Rehydrated Corn Grain Silage and Exogenous Protease: Effects on Dairy Cow Performance, Metabolism, and Starch Digestibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sampling and Chemical Analysis
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diogénes, L.V.; Pereira Filho, J.M.; Edvan, R.L.; de Oliveira, J.P.F.; Nascimento, R.R.d.; Santos, E.M.; Alencar, E.J.S.; Mazza, P.H.S.; Oliveira, R.L.; Bezerra, L.R. Effect of Different Additives on the Quality of Rehydrated Corn Grain Silage: A Systematic Review. Ruminants 2023, 3, 425–444. [Google Scholar] [CrossRef]
- Gandra, J.R.; Oliveira, E.R.; Takiya, C.S.; Del Valle, T.A.; Rennó, F.P.; Goes, R.H.T.B.; Leite, R.S.R.; Garcia, N.F.L.; Batista, J.D.O.; Acosta, A.P.; et al. Amylolytic activity and chemical composition of rehydrated ground maize ensiled with α-amylase or glucoamylase. J. Agric. Sci. 2019, 157, 449–455. [Google Scholar] [CrossRef]
- Oliveira, E.R.; Takiya, C.S.; Del Valle, T.A.; Rennó, F.P.; Goes, R.H.T.B.; Leite, R.S.R.; Oliveira, K.M.P.; Batista, J.D.O.; Araki, H.M.C.; Damiani, J.; et al. Effects of exogenous amylolytic enzymes on fermentation, nutritive value, and in vivo digestibility of rehydrated corn silage. Anim. Feed Sci. Technol. 2019, 251, 86–95. [Google Scholar] [CrossRef]
- Castro, L.P.; Pereira, M.N.; Dias, J.D.L.; Lage, D.V.D.; Barbosa, E.F.; Melo, R.P.; Ferreira, K.; Carvalho, J.T.R.; Cardoso, F.F.; Pereira, R.A.N. Lactation performance of dairy cows fed rehydrated and ensiled corn grain differing in particle size and proportion in the diet. J. Dairy Sci. 2019, 102, 9857–9869. [Google Scholar] [CrossRef]
- Kung Jr, L.; Windle, M.C.; Walker, N. The effect of an exogenous protease on the fermentation and nutritive value of high-moisture corn. J. Dairy Sci. 2014, 97, 1707–1712. [Google Scholar] [CrossRef]
- Windle, M.C.; Walker, N.; Kung, L. Effects of an exogenous protease on the fermentation and nutritive value of corn silage harvested at different dry matter contents and ensiled for various lengths of time. J. Dairy Sci. 2014, 97, 3053–3060. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, C.M. The Effect of Feed Processing on Ruminant Performance: Methods and Applications. J. Dairy Sci. 1993, 76, 1412–1424. [Google Scholar]
- Fredin, S.M.; Ferraretto, L.F.; Akins, M.S.; Hoffman, R.D.; Shaver, R.D. Fecal starch as an indicator of total-tract starch digestibility by lactating dairy cows. J. Dairy Sci. 2014, 97, 1862–1871. [Google Scholar] [CrossRef]
- Wildman, E.E.; Jones, G.W.; Wagner, P.E.; Boulton, A.E.; Davis, J.D. A Dairy Herd Milk Production and Evaluation Program. J. Dairy Sci. 1982, 65, 785–792. [Google Scholar]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Valadares, R.F.D.; Valadares Filho, S.D.C.; Cecon, P.R.; Rennó, L.N.; Queiroz, A.C.D.; Chizzotti, M.L. Microbial protein production and estimation of purine and urea excretion in lactating cows fed isoproteic diets containing different levels of non-protein nitrogen compounds. Rev. Bras. Zootec. 2001, 30, 1621–1629. [Google Scholar] [CrossRef]
- Chen, X.B.; Chen, Y.K.; Franklin, M.F.; Ørskov, E.R.; Shand, W.J. The Effect of Feed Intake and Body Weight on Purine Derivative Excretion and Microbial Protein Supply in Sheep. J. Anim. Sci. 1992, 70, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, T.; Ørskov, E.R.; Reeds, P.J.; Kyle, D.J. The effect of protein infusion on urinary excretion of purine derivatives in ruminants nourished by intragastric nutrition. J. Dairy Sci. 1987, 109, 7–12. [Google Scholar] [CrossRef]
- Gonzalez-Ronquillo, M.; Balcells, J.; Guada, J.A.; Vicente, F. Purine derivative excretion in dairy cows: Endogenous excretion and the effect of exogenous nucleic acid supply. J. Dairy Sci. 2003, 86, 1282–1291. [Google Scholar] [CrossRef]
- Valadares, R.; Broderick, G.; Filho, S.V.; Clayton, M. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 1999, 82, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lock, A.L.; Garnsworthy, P.C. Technical Note: A Rapid Lipid Separation Method for Determining Fatty Acid Composition of Milk. J. Dairy Sci. 2004, 87, 3785–3788. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Fellner, V.; Dugan, M.E.R.; Sauer, F.D.; Mossoba, M.M.; Yurawecz, M.P. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 1997, 32, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biolog. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Huntington, G.B. Starch Utilization by Ruminants: From Basics to the Bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar] [CrossRef]
- Kozloski, V.G. Ruminal Microbial Metabolism, 3rd ed.; UFSM Press: Santa Maria, Brazil, 2011. [Google Scholar]
- Guimarães, G.S.; Azevedo, J.A.G.; Cairo, F.C.; Mota, P.F.; Lima, D.C.; Pereira, J.D.A.; Silva, R.A.; Souza, M.P.; Azevedo, R.M.; Ribeiro, L.V.; et al. Proportions of Concentrate and Rehydrated Ground Grain Corn Silage at Different Storage Times for Better Use of Starch by Lambs. Trop. Anim. Health Prod. 2022, 54, 297. [Google Scholar] [CrossRef]
- Arcari, M.A.; Silva, G.; Martins, R.D.; Souza, J.; Oliveira, S.; Ribeiro, L. Effect of Substituting Dry Corn with Rehydrated Ensiled Corn on Dairy Cow Milk Yield and Nutrient Digestibility. Anim. Feed Sci. Technol. 2016, 221, 167–173. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Crump, P.M.; Shaver, R.D. Effect of cereal grain type and corn grain harvesting and processing methods on intake, digestion, and milk production by dairy cows through a meta-analysis. J. Dairy Sci. 2013, 96, 533–550. [Google Scholar] [CrossRef]
- Batista, J.D.O.; Gandra, J.R.; Del Valle, T.A.; Takiya, C.S.; de Goes, R.H.T.B.; Oliveira, E.R.; Pedrini, C.A. Effects of Amylase and Glucoamylase on Rehydrated Corn Ensiled for an Extended Period: Nutritive Value, Fermentation Profile, and Amylolytic Activity. N. Z. J. Agric. Res. 2021, 65, 520–533. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Effects of Corn Grain Conservation Method on Feeding Behavior and Productivity of Lactating Dairy Cows at Two Dietary Starch Concentrations. J. Dairy Sci. 2003, 86, 174–183. [Google Scholar] [CrossRef]
- Passini, R.; Jorge, A.M.; Resende, F.D.; Moreira, F.B.; Gomes, J.D.; Nogueira, J.R. Digestibilidade de dietas à base de grão úmido de milho ou de sorgo ensilados. Acta Sci. Anim. Sci. 2008, 24, 1147. [Google Scholar] [CrossRef]
- Corrêa, C.E.S.; Shaver, R.D.; Pereira, M.N.; Lauer, J.G.; Kohn, K. Relationship Between Corn Vitreousness and Ruminal In Situ Starch Degradability. J. Dairy Sci. 2002, 85, 3008–3012. [Google Scholar] [CrossRef] [PubMed]
- Villot, C.; Maillard, M.B.; Bernaud, J.; Chauveau, L.; Gaillard, J.L.; Leduc, C.; Giger-Reverdin, S.; Martin, C.; Leroux, C.; Maton, C. Combinations of Non-Invasive Indicators to Detect Dairy Cows Submitted to High-Starch-Diet Challenge. Animal 2020, 14, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.; Soderlund, S. Ruminal and Postruminal Starch Digestion by Cattle. In Cattle Grain Processing Symposium; Pioneer Hi-Bred—A DuPont Business: Johnston, IA, USA, 2007; pp. 1–23. [Google Scholar]
- Durães, H.F.; Oliveira, E.R.; Silva, J.T.; Gandra, J.R.; Teixeira, R.A.; Neves, N.F.; Gabriel, A.M.A.; Monção, F.P.; Rigueira, J.P.S.; Carvalho, C.D.C.S.; et al. Rehydrated Corn Ensiled with Different Concentrations of Protease in the Diet of Dairy Cows: Impacts on Intake, Digestibility, Ruminal and Blood Parameters, and Milk Yield and Composition. N. Z. J. Agric. Res. 2024, 67, 1–17 . [Google Scholar] [CrossRef]
- Mombach, M.A.; Pereira, D.H.; Pina, D.S.; Bolson, D.C.; Pedreira, B.C. Silage of Rehydrated Corn Grain. Arq. Bras. Med. Vet. Zootec. 2019, 71, 959–966. [Google Scholar] [CrossRef]
- Piran Filho, F.A.; Bragatto, J.M.; Parra, C.S.; Silva, S.M.S.; Pinto, R.C.C.; Moraes, A.; Santos, T.C.; Jobim, C.C.; Owens, F.; Daniel, J.L.P. Effect of Processing Method of Rehydrated Flint Corn Grain Silage on Finishing Performance of Crossbred Angus × Nellore Bulls. Rev. Bras. Zootec. 2024, 53, e20230140. [Google Scholar] [CrossRef]
- Rosmalia, A.; Permana, I.G.; Despal, D. Synchronization of Rumen Degradable Protein with Non-Fiber Carbohydrate on Microbial Protein Synthesis and Dairy Ration Digestibility. Vet. World 2022, 15, 252–261. [Google Scholar] [CrossRef]
- Broderick, G.A. Effects of Varying Dietary Protein and Energy Levels on the Production of Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Lim, J.; Kung, L., Jr. Effect of Exogenous Protease Enzymes on the Fermentation and Nutritive Value of Corn Silage. J. Dairy Sci. 2012, 95, 6687–6694. [Google Scholar] [CrossRef]
- Arcari, M.A.; Martins, C.M.D.M.R.; Tomazi, T.; dos Santos, M.V. Effect of the ensiling time of hydrated ground corn on silage composition and in situ starch degradability. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 60. [Google Scholar] [CrossRef]
- Dhiman, T.R.; Bal, M.A.; Wu, Z.H.; Moreira, V.R.; Shaver, R.D.; Satter, L.D.; Shinners, K.J.; Walgenbach, R.P. Influence of mechanical processing on utilization of corn silage by lactating dairy cows. J. Dairy Sci. 2000, 83, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.F.; Shaver, R.D. Meta-analysis: Effect of corn silage harvest practices on intake, digestion, and milk production by dairy cows. Prof. Anim. Sci. 2012, 28, 141–149. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Reinhardt, C.D.; Schingoethe, D.J. Effects of dietary protein source on milk production and amino acid metabolism in lactating dairy cows. J. Dairy Sci. 2009, 92, 4872–4880. [Google Scholar]
- Theurer, C.B.; Oba, M.; McGuire, M.A.; Bauman, D.E.; McBride, B.W.; Huber, J.T. Invited Review: Summary of Steam-Flaking Corn or Sorghum Grain for Lactating Dairy Cows. J. Dairy Sci. 1999, 82, 1950–1959. [Google Scholar] [CrossRef]
- Grant, R. Optimizing Starch Concentrations in Dairy Rations; Tri-State Dairy Nutrition Conference: New York, NY, USA, 2005; Volume 518, pp. 73–79. [Google Scholar]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal Descriptors of Body Condition Score in Holstein Cows. J. Dairy Sci. 1999, 77, 2695–2703. [Google Scholar] [CrossRef]
- Yu, P.; Murphy, M.R.; Shaver, R.D.; Drouillard, J.S.; McGuire, M.A. Effects of Ground, Steam-Flaked, and Steam-Rolled Corn Grains on Performance of Lactating Cows. J. Dairy Sci. 1998, 81, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Lipogenesis and Adipose Tissue Cellularity in Steers Switched from Alfalfa Hay to High Concentrate Diets. J. Dairy Sci. 1983, 56, 483–492. [Google Scholar]
- Ribeiro, C.G.S.F.; Lopes, F.C.F.; Gama, M.A.S.; Morenz, M.J.F.; Rodriguez, N.M. Productive Performance and Fatty Acid Profile of Milk from Cows Fed Increasing Levels of Sunflower Oil in Elephant Grass-Based Diets. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1513–1521. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Recent Advances in Biohydrogenation of Unsaturated Fatty Acids within the Rumen Microbial Ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.; Fonseca, A.J.; Dewhurst, R.J. Factors Affecting Odd- and Branched-Chain Fatty Acids in Milk: A Review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Jorjong, S.; Van Knegsel, A.T.; Verwaeren, J.; Lahoz, M.V.; Bruckmaier, R.M.; De Baets, B.; Kemp, B.; Fievez, V. Milk Fatty Acids as Possible Biomarkers to Early Diagnose Elevated Concentrations of Blood Plasma Nonesterified Fatty Acids in Dairy Cows. J. Dairy Sci. 2014, 97, 7054–7064. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Griinari, J.M. Nutritional Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Klusmeyer, T.H.; Cameron, M.R. Microbial Protein Synthesis and Flows of Nitrogen Fractions to the Duodenum of Dairy Cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Schwab, E.C.; Shaver, R.D.; Shinners, K.J.; Lauer, J.G.; Coors, J.G. Processing and Chop Length Effects in Brown-Midrib Corn Silage on Intake, Digestion, and Milk Production by Dairy Cows. J. Dairy Sci. 2002, 85, 613–623. [Google Scholar] [CrossRef]
- Hoffman, P.C.; Esser, N.M.; Shaver, R.D.; Coblentz, W.K.; Scott, M.P.; Bodnar, A.L.; Schmidt, R.J.; Charley, R.C. Influence of Ensiling Time and Inoculation on Alteration of the Starch-Protein Matrix in High-Moisture Corn. J. Dairy Sci. 2011, 94, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.C.; Shaver, R.D.; Hoffman, P.C.; Akins, M.S.; Bertics, S.J.; Gencoglu, H.; Coors, J.G. Type of Corn Endosperm Influences Nutrient Digestibility in Lactating Dairy Cows. J. Dairy Sci. 2009, 92, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.L.; Eastridge, M.L.; St-Pierre, N.R.; Noftsger, S.M. Effects of Grain Variability and Processing on Starch Utilization by Lactating Dairy Cattle. J. Anim. Sci. 2001, 79, 218–238. [Google Scholar] [CrossRef]
- Nocek, J.E.; Tamminga, S. Site of Digestion of Starch in the Gastrointestinal Tract of Dairy Cows and Its Effect on Milk Yield and Composition. J. Dairy Sci. 1991, 74, 3598–3629. [Google Scholar] [CrossRef]
- Cooke, K.M.; Bernard, J.K. Effect of Length of Cut and Kernel Processing on Use of Corn Silage by Lactating Dairy Cows. J. Dairy Sci. 2005, 88, 310–316. [Google Scholar] [CrossRef]

| Ingredients | Experimental Diets 1 | ||
|---|---|---|---|
| CON | RCS | RCSP | |
| Corn silage | 60.00 | 60.00 | 60.00 |
| Corn meal | 26.00 | - | - |
| Rehydrated corn grain silage 2 | - | 26.00 | 26.00 |
| Rehydrated corn grain silage + protease | - | - | 26.00 |
| Soybean meal | 11.00 | 11.00 | 11.00 |
| Urea | 1.00 | 1.00 | 1.00 |
| Mineral mix 3 | 2.00 | 2.00 | 2.00 |
| Chemical composition | |||
| Dry matter | 51.70 | 49.09 | 49.13 |
| Organic matter (%DM) | 93.84 | 95.47 | 95.46 |
| Crude protein (%DM) | 15.10 | 15.24 | 15.52 |
| Fat (%DM) | 3.81 | 3.17 | 3.31 |
| Non-fiber carbohydrate (%DM) | 39.26 | 39.12 | 39.24 |
| Starch (%DM) | 23.45 | 23.25 | 23.32 |
| Neutral detergent fiber (%DM) | 35.63 | 30.95 | 31.23 |
| Neutral detergent fiber (%DM), physically effective | 24.15 | 23.56 | 23.67 |
| Acid detergent fiber (%DM) | 19.90 | 17.82 | 17.96 |
| Lignin (%DM) | 4.09 | 4.45 | 4.59 |
| Ash (%DM) | 6.16 | 4.53 | 4.54 |
| Total nutrient digestible (%DM) 4 | 64.59 | 66.38 | 66.46 |
| Net energy lactation 4 (Mcal/kg DM) | 1.44 | 1.49 | 1.49 |
| Item | Experimental Diets 1 | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | RCS | RCSP | Diet | Time | Int | C1 | C2 | ||
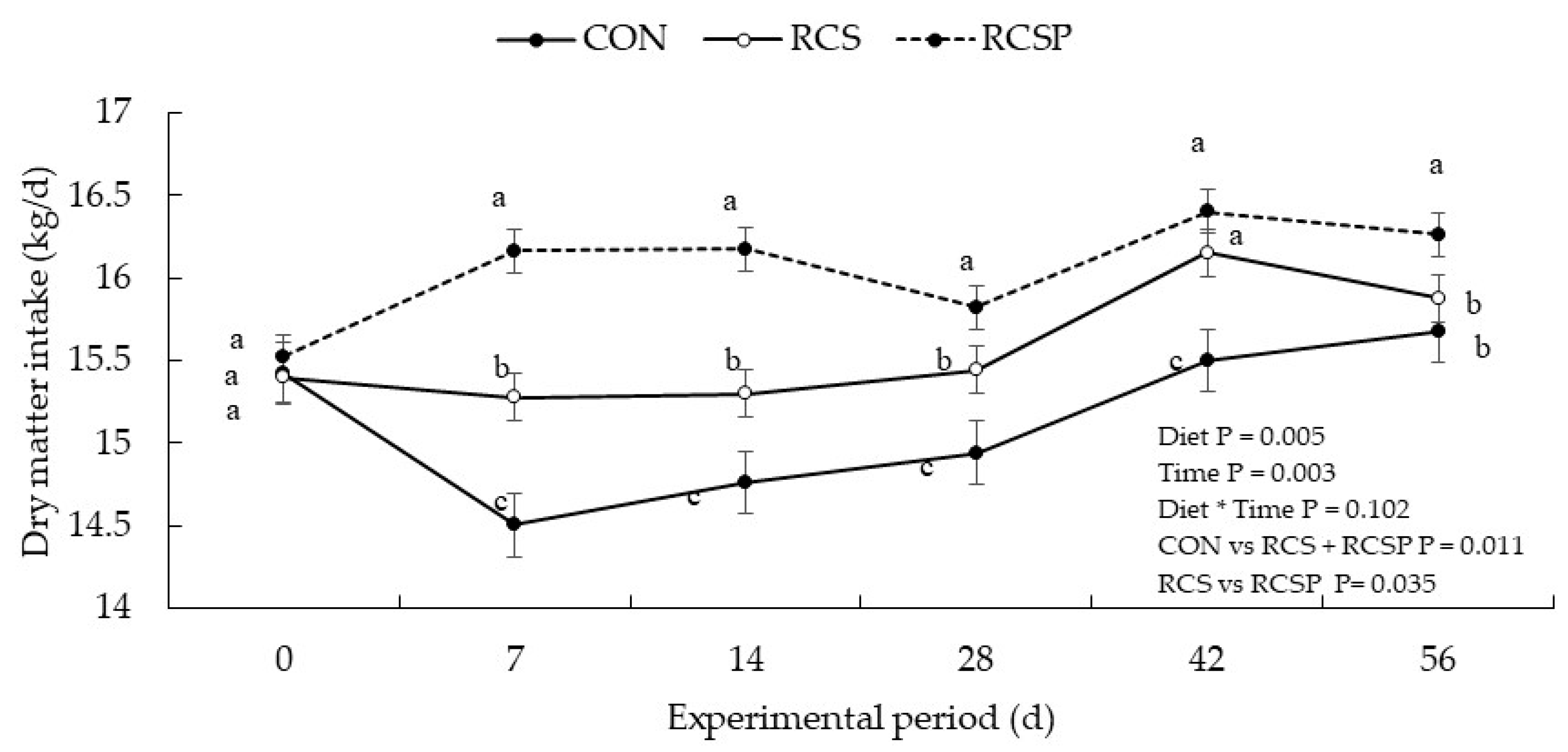
| Dry matter intake, kg/d | 15.13 | 15.57 | 16.39 | 0.207 | 0.005 | 0.003 | 0.012 | 0.011 | 0.035 |
| Dry matter intake, %BW | 2.83 | 2.92 | 3.03 | 0.029 | 0.018 | 0.075 | 0.010 | 0.030 | 0.106 |
| Efficiency | |||||||||
| MY/DMI 4 | 1.09 | 1.10 | 1.22 | 0.025 | 0.048 | 0.033 | 0.096 | 0.042 | 0.046 |
| FCM/DMI 5 | 1.31 | 1.33 | 1.35 | 0.028 | 0.231 | 0.515 | 0.335 | 0.565 | 0.423 |
| ECM/DMI 6 | 1.32 | 1.34 | 1.36 | 0.026 | 0.831 | 0.231 | 0.437 | 0.118 | 0.528 |
| Fecal starch, (%) | 11.45 | 7.51 | 4.69 | 0.012 | <0.0001 | 0.088 | 0.730 | <0.0001 | 0.015 |
| Total starch digestibility, (%) | 88.55 | 92.49 | 95.31 | 0.254 | <0.0001 | 0.088 | 0.730 | <0.0001 | 0.015 |
| Item | Experimental Diets 1 | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | RCS | RCSP | Diet | Time | Int | C1 | C2 | ||
| kg/d | |||||||||
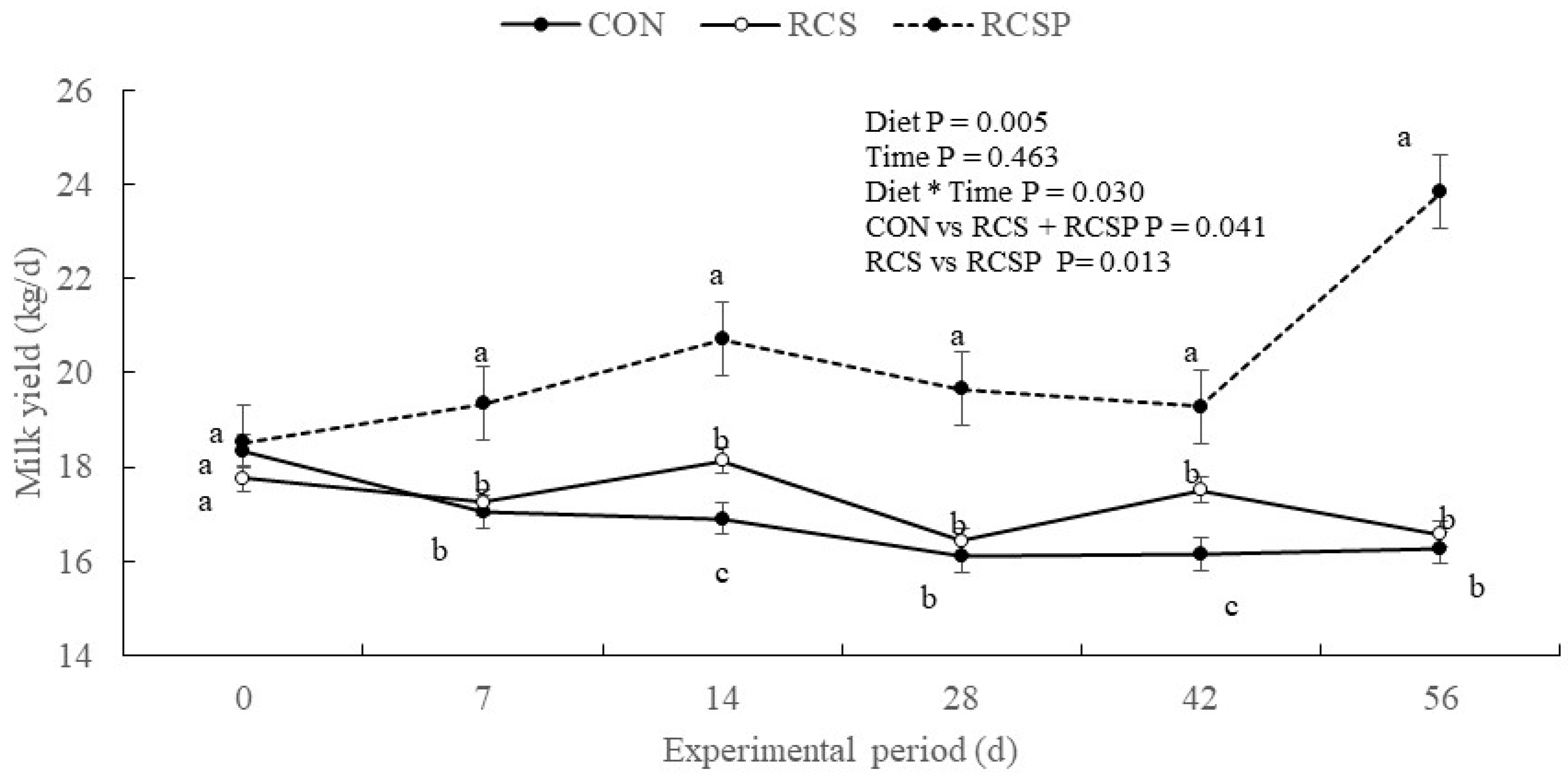
| Milk yield | 16.80 | 17.26 | 20.22 | 0.518 | 0.005 | 0.463 | 0.030 | 0.041 | 0.013 |
| Fat correct milk 3.5% | 18.56 | 17.56 | 22.66 | 0.574 | 0.001 | 0.564 | 0.055 | 0.021 | 0.002 |
| Energy correct (Mcal/d) | 19.03 | 17.89 | 22.95 | 0.552 | 0.002 | 0.502 | 0.461 | 0.032 | 0.011 |
| Fat | 0.677 | 0.614 | 0.819 | 0.022 | 0.012 | 0.547 | 0.214 | 0.124 | 0.002 |
| Protein | 0.557 | 0.605 | 0.721 | 0.015 | 0.005 | 0.321 | 0.154 | 0.002 | 0.231 |
| Lactose | 0.809 | 0.747 | 0.996 | 0.025 | 0.002 | 0.150 | 0.021 | 0.278 | 0.001 |
| Total solids | 2.41 | 2.25 | 2.61 | 0.064 | 0.111 | 0.192 | 0.065 | 0.912 | 0.038 |
| Percentage (%) | |||||||||
| Fat | 4.03 | 3.56 | 4.05 | 0.083 | 0.001 | 0.815 | 0.435 | 0.775 | 0.003 |
| Protein | 3.32 | 3.51 | 3.57 | 0.040 | 0.031 | 0.371 | 0.037 | 0.008 | 0.318 |
| Lactose | 4.37 | 4.42 | 4.55 | 0.025 | 0.052 | 0.013 | 0.390 | 0.074 | 0.098 |
| Total solids | 13.27 | 13.15 | 13.09 | 0.109 | 0.338 | 0.698 | 0.146 | 0.132 | 0.124 |
| Milk urea nitrogen (mg/dL) | 12.78 | 10.78 | 10.45 | 0.533 | 0.002 | 0.005 | 0.078 | 0.005 | 0.962 |
| Somatic cell count (Log10) | 2.62 | 2.83 | 2.30 | 0.057 | 0.023 | 0.209 | 0.069 | 0.770 | 0.032 |
| Body weight, kg | 545 | 534 | 541 | 7.800 | 0.125 | <0.0001 | 0.459 | 0.137 | 0.160 |
| Body weight movement, kg | 7.25 | 4.70 | 5.17 | 2.168 | 0.562 | 0.001 | 0.447 | 0.291 | 0.850 |
| Body condition score | 3.12 | 3.04 | 2.98 | 0.035 | 0.166 | 0.007 | 0.136 | 0.106 | 0.356 |
| Body condition score movement | 0.031 | 0.068 | 0.037 | 0.024 | 0.485 | 0.312 | 0.112 | 0.450 | 0.350 |
| Fatty Acids (g/100 g) | Experimental Diets 1 | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | RCS | RCSP | Diet | Time | Int | C1 | C2 | ||
| C4:0 | 1.615 | 1.629 | 1.603 | 0.008 | 0.312 | <0.0001 | 0.056 | 0.925 | 0.129 |
| C6:0 | 1.614 | 1.583 | 1.584 | 0.007 | 0.036 | 0.023 | 0.075 | 0.010 | 0.939 |
| C8:0 | 2.929 | 2.900 | 2.926 | 0.008 | 0.101 | 0.295 | 0.259 | 0.206 | 0.088 |
| C10:0 | 6.802 | 6.754 | 6.733 | 0.023 | 0.382 | 0.036 | 0.341 | 0.185 | 0.672 |
| C12:0 | 4.198 | 4.206 | 4.213 | 0.004 | 0.389 | 0.004 | 0.451 | 0.222 | 0.520 |
| C14:0 | 11.31 | 11.29 | 11.28 | 0.018 | 0.509 | <0.0001 | 0.007 | 0.285 | 0.626 |
| C14:1 | 0.055 | 0.053 | 0.054 | 0.001 | 0.151 | 0.001 | 0.004 | 0.053 | 0.895 |
| C15:0 | 1.461 | 1.462 | 1.463 | 0.004 | 0.957 | 0.076 | 0.225 | 0.861 | 0.810 |
| C15:1 | 0.195 | 0.201 | 0.210 | 0.002 | 0.003 | <0.0001 | 0.186 | 0.024 | 0.006 |
| C16:0 | 27.41 | 27.47 | 27.68 | 0.046 | 0.041 | <0.0001 | 0.054 | 0.114 | 0.034 |
| C16:1 | 0.976 | 0.961 | 0.956 | 0.004 | 0.043 | 0.790 | 0.666 | 0.029 | 0.601 |
| C17:0 | 0.175 | 0.168 | 0.167 | 0.002 | 0.214 | <0.0001 | 0.897 | 0.223 | 0.334 |
| C17:1 | 0.414 | 0.423 | 0.412 | 0.004 | 0.354 | <0.0001 | 0.702 | 0.666 | 0.173 |
| C18:0 | 14.30 | 14.29 | 14.16 | 0.029 | 0.113 | 0.115 | 0.021 | 0.218 | 0.088 |
| cis 11,C18:1 | 7.18 | 7.15 | 7.19 | 0.013 | 0.568 | 0.439 | 0.030 | 0.545 | 0.387 |
| cis 9,C18:1 | 13.51 | 13.60 | 13.56 | 0.031 | 0.544 | 0.598 | 0.088 | 0.329 | 0.619 |
| trans-10,cis-12 C18:2 | 1.546 | 1.553 | 1.532 | 0.007 | 0.404 | <0.0001 | 0.449 | 0.776 | 0.187 |
| cis-9,cis-12,cis-15 C18:3 | 1.553 | 1.561 | 1.536 | 0.002 | 0.177 | 0.008 | 0.334 | 0.701 | 0.085 |
| C20:0 | 0.847 | 0.852 | 0.857 | 0.006 | 0.028 | 0.897 | 0.959 | 0.041 | 0.243 |
| C22:0 | 0.851 | 0.855 | 0.850 | 0.002 | 0.557 | 0.224 | 0.554 | 0.557 | 0.225 |
| C24:0 | 0.160 | 0.161 | 0.163 | 0.002 | 0.447 | 0.225 | 0.521 | 0.412 | 0.842 |
| Summary | |||||||||
| Ʃ 4-a 14-C 4 | 28.53 | 28.42 | 28.40 | 0.033 | 0.146 | 0.008 | 0.161 | 0.112 | 0.724 |
| Ʃ above de 16-C 5 | 69.80 | 69.91 | 69.92 | 0.033 | 0.180 | 0.005 | 0.158 | 0.066 | 0.822 |
| Ʃ SFA 6 | 73.69 | 73.63 | 73.70 | 0.034 | 0.740 | 0.752 | 0.261 | 0.716 | 0.504 |
| Ʃ UFA 7 | 25.32 | 25.40 | 25.34 | 0.034 | 0.686 | 0.707 | 0.221 | 0.559 | 0.527 |
| Ʃ MUFA 8 | 22.34 | 22.39 | 22.37 | 0.033 | 0.860 | 0.808 | 0.147 | 0.623 | 0.870 |
| Ʃ PUFA 9 | 3.95 | 3.96 | 3.92 | 0.010 | 0.452 | 0.002 | 0.564 | 0.087 | 0.124 |
| Ʃ OCFA 10 | 2.251 | 2.253 | 2.252 | 0.005 | 0.124 | 0.225 | 0.874 | 0.556 | 0.789 |
| Sat/insat 11 | 2.91 | 2.89 | 2.90 | 0.006 | 0.224 | 0.087 | 0.665 | 0.335 | 0.442 |
| Item | Experimental Diets 1 | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | RCS | RCSP | Diet | Time | Int | C1 | C2 | ||
| Glucose (mg/dL) | 60.98 | 75.56 | 76.39 | 4.094 | <0.0001 | 0.078 | 0.810 | 0.024 | 0.912 |
| Total cholesterol (mg/dL) | 155.83 | 134.00 | 127.20 | 7.566 | 0.042 | <0.0001 | 0.319 | 0.033 | 0.616 |
| Triglycerides (mg/dL) | 217.98 | 204.36 | 209.44 | 3.772 | 0.320 | 0.003 | 0.647 | 0.161 | 0.575 |
| Total protein (g/L) | 8.66 | 9.10 | 8.46 | 0.156 | 0.149 | <0.0001 | 0.047 | 0.672 | 0.057 |
| Albumin (g/L) | 3.36 | 3.28 | 3.64 | 0.096 | 0.170 | 0.016 | 0.750 | 0.582 | 0.072 |
| Urea (mg/dL) | 28.42 | 21.24 | 23.90 | 0.752 | <0.0001 | 0.072 | 0.105 | 0.033 | 0.396 |
| Blood urea nitrogen (mg/dL) | 12.33 | 9.22 | 10.37 | 0.326 | <0.0001 | 0.072 | 0.105 | 0.033 | 0.396 |
| Item | Experimental Diets 1 | SEM 2 | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | RCS | RCSP | Diet | Time | Int | C1 | C2 | ||
| Uric acid (mmol/L) | 1.35 | 1.38 | 1.74 | 0.057 | 0.168 | 0.029 | 0.959 | 0.296 | 0.115 |
| Urine allantoin (mmol/L) | 40.98 | 39.37 | 42.21 | 1.475 | 0.746 | 0.429 | 0.025 | 0.954 | 0.447 |
| Allantoin milk (mmol/L) | 0.901 | 0.866 | 0.928 | 0.032 | 0.658 | 0.451 | 0.065 | 0.984 | 0.445 |
| Total purines (mmol/L) | 43.23 | 41.62 | 44.88 | 1.510 | 0.694 | 0.377 | <0.0001 | 0.995 | 0.394 |
| Uric acid (mmol/d) | 20.79 | 21.18 | 27.04 | 0.962 | 0.144 | 0.003 | 0.506 | 0.278 | 0.099 |
| Urine allantoin (mmol/d) | 656.28 | 592.96 | 659.20 | 26.085 | 0.381 | 0.239 | <0.0001 | 0.287 | 0.064 |
| Allantoin milk (mmol/d) | 15.67 | 13.04 | 19.93 | 0.824 | 0.387 | 0.235 | 0.002 | 0.288 | 0.075 |
| Total purines (mmol/d) | 692.75 | 627.19 | 706.18 | 26.665 | 0.021 | 0.226 | 0.023 | 0.085 | 0.001 |
| Absorbable purines (mmol/d) | 674.71 | 607.68 | 686.52 | 26.670 | 0.018 | 0.335 | 0.003 | 0.095 | 0.015 |
| Microbial synthesis (g/d) | |||||||||
| Nitrogen | 490.54 | 441.81 | 499.13 | 19.390 | 0.002 | 0.338 | 0.021 | 0.012 | 0.036 |
| Crude protein | 3065 | 2761 | 3119 | 121.19 | 0.002 | 0.338 | 0.021 | 0.012 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandra, J.R.; Mattos, R.M.; Soares, T.M.D.M.; Pedrini, C.A.; Martinez, A.C.; Oliveira, E.R.; Gandra, E.R.S.; Vasconcelos, W.R.F.; Andrade, A.C. Rehydrated Corn Grain Silage and Exogenous Protease: Effects on Dairy Cow Performance, Metabolism, and Starch Digestibility. Dairy 2025, 6, 1. https://doi.org/10.3390/dairy6010001
Gandra JR, Mattos RM, Soares TMDM, Pedrini CA, Martinez AC, Oliveira ER, Gandra ERS, Vasconcelos WRF, Andrade AC. Rehydrated Corn Grain Silage and Exogenous Protease: Effects on Dairy Cow Performance, Metabolism, and Starch Digestibility. Dairy. 2025; 6(1):1. https://doi.org/10.3390/dairy6010001
Chicago/Turabian StyleGandra, Jefferson R., Rafael M. Mattos, Thais M. D. M. Soares, Cibeli A. Pedrini, Antônio C. Martinez, Euclides R. Oliveira, Erika R. S. Gandra, Wallison R. F. Vasconcelos, and André C. Andrade. 2025. "Rehydrated Corn Grain Silage and Exogenous Protease: Effects on Dairy Cow Performance, Metabolism, and Starch Digestibility" Dairy 6, no. 1: 1. https://doi.org/10.3390/dairy6010001
APA StyleGandra, J. R., Mattos, R. M., Soares, T. M. D. M., Pedrini, C. A., Martinez, A. C., Oliveira, E. R., Gandra, E. R. S., Vasconcelos, W. R. F., & Andrade, A. C. (2025). Rehydrated Corn Grain Silage and Exogenous Protease: Effects on Dairy Cow Performance, Metabolism, and Starch Digestibility. Dairy, 6(1), 1. https://doi.org/10.3390/dairy6010001







