The Calci-Inflammatory Network: A Paradigm Shift in Understanding Milk Fever
Abstract
1. Introduction
2. Evolving Hypotheses of Milk Fever: A Historical and Contemporary Overview
2.1. Historical Context and Early Theories
2.1.1. Hibbs Review and Subsequent Hypotheses
2.1.2. Development of the Hypocalcemia Theory
2.1.3. The DCAD and Potassium Hypothesis: A Shift in Thinking
2.1.4. The Endotoxin Hypothesis and Systemic Inflammation: A New Perspective
2.1.5. Endotoxins and Systemic Inflammation
3. Inflammatory Response in Milk Fever Cows
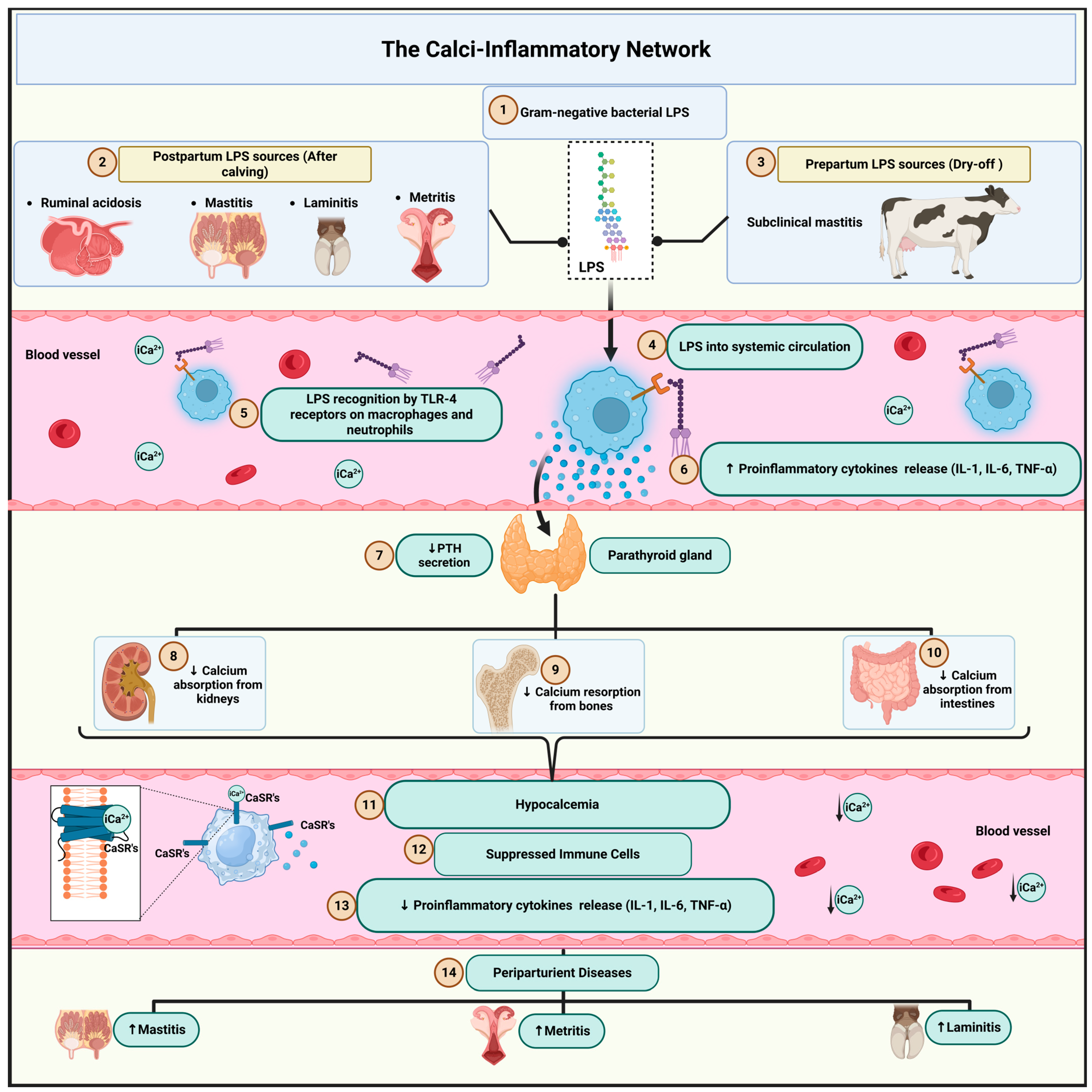
4. Re-Evaluating Traditional Theories in Light of the Endotoxin Hypothesis: The Calci-Inflammatory Network
4.1. Multiple Sources of Endotoxin and Early Immune Activation
4.2. Mechanisms of the Calci-Inflammatory Network
4.3. Clinical Consequences
4.4. Hypocalcemia in Multiple Periparturient Diseases: The Common Role of Inflammation
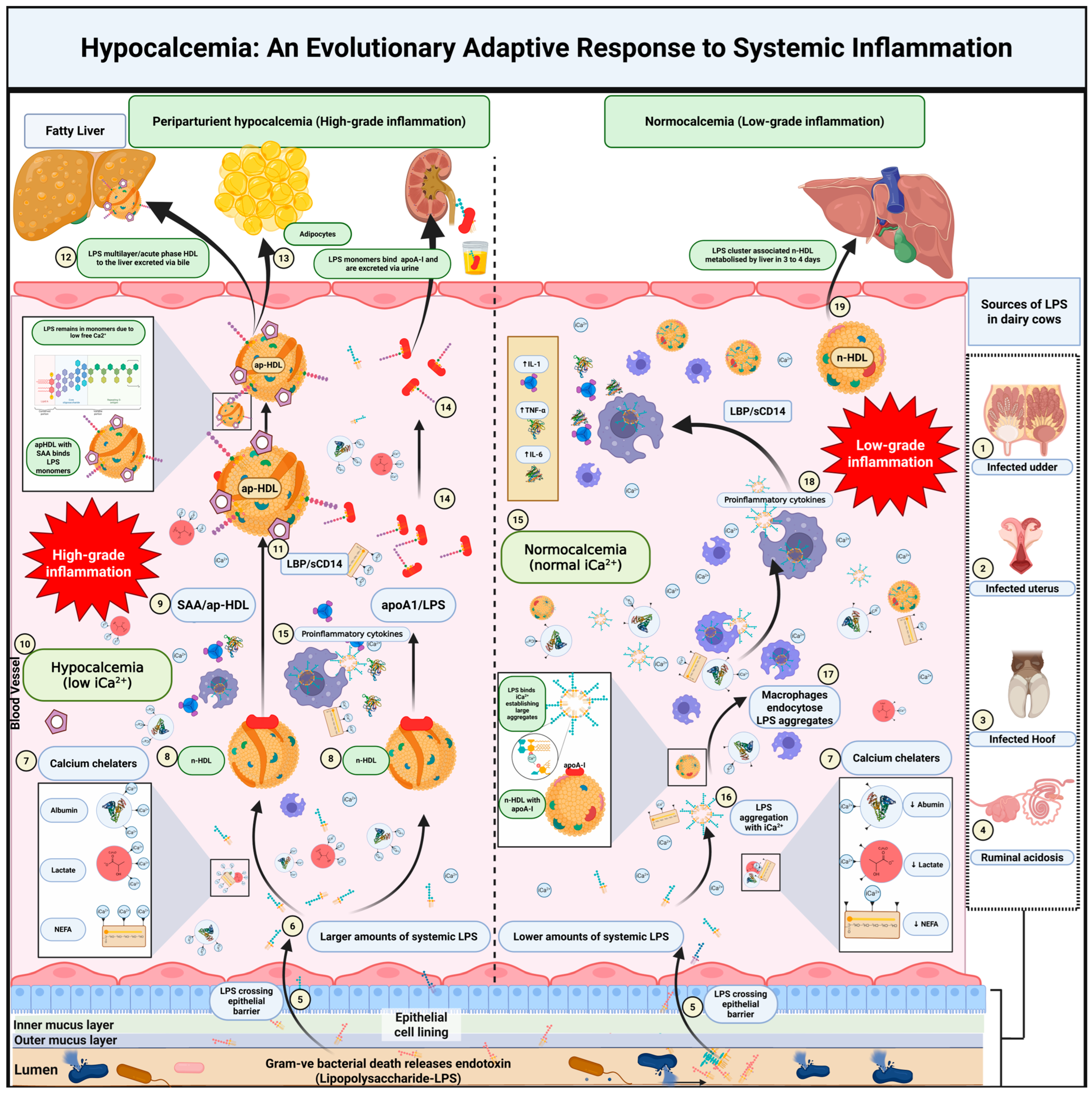
5. A New Hypothesis on Hypocalcemia and Its Role in Inflammation
5.1. Fate of Endotoxin in the Circulation
5.2. Calcium’s Role in LPS Aggregation and Immune Activation
5.3. Hypocalcemia as a Protective Mechanism
5.4. Lipoproteins and LPS Clearance
5.5. Modulation of the Inflammatory Response
5.6. Interactions of Calcium with Lactate, Fatty Acids, and Albumin
5.7. Lactate Binding to Ionized Calcium
5.8. Non-Esterified Fatty Acids Binding to Ionized Calcium
5.9. Albumin Binding to Ionized Calcium
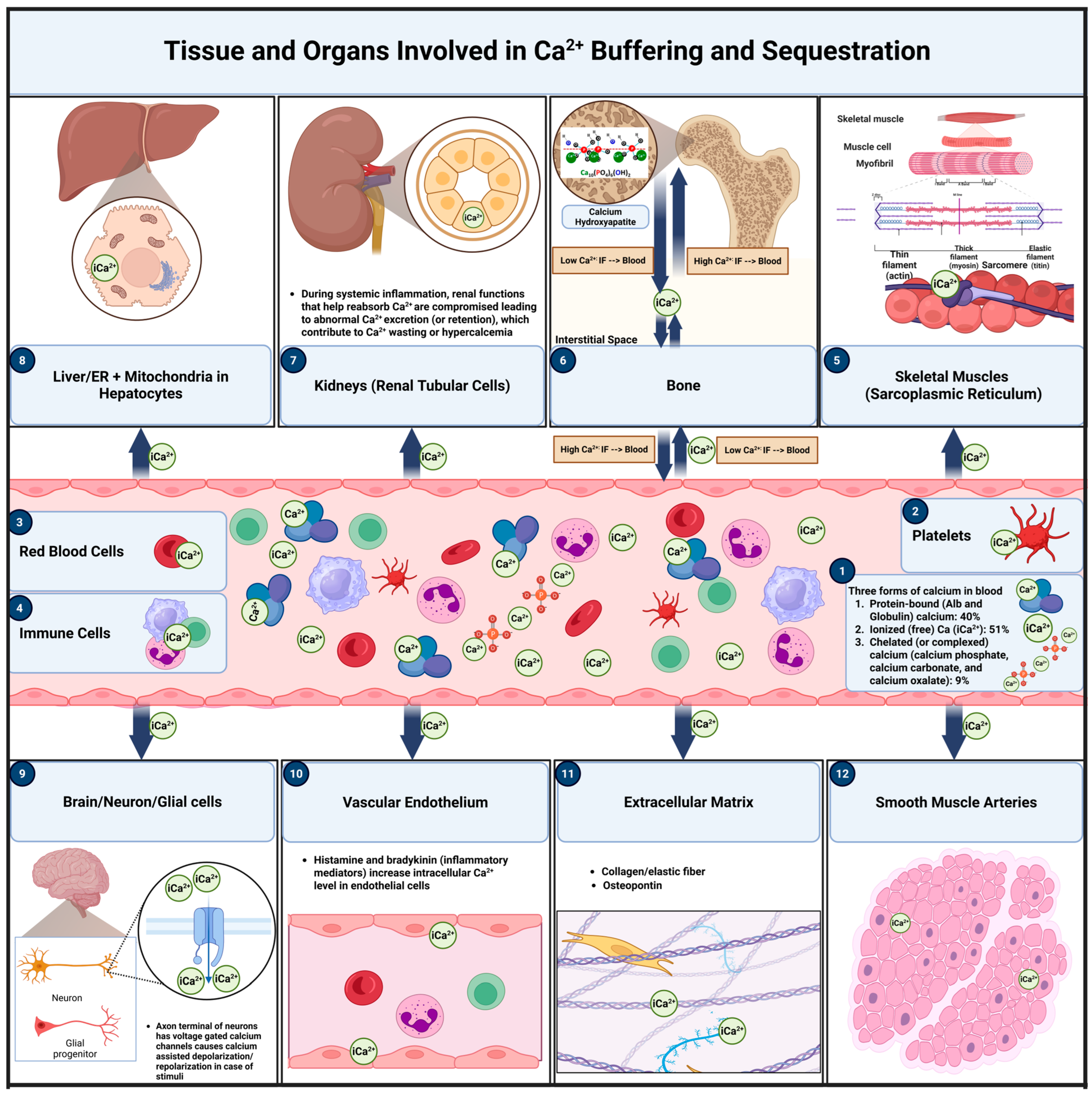
6. Calcium Buffering and Sequestration: Distinct Mechanisms and Key Locations in Calcium Homeostasis During Inflammation
6.1. Calcium Buffering
6.1.1. Primary Sites of Calcium Buffering
Blood Plasma
Extracellular Matrix (ECM)
Intracellular Compartments
6.2. Calcium Sequestration
6.2.1. Primary Sites of Calcium Sequestration
Bones
Skeletal Muscles (Sarcoplasmic Reticulum)
Liver (Hepatocytes)
Kidneys (Renal Tubular Cells)
Brain (Neurons and Glial Cells)
Mitochondria
7. Impact of Systemic Inflammation on Calcium Buffering and Sequestration
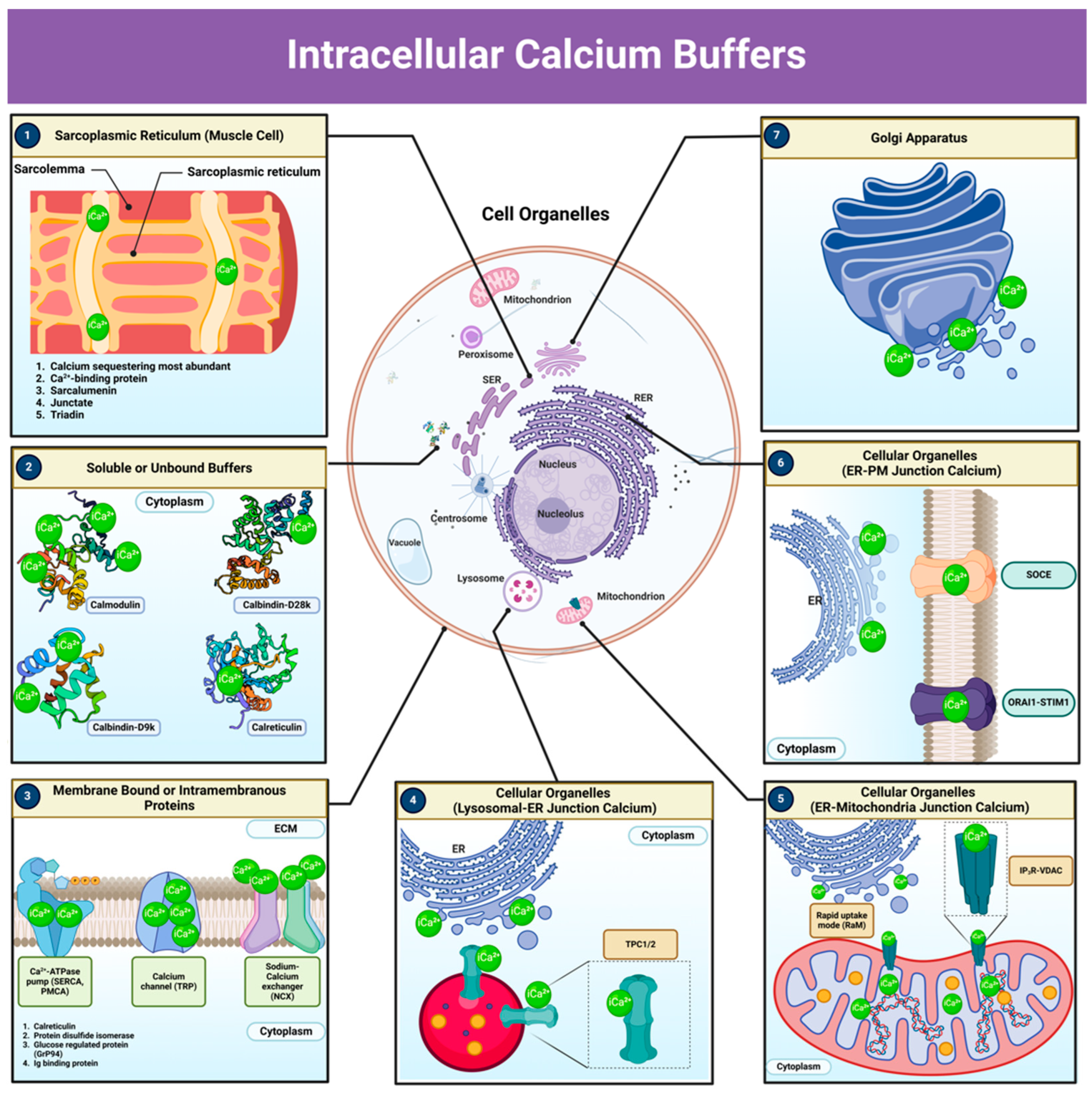
8. Intracellular Calcium Buffers: Pathways and Components Involved in Calcium Homeostasis
9. The Calcium-Sensing Receptor: Functions, Mechanisms, and Role in Immune Modulation
9.1. CaSR Function in Calcium Homeostasis
9.2. CaSR in Immune Regulation and Inflammation
9.3. Hypocalcemia, Calcium Sequestration, and Immune Cell Impairment
9.4. Sequestration Benefits and Risks
10. Systems Integration of Calcium Buffers and Inflammatory Networks
10.1. Cross-Talk Between Calcium Buffering and Inflammatory Signaling
10.2. HDL and Apolipoproteins: Facilitators of Endotoxin Clearance
10.3. Inorganic Anions and the pH–Calcium–Inflammation Network
10.4. Emergent Network Properties and Translational Potential
11. Evidence Linking Hypocalcemia to Inflammatory Responses in Dairy Cows
11.1. Recent Research in Dairy Cows Supporting the Hypothesis
11.2. Exacerbation of Inflammation by High Calcium Levels
11.3. Experimental Designs Inducing Endotoxemia
11.4. Hypocalcemia as a Response to Endotoxin-Induced Systemic Inflammation
11.5. Pre-Existing Systemic Inflammation in Cows Prone to Milk Fever
11.6. Calcium Oral Supplementation and Its Role in Postpartum Inflammation in Dairy Cows
12. Translational Implications of the Calci-Inflammatory Network
13. The Role of Calcium in LPS Aggregation and Clearance Without Inducing Inflammation
14. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. J. Dairy Sci. 2011, 94, 2928–2934. [Google Scholar] [CrossRef]
- Block, E. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J. Dairy Sci. 1984, 67, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; DeGaris, P.J.; McNeil, D.M.; Block, E. Hypocalcemia in dairy cows: Pathophysiology and preventive strategies. J. Dairy Sci. 2006, 89, 1094–1108. [Google Scholar] [CrossRef]
- Block, E. Manipulation of dietary cation-anion difference on nutritionally related production diseases, productivity, and metabolic responses of dairy cows. J. Dairy Sci. 1994, 77, 1437–1450. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—Cow-level prevalence and preventive strategies in German dairy herds. J. Dairy Sci. 2017, 100, 9258–9266. [Google Scholar] [CrossRef]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Serrenho, R.C.; DeVries, T.J.; Duffield, T.F.; LeBlanc, S.J. Graduate student literature review: What do we know about the effects of clinical and subclinical hypocalcemia on health and performance of dairy cows? J. Dairy Sci. 2021, 104, 6304–6326. [Google Scholar] [CrossRef]
- Zebeli, Q.; Beitz, D.C.; Bradford, B.J.; Dunn, S.M.; Ametaj, B.N. Peripartal alterations of calcitonin gene-related peptide and minerals in dairy cows affected by milk fever. Vet. Clin. Pathol. 2013, 42, 70–77. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Rev. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, J.W. Milk fever (Parturient paresis) in dairy cows: A review. J. Dairy Sci. 1950, 33, 758–789. [Google Scholar] [CrossRef]
- Hutyra, F.; Marek, J.; Manniger, R. Special Pathology and Therapeutics of the Diseases of Domestic Animals, 4th ed.; Greig, J.R., Ed.; Forgotten Books: London, UK, 1938. [Google Scholar]
- Dryerre, H.; Greig, J.R. Milk fever: Its possible association with derangements in the internal secretions. Vet. Rec. 1925, 5, 225–231. [Google Scholar]
- Auger, M.L. Réalisation expérimentale de la fièvre vitulaire. Compt. Rend. 1926, 182, 348–350. [Google Scholar]
- Little, W.L.; Wright, N.C. The aetiology of milk fever in cattle. Br. J. Exp. Pathol. 1925, 6, 129. [Google Scholar]
- Dryrre, H.; Greig, J.R. The specific chemotherapy of milk fever by the parenteral administration of Ca-boro-gluconate. Vet. Med. 1935, 30, 234–238. [Google Scholar]
- Ender, F.; Dishington, I.W.; Helgebostad, A. Calcium balance studies in dairy cows under experimental induction and prevention of hypocalcaemic paresis puerperalis. The solution of the aetiology and the prevention of milk fever by dietary means. Z. Tierphysiol. Tierernaehr. Futtermittelkd. 1971, 28, 233. [Google Scholar]
- Dishington, I.W. Prevention of milk fever (Hypocalcaemia paresis puerperalis) by dietary salt supplements. Acta Vet. Scand. 1975, 16, 503–512. [Google Scholar] [CrossRef]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A.; Buxton, D.R. Strategies for preventing milk fever in dairy cattle. J. Dairy Sci. 1997, 80, 1269–1280. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.Y.; Yu, Y.N.; Yang, L.Y.; Zhang, P.H.; He, J.H.; Shen, W.J.; Tan, Z.L.; Feng, B.L.; Tang, S.X. Enhancing dietary cation-anion difference reshaped the distribution of endotoxin across different biofluids and influenced inflammatory response in dairy cows exposed to heat stress. Anim. Feed. Sci. Technol. 2020, 262, 114444. [Google Scholar] [CrossRef]
- Aiumlamai, S.; Kindahl, H.; Fredriksson, G.; Edqvist, L.E.; Kulander, L.; Eriksson, O. The role of endotoxins in induced ruminal acidosis in calves. Acta Vet. Scand. 1992, 33, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.H. Bovine endotoxicosis: Some aspects of relevance to production diseases. A review. Acta Vet. Scand. Suppl. 2003, 98, 141–155. [Google Scholar] [CrossRef]
- Ametaj, B.N. A new understanding of the causes of fatty liver in dairy cows. Adv. Dairy Technol. 2005, 17, 97–112. [Google Scholar]
- Ametaj, B.N. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Dairy Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef]
- Shandilya, U.; Sharma, A.; Mallikarjunappa, S.; Guo, J.; Mao, Y.; Meade, K.; Karrow, N. CRISPR-Cas9-mediated knockout of TLR4 modulates Mycobacterium avium ssp. paratuberculosis cell lysate-induced inflammation in bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 11135–11146. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Reuter, R.; Chase, C.; Coleman, S.; Riley, D.; Spiers, D.; Arthington, J.; Galyean, M. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immun. 2009, 15, 81–89. [Google Scholar] [CrossRef]
- Jermann, P.; Wagner, L.; Fritsche, D.; Gross, J.; Wellnitz, O.; Bruckmaier, R. Acute phase reaction to LPS-induced mastitis in early lactation dairy cows fed nitrogenic, glucogenic or lipogenic diets. J. Dairy Sci. 2023, 106, 9879–9891. [Google Scholar] [CrossRef] [PubMed]
- Augustine, M.; Leonard, M.; Thayu, M.; Baldassano, R.; De Boer, I.; Shults, J.; Denson, L.; DeBoer, M.; Herskovitz, R.; Denburg, M. Changes in vitamin D-related mineral metabolism after induction with anti-tumor necrosis factor-α therapy in Crohn’s disease. J. Clin. Endocrinol. Metab. 2014, 99, E991–E998. [Google Scholar] [CrossRef]
- Meurer, M.; Höcherl, K. Endotoxaemia differentially regulates the expression of renal Ca2+ transport proteins in mice. Acta Physiol. 2018, 225, e13175. [Google Scholar] [CrossRef]
- Klein, G. The role of calcium in inflammation-associated bone resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Ametaj, B.N. Milk fever: Reductionist versus systems veterinary approach. In Periparturient Diseases of Dairy Cows; Ametaj, B.N., Ed.; Springer: Berlin, Germany, 2017; pp. 247–266. [Google Scholar]
- Ametaj, B.N.; Goff, J.P.; Horst, R.L.; Bradford, B.J.; Beitz, D.C. Presence of acute phase response in normal and milk fever dairy cows around parturition. Acta Vet. Scand. Suppl. 2003, 98, 241. [Google Scholar] [CrossRef]
- Thaveeratitham, P.; Khovidhunkit, W.; Patumraj, S. High-density lipoproteins (HDL) inhibit endotoxin-induced leukocyte adhesion on endothelial cells in rats: Effect of the acute-phase HDL. Clin. Hemorheol. Microcirc. 2007, 36, 1–12. [Google Scholar] [PubMed]
- Collage, R.D.; Howell, G.M.; Zhang, X.; Stripay, J.L.; Lee, J.S.; Angus, D.C.; Rosengart, M.R. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit. Care Med. 2013, 41, e352–e360. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, D.G.; Madsen, K.L.; Churchill, T.A.; Dunn, S.M.; Ametaj, B.N. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef]
- Su, G.; Klein, R.; Aminlari, A.; Zhang, H.; Steinstraesser, L.; Alarcon, W.; Remick, D.; Wang, S. Kupffer cell activation by lipopolysaccharide in rats: Role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 2000, 31, 932–936. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fu, Y. The rumen microbiota contributes to the development of mastitis in dairy cows. Microbiol. Spectr. 2022, 10, e0251221. [Google Scholar] [CrossRef]
- Papaioannou, N.; Voutsas, I.; Samara, P.; Tsitsilonis, O. A flow cytometric approach for studying alterations in the cytoplasmic concentration of calcium ions in immune cells following stimulation with thymic peptides. Cell. Immunol. 2016, 302, 32–40. [Google Scholar] [CrossRef]
- Yang, R.; Mark, M.; Gray, A.; Huang, A.; Xie, M.; Zhang, M.; Goddard, A.; Wood, W.; Gurney, A.; Godowski, P. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef]
- Iamartino, L.; Brandi, M. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef]
- Hendy, G.; Canaff, L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 2016, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Nat. Rev. Endocrinol. 2013, 9, 391–403. [Google Scholar] [CrossRef]
- Kehrli, M.E., Jr.; Goff, J.P. Periparturient hypocalcemia in cows: Effects on peripheral blood neutrophil and lymphocyte function. J. Dairy Sci. 1989, 72, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Canaff, L.; Hendy, G. Human calcium-sensing receptor gene. J. Biol. Chem. 2002, 277, 30337–30350. [Google Scholar] [CrossRef] [PubMed]
- Venjakob, P.; Staufenbiel, R.; Heuwieser, W.; Borchardt, S. Association between serum calcium dynamics around parturition and common postpartum diseases in dairy cows. J. Dairy Sci. 2020, 104, 2243–2253. [Google Scholar] [CrossRef]
- Venjakob, P.; Staufenbiel, R.; Heuwieser, W.; Borchardt, S. Serum calcium dynamics within the first 3 days in milk and the associated risk of acute puerperal metritis. J. Dairy Sci. 2019, 102, 11428–11438. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef]
- Atalay, H. The effect of serum β-hydroxybutyric acid and calcium levels on left displaced abomasum in Holstein cows on transition period. J. Istanbul Vet. Sci. 2019, 3, 43–48. [Google Scholar] [CrossRef]
- Curtis, C.; Erb, H.; Sniffen, C.; Smith, R.; Powers, P.; Smith, M.; Me, W.; Rb, H.; Pearson, E. Association of parturient hypocalcemia with eight periparturient disorders in Holstein cows. J. Am. Vet. Med. Assoc. 1983, 183, 559–561. [Google Scholar] [CrossRef]
- Kantham, L.; Quinn, S.; Egbuna, O.; Baxi, K.; Butters, R.; Pang, J.; Pollak, M.; Goltzman, D.; Brown, E. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E915–E923. [Google Scholar] [CrossRef]
- Marmalyuk, D.; Runova, G.; Fadeyev, V. The role of the calcium-sensing receptor in the regulation of parathyroid hormone secretion in physiology and in calcitropic diseases. Osteoporos. Bone Dis. 2023, 26, 25–32. [Google Scholar] [CrossRef]
- Zhang, B.X.; Huang, B.; Jiang, Q.; Loor, J.; Lv, X.; Zhang, W.; Li, M.; Wen, J.; Yin, Y.; Wang, J.; et al. Transcriptomics of circulating neutrophils in dairy cows with subclinical hypocalcemia. Front. Vet. Sci. 2022, 9, 959831. [Google Scholar] [CrossRef]
- Martinez, N.; Sinedino, L.; Bisinotto, R.; Ribeiro, E.; Gomes, G.; Lima, F.; Greco, L.; Risco, C.; Galvão, K.; Taylor-Rodriguez, D.; et al. Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J. Dairy Sci. 2014, 97, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; et al. Inflammatory biomarkers are associated with ketosis in periparturient Holstein cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Kinet, J. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef]
- Trebicka, J.; Krag, A.; Gansweid, S.; Appenrodt, B.; Schiedermaier, P.; Sauerbruch, T.; Spengler, U. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1218–1225. [Google Scholar] [CrossRef]
- Munford, R.S.; Hall, C.L.; Dietschy, J.M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect. Immun. 1981, 34, 835–843. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Wright, S.D. Lipopolysaccharide (LPS) binding protein catalyzes binding of LPS to lipoproteins. Prog. Clin. Biol. Res. 1995, 392, 287–295. [Google Scholar] [PubMed]
- Kitchens, R.L.; Wolfbauer, G.; Albers, J.J.; Munford, R.S. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 1999, 274, 34116–34122. [Google Scholar] [CrossRef]
- Tobias, P.S.; Soldau, K.; Ulevitch, R.J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J. Biol. Chem. 1989, 264, 10867–10871. [Google Scholar] [CrossRef]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P. Ionized hypocalcemia during sepsis. Crit. Care Med. 2000, 28, 266–268. [Google Scholar] [CrossRef]
- Skarnes, R.C.; Chedid, L. Biological degradation and inactivation of endotoxin (chromate-labeled). In Bacterial Endotoxins; Landy, M., Braun, W., Eds.; Rutgers University Press: New Brunswick, NJ, USA, 1964; pp. 575–587. [Google Scholar]
- Hotchkiss, R.S.; Karl, I.E. Calcium: A regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996, 4, 58–71. [Google Scholar]
- Feingold, K.R.; Grunfeld, C. Lipids: A key player in the battle between the host and microorganisms. J. Lipid Res. 2012, 53, 2487–2489. [Google Scholar] [CrossRef]
- Gibot, S. On the origins of lactate during sepsis. Crit. Care 2012, 16, 151. [Google Scholar] [CrossRef]
- Toffaletti, J.; Abrams, B. Effects of in vivo and in vitro production of lactic acid on ionized, protein-bound, and complex-bound calcium in blood. Clin. Chem. 1989, 35, 935–938. [Google Scholar] [CrossRef]
- Khatua, B.; Yaron, J.R.; El-Kurdi, B.; Kostenko, S.; Papachristou, G.I.; Singh, V.P. Ringer’s lactate prevents early organ failure by providing extracellular calcium. J. Clin. Med. 2020, 9, 263. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Willey, S.; Tomasic, P.; Chernow, B. Free fatty acids alter calcium binding: A cause for misinterpretation of serum calcium values and hypocalcemia in critical illness. J. Clin. Endocrinol. Metab. 1987, 64, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.; Tsang, R.C. In vitro effects of fatty acids on serum-ionized calcium. J. Pediatr. 1977, 91, 233–236. [Google Scholar] [CrossRef]
- Chapinal, N.; Leblanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.O. Protein-bound calcium in human serum: Quantitative examination of binding and its variables by a molecular binding model and clinical chemical implications for measurement of ionized calcium. Scand. J. Clin. Lab. Investig. 1972, 30, 321–329. [Google Scholar] [CrossRef]
- Dimeski, G.; Treacy, O. The influence of albumin and pH on total and ionized calcium and magnesium. Point Care 2018, 17, 123–126. [Google Scholar] [CrossRef]
- Dalal, P.; Muller, W.; Sullivan, D. Endothelial cell calcium signaling during barrier function and inflammation. Am. J. Pathol. 2019, 190, 535–542. [Google Scholar] [CrossRef]
- Ridefelt, P.; Helmersson-Karlqvist, J. Albumin adjustment of total calcium does not improve the estimation of calcium status. Scand. J. Clin. Lab. Investig. 2017, 77, 442–447. [Google Scholar] [CrossRef]
- Buege, M.; Do, B.; Lee, H.; Weber, D.; Horowitz, S.; Feng, L.; Qing, Y.; Shank, B. Corrected calcium versus ionized calcium measurements for identifying hypercalcemia in patients with multiple myeloma. Cancer Treat. Res. Commun. 2019, 21, 100159. [Google Scholar] [CrossRef]
- Gilabert, J. Cytoplasmic calcium buffering: An integrative crosstalk. Adv. Exp. Med. Biol. 2020, 1131, 163–182. [Google Scholar] [CrossRef]
- Kraus-Friedmann, N. Calcium sequestration in the liver. Cell Calcium 1990, 11, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Baird, G.S. Ionized calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Contri, M.; Boraldi, F.; Taparelli, F.; Paepe, A.; Ronchetti, I. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am. J. Pathol. 1996, 148, 569–577. [Google Scholar]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Bazil, J.N.; Blomeyer, C.A.; Pradhan, R.K.; Camara, A.K.; Dash, R.K. Modeling the calcium sequestration system in isolated guinea pig cardiac mitochondria. J. Bioenerg. Biomembr. 2013, 45, 177–188. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef]
- Zikan, V.; Stepan, J.J. Marker of bone resorption in acute response to exogenous or endogenous parathyroid hormone. Biomark. Insights 2008, 3, 19–24. [Google Scholar] [CrossRef]
- Cianferotti, L.; Gomes, A.R.; Fabbri, S.; Tanini, A.; Brandi, M.L. The calcium-sensing receptor in bone metabolism: From bench to bedside and back. Osteoporos. Int. 2015, 26, 2055–2071. [Google Scholar] [CrossRef]
- Hatate, K.; Kawashima, C.; Hanada, M.; Kayano, M.; Yamagishi, N. Short communication: Serum osteoprotegerin concentrations in periparturient dairy cows. J. Dairy Sci. 2018, 101, 6622–6626. [Google Scholar] [CrossRef]
- Roux, E.; Marhl, M. Role of sarcoplasmic reticulum and mitochondria in Ca2+ removal in airway myocytes. Biophys. J. 2004, 86, 2583–2595. [Google Scholar] [CrossRef]
- Stammers, A.; Susser, S.; Hamm, N.; Hlynsky, M.; Kimber, D.; Kehler, D.; Duhamel, T. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA). Can. J. Physiol. Pharmacol. 2015, 93, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Treves, S.; Jungbluth, H.; Muntoni, F.; Zorzato, F. Congenital muscle disorders with cores: The ryanodine receptor calcium channel paradigm. Curr. Opin. Pharmacol. 2008, 8, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Barritt, G. Calcium signalling in liver cells. In Calcium Signalling in Liver Cells; Springer: Berlin/Heidelberg, Germany, 2000; pp. 73–94. [Google Scholar] [CrossRef]
- Rieusset, J. Endoplasmic reticulum–mitochondria calcium signaling in hepatic metabolic diseases. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 865–876. [Google Scholar] [CrossRef]
- Oliva-Vilarnau, N.; Hankeova, S.; Vorrink, S.U.; Mkrtchian, S.; Andersson, E.R.; Lauschke, V.M. Calcium signaling in liver injury and regeneration. Front. Med. 2018, 5, 192. [Google Scholar] [CrossRef]
- Jagtap, Y.; Adlakha, N. Simulation of buffered advection diffusion of calcium in a hepatocyte cell. Math. Biol. Bioinform. 2018, 13, 609–619. [Google Scholar] [CrossRef]
- Kang, Y.; McKenna, T.; Watson, L.; Williams, R.; Holt, M. Cytochemical changes in hepatocytes of rats with endotoxemia or sepsis: Localization of fibronectin, calcium, and enzymes. J. Histochem. Cytochem. 1988, 36, 665–678. [Google Scholar] [CrossRef]
- Bernardo, J.; Friedman, P. Renal calcium metabolism. In Renal Physiology; Academic Press: London, UK, 2013; pp. 2225–2247. [Google Scholar] [CrossRef]
- Lim, D.; Semyanov, A.; Genazzani, A.; Verkhratsky, A. Calcium signaling in neuroglia. Int. Rev. Cell Mol. Biol. 2021, 362, 1–53. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, T.; Pereira, A.; Verkhratsky, A.; Huang, J. Glial cells and synaptic plasticity. Neural Plast. 2016, 2016, 5042902. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Racchetti, G.; Melino, G.; Meldolesi, J. Cytosolic Ca2+ buffering, a cell property that in some neurons markedly decreases during aging, has a protective effect against NMDA/nitric oxide-induced excitotoxicity. Life Sci. 1996, 59, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Watanabe, A.; Yamamoto, T. Regulatory mechanisms of mitochondrial calcium uptake by the calcium uniporter complex. Biophys. Physicobiol. 2023, 20, e200004. [Google Scholar] [CrossRef] [PubMed]
- Denton, R. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef]
- Alvarez, S.; Evelson, P.; Vanasco, V.; Magnani, N.; Cimolai, M.; Marchini, T. The role of mitochondria in inflammatory syndromes. In Mitochondria in Inflammation; Tech Science Press: Nanjing, China, 2016; pp. 233–247. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta 2015, 1852, 792–804. [Google Scholar] [CrossRef]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; et al. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef]
- Li, F.; Guan, Z.; Gao, Y.; Bai, Y.; Zhan, X.; Ji, X.; Xu, J.; Zhou, H.; Rao, Z. ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury. Hepatol. Commun. 2024, 8, e0399. [Google Scholar] [CrossRef]
- Briones-Suarez, L.; Cifuentes, M.; Bravo-Sagua, R. Secretory factors from calcium-sensing receptor-activated SW872 pre-adipocytes induce cellular senescence and a mitochondrial fragmentation-mediated inflammatory response in HepG2 cells. Int. J. Mol. Sci. 2023, 24, 5217. [Google Scholar] [CrossRef]
- Williams, G.S.; Boyman, L.; Chikando, A.C.; Khairallah, R.J.; Lederer, W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef]
- Zhou, Y.; Greka, A. Calcium-permeable ion channels in the kidney. Am. J. Physiol. Renal Physiol. 2016, 310, F1157–F1167. [Google Scholar] [CrossRef][Green Version]
- Woudenberg-Vrenken, T.; Bindels, R.; Hoenderop, J. The role of transient receptor potential channels in kidney disease. Nat. Rev. Nephrol. 2009, 5, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Castro, S.; Garofalo, R. The calcium-sensing receptor as a mediator of inflammation. Semin. Cell Dev. Biol. 2016, 49, 52–56. [Google Scholar] [CrossRef]
- Zhao, B. Does TNF promote or restrain osteoclastogenesis and inflammatory bone resorption? Crit. Rev. Immunol. 2018, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tupling, A. The sarcoplasmic reticulum in muscle fatigue and disease: Role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can. J. Appl. Physiol. 2004, 29, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Premkumar, P.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H. Restoration of SERCA ATPase as an intervention to muscle impairment associated with oxidative stress. FASEB J. 2018, 32, 618-15. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Esmon, C. The impact of the inflammatory response on coagulation. Thromb. Res. 2004, 114, 321–327. [Google Scholar] [CrossRef]
- Mohanty, J.; Barodka, V.; Berkowitz, D.; Rifkind, J. Intracellular calcium mediated stiffness of red blood cells is reversed by hypoxic pre-incubation with nitrite ions. Biophys. J. 2010, 98, 77a. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Kirdajova, D.; Kriska, J.; Tureckova, J.; Anděrová, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Muller, W. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Shannon, T.; Ginsburg, K.; Bers, D. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 2000, 78, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Giacomo, V. Extracellular matrix: Immunity and inflammation. In Extracellular Matrix; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–109. [Google Scholar] [CrossRef]
- Touyz, R.; Alves-Lopes, R.; Rios, F.; Camargo, L.; Anagnostopoulou, A.; Arner, A.; Montezano, A. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in vascular smooth muscle cell elasticity and adhesion: Novel insights into the mechanism of action. Front. Physiol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Carlstedt, F.; Lind, L. Hypocalcemic syndromes. Crit. Care Clin. 2001, 17, 139–153. [Google Scholar] [CrossRef]
- Klein, G. Phosphate as an adjunct to calcium in promoting coronary vascular calcification in chronic inflammatory states. eLife 2024, 13, e91808. [Google Scholar] [CrossRef]
- Edwards, F.; Taheri, A.; Dann, S.; Dye, J. Characterization of cytolytic neutrophil activation in vitro by amorphous hydrated calcium phosphate as a model of biomaterial inflammation. J. Biomed. Mater. Res. Part A 2011, 96, 552–565. [Google Scholar] [CrossRef]
- Chen, J.; Sitsel, A.; Benoy, V.; Sepúlveda, M.; Vangheluwe, P. Primary active Ca2+ transport systems in health and disease. Cold Spring Harb. Perspect. Biol. 2019, 12, a035113. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac Excitation-Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Wong, P.T. Isolation of a High Capacity Ca2+-Binding Protein from Sarcoplasmic Reticulum. Nat. New Biol. 1971, 231, 198–200. [Google Scholar]
- Beard, N.A.; Laver, D.R.; Dulhunty, A.F. Calsequestrin and the Calcium Release Channel of Skeletal and Cardiac Muscle. Prog. Biophys. Mol. Biol. 2004, 85, 33–69. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Neuronal Calcium Signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A Prototypical Calcium Sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Kalyanasundaram, A. SERCA Pump Isoforms: Their Role in Calcium Transport and Disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. The Puzzle of TRPV4 Channelopathies. EMBO Rep. 2013, 14, 152–163. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Lederer, W.J. Sodium/Calcium Exchange: Its Physiological Implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. The Plasma Membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb. Perspect. Biol. 2011, 3, a004168. [Google Scholar] [CrossRef]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ Signalling in the Golgi Apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pozzan, T. Microdomains of Intracellular Ca2+: Molecular Determinants and Functional Consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Pozzan, T. Golgi Ca2+, Messengers and Function. Cell Calcium 2007, 42, 405–412. [Google Scholar] [CrossRef]
- Brown, E.M. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 1991, 71, 371–411. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.M.; Brown, E.M. Extracellular calcium sensing and signalling. In Calcium as a Cellular Regulator; Carafoli, E., Klee, C.B., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 233–261. [Google Scholar]
- Conigrave, A.D.; Ward, D.T. Calcium-sensing receptor (CaSR): Pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 315–331. [Google Scholar] [CrossRef]
- Brennan, S.C.; Thiem, U.; Roth, S.; Aggarwal, A.; Fetahu, I.S.; Tennakoon, S.; Gomes, A.R.; Brandi, M.L.; Bruggeman, F.; Mentaverri, R.; et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 1732–1744. [Google Scholar] [CrossRef]
- Hannan, F.M.; Babinsky, V.N.; Thakker, R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2018, 61, R1–R22. [Google Scholar] [CrossRef]
- Hofer, A.M.; Brown, E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003, 2, 521–527. [Google Scholar] [CrossRef]
- Hendy, G.N.; Canaff, L. Calcium-Sensing Receptor Gene: Regulation of Expression. Front Physiol. 2016, 7, 394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Busic-Pavlek, I.; Dumic-Cule, I.; Kovacevic, L.; Milosevic, M.; Delimar, P.; Korsa, L.; Marusic, Z.; Prutki, M. Calcium-Sensing Receptor Expression in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 11678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olszak, I.T.; Poznansky, M.C.; Evans, R.H.; Olson, D.; Kos, C.; Pollak, M.R.; Brown, E.M.; Scadden, D.T. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J. Clin. Investig. 2000, 105, 1299–1305. [Google Scholar] [CrossRef]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schöneberg, T.; Schaefer, M.; Krügel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Schlam, D.; Breuer, C.; Gütschow, M.; Glogauer, M.; Grinstein, S. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of large particles and bacteria by macrophages. Cell. Mol. Life Sci. 2016, 73, 3749–3763. [Google Scholar]
- Zhai, T.Y.; Cui, B.H.; Zou, L.; Zeng, J.Y.; Gao, S.; Zhao, Q.; Wang, Y.; Xie, W.L.; Sun, Y.H. Expression and Role of the Calcium-Sensing Receptor in Rat Peripheral Blood Polymorphonuclear Neutrophils. Oxid. Med. Cell. Longev. 2017, 2017, 3869561. [Google Scholar] [CrossRef] [PubMed]
- Samakai, E.; Go, C.; Soboloff, J. Defining the Roles of Ca2+ Signals during T Cell Activation. In Signaling Mechanisms Regulating T Cell Diversity and Function; Soboloff, J., Kappes, D.J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; Chapter 10. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532321/ (accessed on 5 December 2024). [CrossRef]
- Werner, L.E.; Wagner, U. Calcium-Sensing Receptor-Mediated NLRP3 Inflammasome Activation in Rheumatoid Arthritis and Autoinflammation. Front. Physiol. 2023, 13, 1078569. [Google Scholar] [CrossRef]
- Liu, W.; Guo, Y.; Liu, Y.; Sun, J.; Yin, X. Calcium-Sensing Receptor of Immune Cells and Diseases. Cardiovasc. Innov. Appl. 2021, 5, 257–266. [Google Scholar] [CrossRef]
- Jäger, E.; Murthy, S.; Schmidt, C.; Hahn, M.; Strobel, S.; Peters, A.; Stäubert, C.; Sungur, P.; Venus, T.; Geisler, M.; et al. Calcium-Sensing Receptor-Mediated NLRP3 Inflammasome Response to Calciprotein Particles Drives Inflammation in Rheumatoid Arthritis. Nat. Commun. 2020, 11, 4243. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Hu, Z.; He, L.; Wang, W.; Xu, J.; Fan, Z.; Liu, C.; Zhang, H.; Zhao, K. Prokineticin 2 via Calcium-Sensing Receptor Activated NLRP3 Inflammasome Pathway in the Testicular Macrophages of Uropathogenic Escherichia coli-Induced Orchitis. Front. Immunol. 2020, 11, 570872. [Google Scholar] [CrossRef]
- Miyazono, K. Transforming Growth Factor-Beta Signaling in Epithelial-Mesenchymal Transition and Progression of Cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 314–323. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhong, H.; Qu, Y.; Xi, D.; Tang, N.; He, F. Calcium-Sensitive Receptors-Mediated Macrophage Polarization via the PLC-Ca2+ and cAMP-NF-κB-NLRP3 Inflammasome Signalling Pathways Impact Cardiac Fibroblast Activation through Paracrine Effects. ESS Open Arch. eprints 2024, 65, 06544864. [Google Scholar] [CrossRef]
- Liu, J.O. Calmodulin-Dependent Phosphatase, Kinases, and Transcriptional Corepressors Involved in T-Cell Activation. Immunol. Rev. 2009, 228, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, A.M.; Humphries, L.A.; Rawlings, D.J. Calcium Signalling and Cell-Fate Choice in B Cells. Nat. Rev. Immunol. 2007, 7, 778–789. [Google Scholar] [CrossRef]
- Kadowaki, N.; Antonenko, S.; Lau, J.Y.; Liu, Y.J. Natural Interferon Alpha/Beta-Producing Cells Link Innate and Adaptive Immunity. J. Exp. Med. 2000, 192, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium Overload and Mitochondrial Metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Zaloga, G.P. Hypocalcemia in critically ill patients. Crit. Care Med. 1992, 20, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.; Barca, J.; Pereira, I.; Meikle, A.; Ruprechter, G. Association between non-esterified fatty acids and calcium concentrations at calving with early lactation clinical diseases, fertility and culling in grazing dairy cows in Uruguay. Prev. Vet. Med. 2024, 230, 106294. [Google Scholar] [CrossRef] [PubMed]
- Duran-Güell, M.; Flores-Costa, R.; Casulleras, M.; López-Vicario, C.; Titos, E.; Díaz, A.; Alcaraz-Quiles, J.; Horrillo, R.; Costa, M.; Fernández, J.; et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J. 2021, 35, e21365. [Google Scholar] [CrossRef]
- Levels, J.H.; Marquart, J.A.; Abraham, P.R.; van den Ende, A.E.; Molhuizen, H.O.; van Deventer, S.J.; Meijers, J.C. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect. Immun. 2005, 73, 2321–2326. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M.; Fitting, C.; Adrie, C.; Payen, D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003, 35, 535–544. [Google Scholar] [CrossRef]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis—Risk factor and therapeutic approach. Front. Pharmacol. 2015, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Chandler, T.L.; Westhoff, T.A.; Behling Kelly, E.L.; Sipka, A.S.; Mann, S. Eucalcemia during lipopolysaccharide challenge in postpartum dairy cows: I. Clinical, inflammatory, and metabolic response. J. Dairy Sci. 2023, 106, 3586–3600. [Google Scholar] [CrossRef]
- Horst, E.A.; Mayorga, E.J.; Al Qaisi, M.; Abeyta, M.A.; Portner, S.L.; McCarthy, C.S.; Goetz, B.M.; Kvidera, S.K.; Baumgard, L.H. Effects of maintaining eucalcemia following immunoactivation in lactating Holstein dairy cows. J. Dairy Sci. 2020, 103, 7472–7486. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.R.; Nonnecke, B.J.; Nishida, T.; Horst, R.L.; Overton, T.R. Effect of lipopolysaccharide infusion on serum macromineral and vitamin D concentrations in dairy cows. J. Dairy Sci. 2003, 86, 3440–3446. [Google Scholar] [CrossRef]
- Seminara, J.A.; Seely, C.R.; McArt, J.A.A. Acute phase responses in clinically healthy multiparous Holsteins with and without calcium dysregulation during the early postpartum period. J. Dairy Sci. 2024, 108, 1930–1939. [Google Scholar] [CrossRef]
- Couto Serrenho, R.; Morrison, E.; Bruinjé, T.C.; LeBlanc, S.J. Assessment of systemic inflammation following oral calcium supplementation in healthy postpartum multiparous dairy cows—A randomized controlled trial. JDS Commun. 2023, 5, 134–138. [Google Scholar] [CrossRef]
- Aberegg, S.K. Ionized Calcium in the ICU: Should It Be Measured and Corrected? Chest 2016, 149, 846–855. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Neves, R.C. Association of transient, persistent, or delayed subclinical hypocalcemia with early lactation disease, removal, and milk yield in Holstein cows. J. Dairy Sci. 2020, 103, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Long-latency deficiency disease: Insights from calcium and vitamin D. Am. J. Clin. Nutr. 2003, 78, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Rappolt, M.; Schromm, A.; Howe, J.; Lohner, K.; Andrä, J.; Koch, M.; Brandenburg, K. Divalent cations affect chain mobility and aggregate structure of lipopolysaccharide from Salmonella minnesota reflected in a decrease of its biological activity. Biochim. Biophys. Acta 2005, 1715, 122–131. [Google Scholar] [CrossRef]
- Redeker, C.; Briscoe, W. Interactions between mutant bacterial lipopolysaccharide (LPS Ra) surface layers: Surface vesicles, membrane fusion, and effect of Ca2+ and temperature. Langmuir 2019, 35, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4 MD2 for efficient LPS recognition and transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef]
- Drago Serrano, M.E.; de la Garza Amaya, M.; Luna, J.S.; Campos Rodríguez, R. Lactoferrin lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Wright, S.D. Lipopolysaccharide binding protein and soluble CD14 transfer lipopolysaccharide to phospholipid bilayers: Preferential interaction with particular classes of lipid. J. Immunol. 1997, 158, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Takeuchi, S.; Kubota, K.; Kobayashi, Y.; Kozakai, S.; Ukai, I.; Shichiku, A.; Okubo, M.; Numasaki, M.; Kanemitsu, Y.; et al. Lipopolysaccharide (LPS) binding protein stimulates CD14 dependent toll-like receptor 4 internalization and LPS-induced TBK1 IKKϵ IRF3 axis activation. J. Biol. Chem. 2018, 293, 10186–10201. [Google Scholar] [CrossRef]
- Uhrig, A.; Banafsche, R.; Kremer, M.; Hegenbarth, S.; Hamann, A.; Neurath, M.; Gerken, G.; Limmer, A.; Knolle, P.A. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 2005, 77, 626–633. [Google Scholar] [CrossRef]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Leelahavanichkul, A.; Kurlander, R.; Chen, Z.; Souza, A.C.; Yuen, P.S.; Star, R.A.; Csako, G.; et al. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J. Immunol. 2012, 188, 1371–1380. [Google Scholar] [CrossRef]
- Van Bossuyt, H.; Wisse, E. Structural changes produced in Kupffer cells in the rat liver by injection of lipopolysaccharide. Cell Tissue Res. 1988, 251, 205–214. [Google Scholar] [CrossRef]
- Tamura, Y.; Osuga, J.; Adachi, H.; Tozawa, R.; Takanezawa, Y.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; et al. Scavenger receptor expressed by endothelial cells I (SREC I) mediates the uptake of acetylated low-density lipoproteins by macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 2004, 279, 30938–30944. [Google Scholar] [CrossRef] [PubMed]
- König, V.; Hopf, U.; Möller, B.; Lobeck, H.; Assmann, G.; Freudenberg, M.; Galanos, C. The significance of high-density lipoproteins (HDL) in the clearance of intravenously administered bacterial lipopolysaccharides (LPS) in mice. Hepatogastroenterology 1988, 35, 111–115. [Google Scholar] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Kumar, P.; Schroder, E.A.; Rajaram, M.V.S.; Harris, E.N.; Ganesan, L.P. The battle of LPS clearance in host defense vs. inflammatory signaling. Cells 2024, 13, 1590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ametaj, B.N. The Calci-Inflammatory Network: A Paradigm Shift in Understanding Milk Fever. Dairy 2025, 6, 22. https://doi.org/10.3390/dairy6030022
Ametaj BN. The Calci-Inflammatory Network: A Paradigm Shift in Understanding Milk Fever. Dairy. 2025; 6(3):22. https://doi.org/10.3390/dairy6030022
Chicago/Turabian StyleAmetaj, Burim N. 2025. "The Calci-Inflammatory Network: A Paradigm Shift in Understanding Milk Fever" Dairy 6, no. 3: 22. https://doi.org/10.3390/dairy6030022
APA StyleAmetaj, B. N. (2025). The Calci-Inflammatory Network: A Paradigm Shift in Understanding Milk Fever. Dairy, 6(3), 22. https://doi.org/10.3390/dairy6030022





