 ± 0.53 | |
| 1.5 | −0.71 ± 0.17 | −1.75 ± 0.17 | −0.84 ± 0.53 | −1.46 ± 0.16 | ||
| 3 | −0.39 ± 0.36 | −1.21 ± 0.77 | −0.68 ± 0.42 | −1.04 ± 0.41 | ||
| 6 | −0.37 ± 0.32 | −1.04 ± 0.35 | −0.32 ± 0.64 | −0.61 ± 0.19 | ||
| 15 | −0.30 ± 0.26 | −0.58 ± 0.50 | −0.56 ± 0.40 | −0.84 ± 0.22 | ||
| b* | 0 | 22.78 ± 0.20 | 25.74 ± 0.90 | 25.31 ± 1.10 | 25.27 ± 0.65 | |
| 1.5 | 21.01 ± 0.17 | 23.66 ± 0.63 | 22.68 ± 0.37 | 23.16 ± 0.62 | ||
| 3 | 20.98 ± 0.36 | 23.19 ± 1.19 | 22.45 ± 1.20 | 22.66 ± 1.09 | ||
| 6 | 21.38 ± 0.32 | 23.35 ± 1.20 | 21.60 ± 1.15 | 22.39 ± 1.72 | ||
| 15 | 19.77 ± 0.26 | 19.04 ± 2.73 | 21.12 ± 2.07 | 20.97 ± 1.11 | ||
| Cheese Core Colour | L* | 0 | 85.62 ± 0.57 | 86.56 ± 1.40 | 86.83 ± 2.47 | 89.11 ± 1.70 |
| 1.5 | 85.14 ± 1.11 | 85.55 ± 3.63 | 84.75 ± 1.53 | 86.71 ± 1.63 | ||
| 3 | 85.76 ± 1.10 | 85.52 ± 1.01 | 85.10 ± 1.40 | 85.12 ± 2.60 | ||
| 6 | 83.52 ± 1.10 | 85.04 ± 1.58 | 85.05 ± 1.27 | 84.62 ± 0.93 | ||
| 15 | 83.25 ± 0.74 | 82.18 ± 0.91 | 83.03 ± 0.70 | 82.97 ± 0.85 | ||
| a* | 0 | −1.19 ± 0.20 | −2.72 ± 0.08 | −2.65 ± 0.16 | −2.66 ± 0.11 | |
| 1.5 | −1.31 ± 0.17 | −2.56 ± 0.16 | −2.56 ± 0.06 | −2.76 ± 0.14 | ||
| 3 | −1.32 ± 0.36 | −2.66 ± 0.08 | −2.59 ± 0.05 | −2.59 ± 0.07 | ||
| 6 | −1.37 ± 0.32 | −2.17 ± 0.21 | −2.38 ± 0.16 | −2.59 ± 0.02 | ||
| 15 | −1.24 ± 0.07 | −1.73 ± 0.19 | −2.14 ± 0.07 | −2.03 ± 0.13 | ||
| b* | 0 | 17.49 ± 0.81 | 21.38 ± 0.46 | 20.94 ± 0.48 | 20.56 ± 0.41 | |
| 1.5 | 18.40 ± 1.86 | 22.12 ± 0.61 | 22.01 ± 0.65 | 22.04 ± 0.81 | ||
| 3 | 18.30 ± 1.37 | 22.86 ± 0.44 | 22.60 ± 0.52 | 22.69 ± 0.84 | ||
| 6 | 21.20 ± 1.10 | 22.69 ± 0.26 | 22.71 ± 0.60 | 23.01 ± 0.60 | ||
| 15 | 22.29 ± 0.34 | 23.29 ± 0.35 | 23.03 ± 0.45 | 22.60 ± 0.28 |

References
- Macedo, A.C.; Malcata, F.X.; Oliveira, J.C. The Technology, Chemistry, and Microbiology of Serra Cheese: A Review. J. Dairy Sci. 1993, 76, 1725–1739. [Google Scholar] [CrossRef]
- Inácio, R.S.; Gomes, A.M.P.; Saraiva, J.A. Serra Da Estrela Cheese: A Review. J. Food Process. Preserv. 2020, 44, e14412. [Google Scholar] [CrossRef]
- Dahl, S.; Tavaria, F.K.; Xavier Malcata, F. Relationships between Flavour and Microbiological Profiles in Serra Da Estrela Cheese throughout Ripening. Int. Dairy J. 2000, 10, 255–262. [Google Scholar] [CrossRef]
- Gabinete de Planeamento e Políticas Caderno de Especificações Do Queijo Serra Da Estrela-DOP 2011. Available online: https://tradicional.dgadr.gov.pt/images/prod_imagens/queijos/docs/CE_Queijo_Serra_Estrela_2022.pdf (accessed on 16 June 2025).
- Barracosa, P.; Simões, I.; Martins, A.P.; Barros, M.; Pires, E. Biochemical Diversity of Cardoon Flowers (Cynara cardunculus L.): Predicting PDO Mediterranean Cheese Textures. Food Biosci. 2021, 39, 100805. [Google Scholar] [CrossRef]
- Barracosa, P.; Rosa, N.; Barros, M.; Pires, E. Selected Cardoon (Cynara cardunculus L.) Genotypes Suitable for PDO Cheeses in Mediterranean Regions. Chem. Biodivers. 2018, 15, e1800110. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X.; Hogg, T.A. Microbiological Profile in Serra Ewes’ Cheese during Ripening. J. Appl. Microbiol. 1995, 79, 1–11. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X. Role of Adventitious Microflora in Proteolysis and Lipolysis of Serra Cheese: Preliminary Screening. Eur. Food Res. Technol. 1997, 205, 25–30. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Malcata, F.X. On the Microbiology of Serra Da Estrela Cheese: Geographical and Chronological Considerations. Food Microbiol. 2000, 17, 293–304. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Reis, P.J.M.; Malcata, F.X. Effect of Dairy Farm and Milk Refrigeration on Microbiological and Microstructural Characteristics of Matured Serra Da Estrela Cheese. Int. Dairy J. 2006, 16, 895–902. [Google Scholar] [CrossRef]
- Macedo, A.C.; Costa, M.L.; Malcata, F.X. Changes in the Microflora of Serra Cheese: Evolution throughout Ripening Time, Lactation Period and Axial Location. Int. Dairy J. 1996, 6, 79–94. [Google Scholar] [CrossRef]
- Guilherme, V.M.P. Contributo Para Uma Avaliação de Risco de Listeria Monocytogenes Em Queijo Serra Da Estrela. Master’s Thesis, Technical University of Lisbon, Lisbon, Portugal, 2012. [Google Scholar]
- Martínez-Rodríguez, Y.; Acosta-Muñiz, C.; Olivas, G.I.; Guerrero-Beltrán, J.; Rodrigo-Aliaga, D.; Sepúlveda, D.R. High Hydrostatic Pressure Processing of Cheese. Compr. Rev. Food. Sci. Food Saf. 2012, 11, 399–416. [Google Scholar] [CrossRef]
- Inácio, R.S.; Barros, R.; Saraiva, J.A.; Gomes, A.M.P. Optimization of Raw Ewes’ Milk High-Pressure Pre-Treatment for Improved Production of Raw Milk Cheese. Foods 2022, 11, 435. [Google Scholar] [CrossRef]
- Inácio, R.S.; Pinto, C.A.; Saraiva, J.A.; Gomes, A.M.P. Effect of High Pressure Pre-Treatment on Raw Ewes’ Milk and on Subsequently Produced Cheese throughout Ripening. J. Sci. Food Agric. 2021, 101, 3975–3980. [Google Scholar] [CrossRef]
- Trujillo, A.J.; Capellas, M.; Saldo, J.; Gervilla, R.; Guamis, B. Applications of High-Hydrostatic Pressure on Milk and Dairy Products: A Review. Innov. Food Sci. Emerg. Technol. 2002, 3, 295–307. [Google Scholar] [CrossRef]
- O’Reilly, C.E.; Kelly, A.L.; Murphy, P.M.; Beresford, T.P. High Pressure Treatment: Applications in Cheese Manufacture and Ripening. Trends Food Sci. Technol. 2001, 12, 51–59. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A. Using High-Pressure Processing for Reduction of Proteolysis and Prevention of over-Ripening of Raw Milk Cheese. Food Bioproc. Tech. 2014, 7, 1404–1413. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. Reducing Biogenic-Amine-Producing Bacteria, Decarboxylase Activity, and Biogenic Amines in Raw Milk Cheese by High-Pressure Treatments. Appl. Environ. Microbiol. 2013, 79, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. High-Pressure Processing for the Control of Lipolysis, Volatile Compounds and Off-Odours in Raw Milk Cheese. Food Bioproc. Tech. 2014, 7, 2207–2217. [Google Scholar] [CrossRef]
- Rodríguez-Pinilla, J.; Márquez, G.; Tabla, R.; Ramírez, R.; Delgado, F.J. Microbiological and Lipolytic Changes in High-Pressure-Treated Raw Milk Cheeses during Refrigerated Storage. Dairy Sci. Technol. 2015, 95, 425–436. [Google Scholar] [CrossRef]
- Delgado, F.J.; Rodríguez-Pinilla, J.; Márquez, G.; Roa, I.; Ramírez, R. Physicochemical, Proteolysis and Texture Changes during the Storage of a Mature Soft Cheese Treated by High-Pressure Hydrostatic. Eur. Food Res. Technol. 2015, 240, 1167–1176. [Google Scholar] [CrossRef]
- Ávila, M.; Gómez-Torres, N.; Delgado, D.; Gaya, P.; Garde, S. Application of High Pressure Processing for Controlling Clostridium Tyrobutyricum and Late Blowing Defect on Semi-Hard Cheese. Food Microbiol. 2016, 60, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Inácio, R.S.; Fidalgo, L.G.; Santos, M.D.; Queirós, R.P.; Saraiva, J.A. Effect of High-Pressure Treatments on Microbial Loads and Physicochemical Characteristics during Refrigerated Storage of Raw Milk Serra Da Estrela Cheese Samples. Int. J. Food Sci. Technol. 2014, 49, 1272–1278. [Google Scholar] [CrossRef]
- Garde, S.; Arqués, J.L.; Gaya, P.; Medina, M.; Nuñez, M. Effect of High-Pressure Treatments on Proteolysis and Texture of Ewes’ Raw Milk La Serena Cheese. Int. Dairy J. 2007, 17, 1424–1433. [Google Scholar] [CrossRef]
- Arqués, J.L.; Garde, S.; Gaya, P.; Medina, M.; Nuñez, M. Short Communication: Inactivation of Microbial Contaminants in Raw Milk La Serena Cheese by High-Pressure Treatments. J. Dairy Res. 2006, 89, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; Delgado, J.; González-Crespo, J.; Cava, R.; Ramírez, R. High-Pressure Processing of a Raw Milk Cheese Improved Its Food Safety Maintaining the Sensory Quality. Food Sci. Technol. Int. 2013, 19, 493–501. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Changes in Microbiology, Proteolysis, Texture and Sensory Characteristics of Raw Goat Milk Cheeses Treated by High-Pressure at Different Stages of Maturation. Food Sci. Technol. 2012, 48, 268–275. [Google Scholar] [CrossRef]
- Sakharam, P.; Prajapati, J.P.; Jana, A.H. High Hydrostatic Pressure Treatment for Dairy Applications. In Proceedings of the National Seminar on Indian Dairy Industry—Opportunities and Challenges, 2014. Available online: https://www.dairyknowledge.in/sites/default/files/ch17_0.pdf (accessed on 16 June 2025).
- Inácio, R.S.; Rodríguez-Alcalá, L.M.; Pimentel, L.L.; Saraiva, J.A.; Gomes, A.M.P. Evolution of Qualitative and Quantitative Lipid Profiles of High-Pressure-Processed Serra Da Estrela Cheese throughout Storage. Appl. Sci. 2023, 13, 5927. [Google Scholar] [CrossRef]
- Inácio, R.S.; Monteiro, M.J.; Lopes-Da-Silva, J.A.; Saraiva, J.A.; Gomes, A.M.P. Effect of High Pressure Processing on Proteolysis, Texture and Sensorial Attributes of Raw Ewe’s Cheeses throughout Storage. Appl. Sci. 2025, 15, 6562. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. J. Pathol. Bacteriol. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- AOAC. Official Method 920.124 Acidity of Cheese—Titrimetric Method; AOAC: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Instituto Português da Qualidade. Norma Portuguesa NP 2105: Determinação do Teor de Matéria Gorda; Instituto Português da Qualidade (IPQ): Caparica, Portugal, 1983.
- ISO 3433; Cheese—Determination of Fat Content—Van Gulik Method. ISO Central Secretariat: Geneva, Switzerland, 1975.
- AOAC. Official Method 2001.14 Determination of Nitrogen (Total) in Cheese; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- European Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. 2005, Volume L338, pp. 1–19. Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj/eng (accessed on 16 June 2025).
- Macedo, A.C.; Tavares, T.G.; Malcata, F.X. Influence of Native Lactic Acid Bacteria on the Microbiological, Biochemical and Sensory Profiles of Serra Da Estrela Cheese. Food Microbiol. 2004, 21, 233–240. [Google Scholar] [CrossRef]
- Rocha, R.; Couto, N.; Pinto, R.P.; Vaz-Velho, M.; Fernandes, P.; Santos, J. Microbiological Characterization of Protected Designation of Origin Serra Da Estrela Cheese. Foods 2023, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Murchie, L.W.; Cruz-Romero, M.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M.; Kelly, A.L. High Pressure Processing of Shellfish: A Review of Microbiological and Other Quality Aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Abe, F. Exploration of the Effects of High Hydrostatic Pressure on Microbial Growth, Physiology and Survival: Perspectives from Piezophysiology. Biosci. Biotechnol. Biochem. 2007, 71, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of Microorganisms by High Isostatic Pressure Processing in Complex Matrices: A Review. Innov. Food Sci. Emerg. Technol. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Smelt, J.P.P.M. Recent Advances in the Microbiology of High Pressure Processing. Trends Food Sci. Technol. 1998, 9, 152–158. [Google Scholar] [CrossRef]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-Pressure Processing—Effects on Microbial Food Safety and Food Quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Shigehisa, T.; Ohmori, T.; Saito, A.; Taji, S.; Hayashi, R. Effects of High Hydrostatic Pressure on Characteristics of Pork Slurries and Inactivation of Microorganisms Associated with Meat and Meat Products. Int. J. Food Microbiol. 1991, 12, 207–215. [Google Scholar] [CrossRef]
- Huang, H.-W.; Lung, H.M.; Yang, B.B.; Wang, C.-Y. Responses of Microorganisms to High Hydrostatic Pressure Processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X.; Oliveira, J.C. Effect of Production Factors and Ripening Conditions on the Characteristics of Serra Cheese. Int. J. Food Sci. Technol. 1997, 32, 501–511. [Google Scholar] [CrossRef]
- Correia, P.; Vítor, A.; Tenreiro, M.; Correia, A.C.; Madanelo, J.; Guiné, R. Effect of Different Thistle Flower Ecotypes as Milk-Clotting in Serra Da Estrela Cheese. Nutr. Food Sci. 2016, 46, 458–475. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as Functional and Preserving Ingredient in “Serra Da Estrela” Cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barreira, J.C.M.; Bento, A.; Fernández-Ruiz, V.; Morales, P.; Ferreira, I.C.F.R. Chestnut and Lemon Balm Based Ingredients as Natural Preserving Agents of the Nutritional Profile in Matured “Serra Da Estrela” Cheese. Food Chem. 2016, 204, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.C.; Malcata, F.X. Technological Optimization of the Manufacture of Serra Cheese. J. Food Eng. 1997, 31, 433–447. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X. Secondary Proteolysis in Serra Cheese during Ripening and throughout the Cheese-Making Season. Eur. Food Res. Technol. 1997, 204, 173–179. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Tenreiro, M.I.C.; Correia, A.C.; Correia, P.M.R.; Barracosa, P. Analysis of Factors Influencing the Physical, Chemical and Sensorial Properties of Serra Da Estrela Cheeses. J. Food Meas. Charact. 2016, 10, 643–657. [Google Scholar] [CrossRef]
- Sousa, M.J.; Malcata, F.X. Comparison of Plant and Animal Rennets in Terms of Microbiological, Chemical, and Proteolysis Characteristics of Ovine Cheese. J. Agric. Food Chem. 1997, 45, 74–81. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A.; Nuñez, M. Effect of High Pressure Processing on the Lipolysis, Volatile Compounds, Odour and Colour of Cheese Made from Unpasteurized Milk. Food Bioproc. Tech. 2015, 8, 1076–1088. [Google Scholar] [CrossRef]

|
P1
600 MPa/6′ |
P2
450 MPa/6′ |
P3
450 MPa/9′ | |
|---|---|---|---|
| Lactobacillus spp. | 3.55 | 3.20 | 3.47 |
| Lactococcus spp. | 3.60 | 2.71 | 3.41 |
| Enterococcus spp. | 4.93 | 1.21 | 1.24 |
| Total aerobic microorganisms | 5.32 | 2.40 | 2.48 |
| Total anaerobic microorganisms | 4.34 | 1.44 | 1.51 |
| Total psychotropic microorganisms | 4.35 | 1.11 | 1.11 |
| Enterobacteriaceae | >5.9 | >5.9 | >5.9 |
| Coliforms | 3.05 | 0.36 | 0.28 |
| Escherichia coli | >1.1 | >1.1 | >1.1 |
| Staphylococcus spp. | 6.39 | 4.37 | 4.55 |
| Pseudomonas spp. | 4.74 | 4.74 | 4.74 |
| Bacillus spp. | 4.68 | 2.13 | 2.32 |
| Yeasts and moulds | >1.1 | >1.1 | >1.1 |
| ChC | P1—600 MPa/6′ | P2—450 MPa/6′ | P3—450 MPa/9′ | |
|---|---|---|---|---|
| Water Content | % (w/w) STD | % (w/w) STD | % (w/w) STD | % (w/w) STD |
| 0 | 47.8 ± 1.10 b,A | 49.4 ± 0.20 a,A | 47.8 ± 0.89 b,A | 48.0 ± 0.82 b,A |
| 1.5 | 46.0 ± 0.31 a,B | 45.8 ± 0.58 a,B | 45.8 ± 1.02 a,B | 45.4 ± 1.17 a,B |
| 3 | 44.5 ± 0.86 a,C | 44.1 ± 0.41 a,D | 44.8 ± 0.18 a,B,C | 44.4 ± 0.26 a,B,C |
| 6 | 43.6 ± 1.18 b,C | 44.5 ± 0.49 a,b,C,D | 45.1 ± 0.37 a,B,C | 43.9 ± 1.00 a,b,C |
| 15 | 44.5 ± 0.21 a,b,C | 44.9 ± 0.52 a,C | 44.4 ± 0.56 a,b,C | 43.9 ± 0.71 b,C |
| Fat Content | % (w/w) STD | % (w/w) STD | % (w/w) STD | % (w/w) STD |
| 0 | 26.1 ± 1.25 a,B | 24.9 ± 1.97 a,B | 25.4 ± 1.31 a,B | 26.5 ± 1.47 a,A |
| 3 | 27.4 ± 0.25 a,A,B | 28.0 ± 0.71 a,A | 27.9 ± 0.25 a,A | 28.4 ± 1.11 a,A |
| 6 | 28.3 ± 1.19 a,A | 28.9 ± 1.60 a,A | 26.5 ± 0.41 a,A,B | 27.1 ± 1.49 a,A |
| 15 | 28.1 ± 0.48 a,A | 27.6 ± 0.48 a,A,B | 27.9 ± 1.77 a,A | 27.9 ± 0.75 a,A |
| Protein Content | % (w/w) STD | % (w/w) STD | % (w/w) STD | % (w/w) STD |
| 0 | 22.0 ± 0.25 b,B | 22.6 ± 0.15 a,b,B | 23.5 ± 0.29 a,A | 20.4 ± 0.32 c,C |
| 3 | 23.3 ± 0.19 a,A | 22.4 ± 0.44 b,c,B | 23.1 ± 0.19 a,b,A | 21.8 ± 0.56 c,B |
| 6 | 23.7 ± 0.40 a,A | 23.1 ± 0.48 a,A,B | 22.8 ± 0.41 a,A | 23.5 ± 0.55 a,A |
| 15 | 22.5 ± 0.29 b,B | 23.7 ± 0.33 a,A | 23.5 ± 0.34 a,A | 23.8 ± 0.60 a,A |
| pH values | pH STD | pH STD | pH STD | pH STD |
| 0 | 5.36 ± 0.17 A,a | 5.27 ± 0.17 B,a | 5.24 ± 0.01 B,a | 5.23 ± 0.02 B,a |
| 1.5 | 5.39 ± 0.07 A,a | 5.34 ± 0.01 A,a | 5.33 ± 0.02 A,a | 5.36 ± 0.06 A,a |
| 3 | 5.25 ± 0.04 A,a | 5.22 ± 0.01 C,a,b | 5.19 ± 0.02 C,b | 5.21 ± 0.01 B,C,b |
| 6 | 5.29 ± 0.02 A,a | 5.17 ± 0.01 D,b | 5.17 ± 0.01 C,b | 5.15 ± 0.03 C,b |
| 15 | 5.26 ± 0.02 A,a | 5.22 ± 0.01 C,b | 5.17 ± 0.01 C,b | 5.18 ± 0.05 B,C,b |
| Titratable acidity | glactic acid/100 gSTD | glactic acid/100 g STD | glactic acid/100 g STD | glactic acid/100 g STD |
| 0 | 0.69 ± 0.01 E,a | 0.83 ± 0.09 D,a | 0.81 ± 0.18 D,a | 0.80 ± 0.09 E,a |
| 1.5 | 1.09 ± 0.06 D,a | 1.00 ± 0.10 C,a | 1.03 ± 0.03 C,a | 1.02 ± 0.09 D,a |
| 3 | 1.27 ± 0.07 C,a | 1.17 ± 0.05 B,C,b | 1.18 ± 0.06 C,a,b | 1.21 ± 0.05 C,a,b |
| 6 | 1.50 ± 0.10 B,a | 1.20 ± 0.09 B,b | 1.63 ± 0.06 B,a | 1.47 ± 0.17 B,a |
| 15 | 1.94 ± 0.06 A,a | 1.61 ± 0.16 A,b | 1.84 ± 0.10 A,a | 1.88 ± 0.09 A,a |
| Water activity | aw STD | aw STD | aw STD | aw STD |
| 0 | 0.959 ± 0.002 a,A | 0.959 ± 0.004 a,A | 0.957 ± 0.001 a,A | 0.956 ± 0.002 a,A |
| 1.5 | 0.954 ± 0.005 a,A,B | 0.953 ± 0.001 a,B | 0.952 ± 0.002 a,B | 0.951 ± 0.001 a,B |
| 3 | 0.953 ± 0.001 a,b,B | 0.954 ± 0.001 a,A,B | 0.950 ± 0.003 c,B | 0.951 ± 0.001 b,c,B |
| 6 | 0.950 ± 0.002 b,B | 0.954 ± 0.001 a,A,B | 0.951 ± 0.000 b,B | 0.950 ± 0.000 b,B |
| 15 | 0.928 ± 0.004 b,C | 0.935 ± 0.006 a,C | 0.932 ± 0.003 a,b,C | 0.933 ± 0.004 a,b,C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inácio, R.S.; Gomes, A.M.P.; Saraiva, J.A. High Pressure Processing of Raw Ewe’s Cheese Promotes Microbiological Safety and Quality During Prolonged Storage. Dairy 2025, 6, 36. https://doi.org/10.3390/dairy6040036
Inácio RS, Gomes AMP, Saraiva JA. High Pressure Processing of Raw Ewe’s Cheese Promotes Microbiological Safety and Quality During Prolonged Storage. Dairy. 2025; 6(4):36. https://doi.org/10.3390/dairy6040036
Chicago/Turabian StyleInácio, Rita S., Ana M. P. Gomes, and Jorge A. Saraiva. 2025. "High Pressure Processing of Raw Ewe’s Cheese Promotes Microbiological Safety and Quality During Prolonged Storage" Dairy 6, no. 4: 36. https://doi.org/10.3390/dairy6040036
APA StyleInácio, R. S., Gomes, A. M. P., & Saraiva, J. A. (2025). High Pressure Processing of Raw Ewe’s Cheese Promotes Microbiological Safety and Quality During Prolonged Storage. Dairy, 6(4), 36. https://doi.org/10.3390/dairy6040036







