Effect of Cavitation and High-Temperature Nanofiltration of Ultrafiltered Skim Milk on the Functionality of Milk Protein Concentrate Powder †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Spray-Dried MPC80 Powders
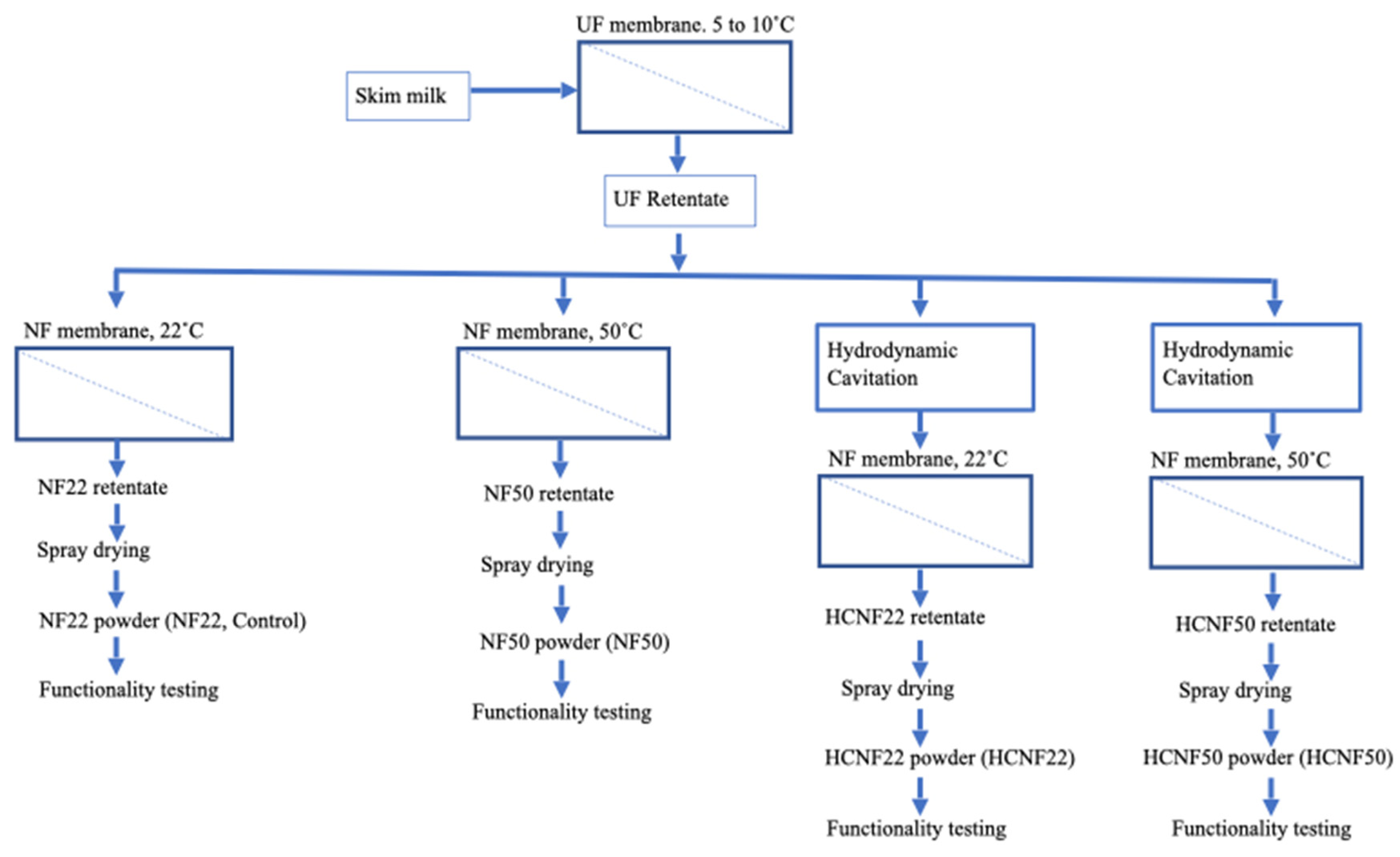
2.2. Experimental Design
2.3. Compositional Analysis
2.4. Morphological Characteristics
2.5. Physicochemical Properties
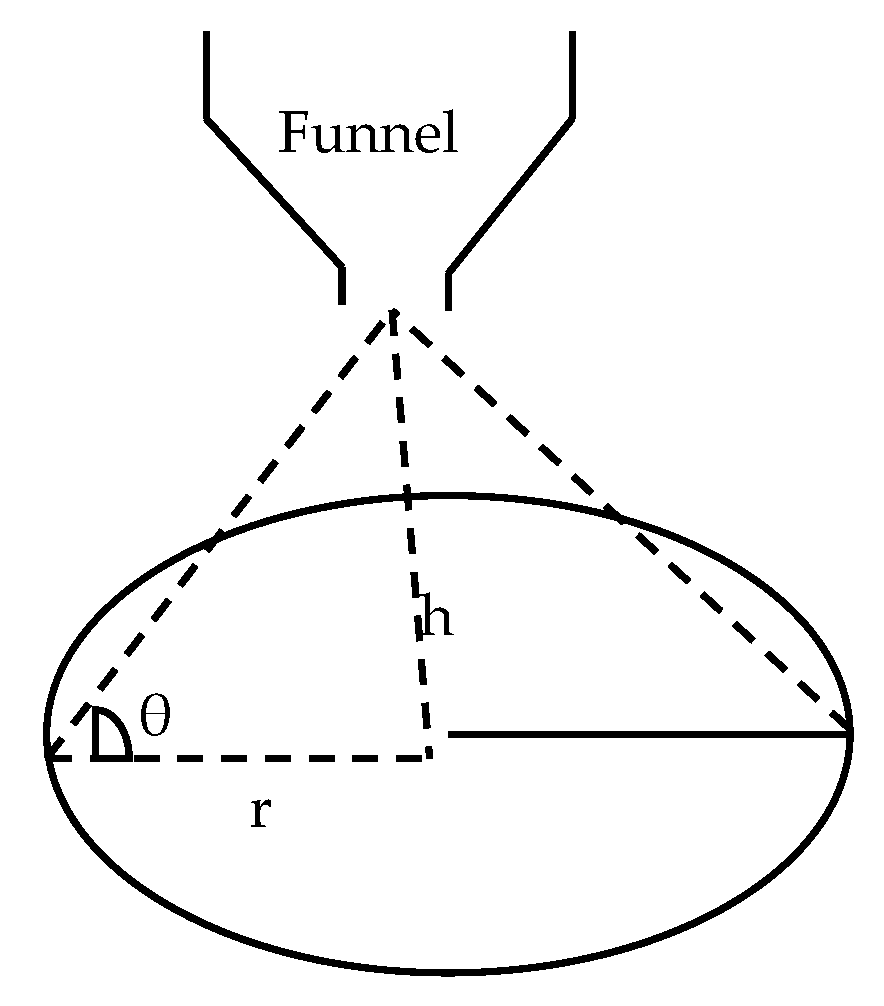
2.6. Functional Properties
2.7. Microbial Examination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Compositional Analysis
3.2. Morphological Characteristics
3.3. Physicochemical Properties
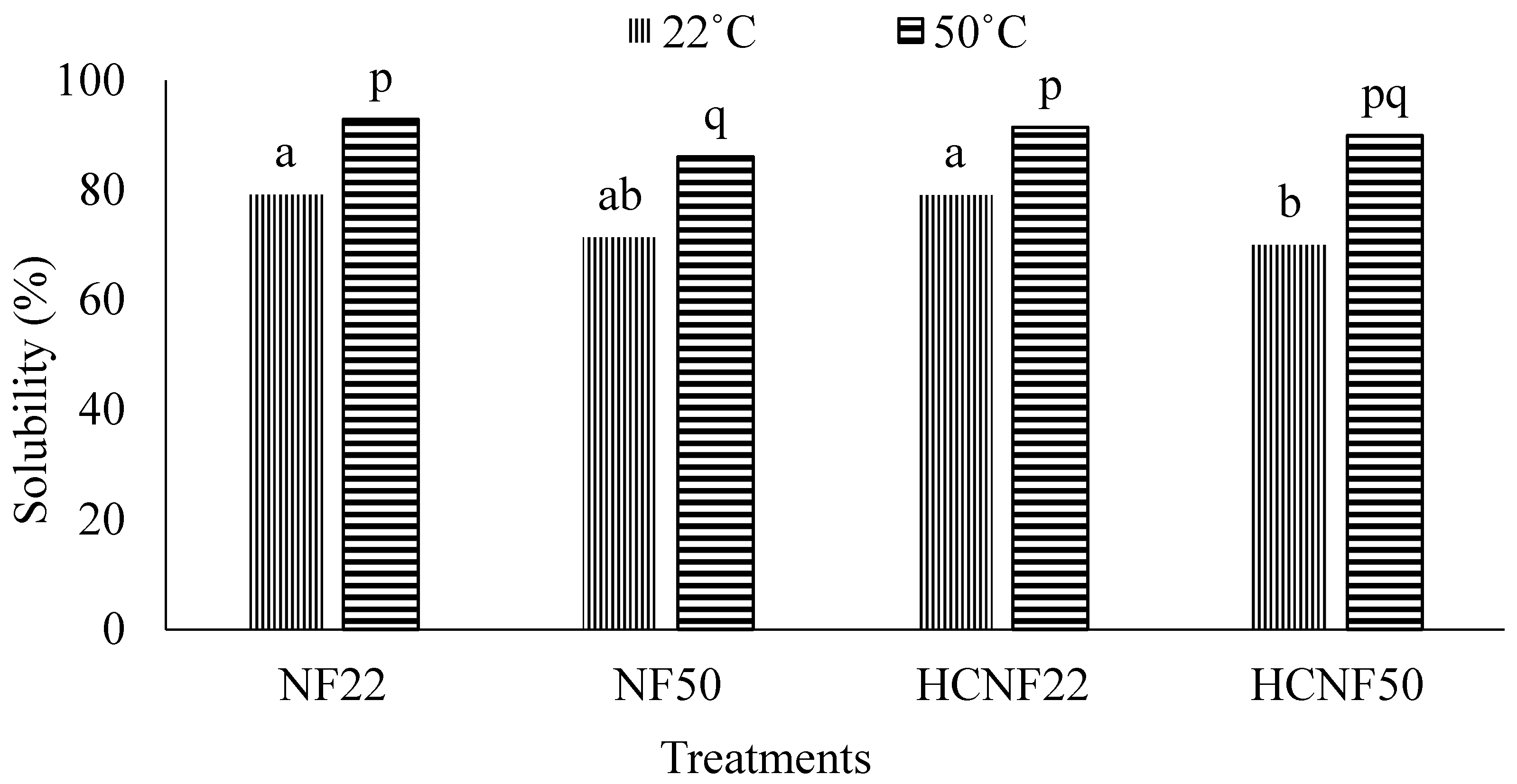
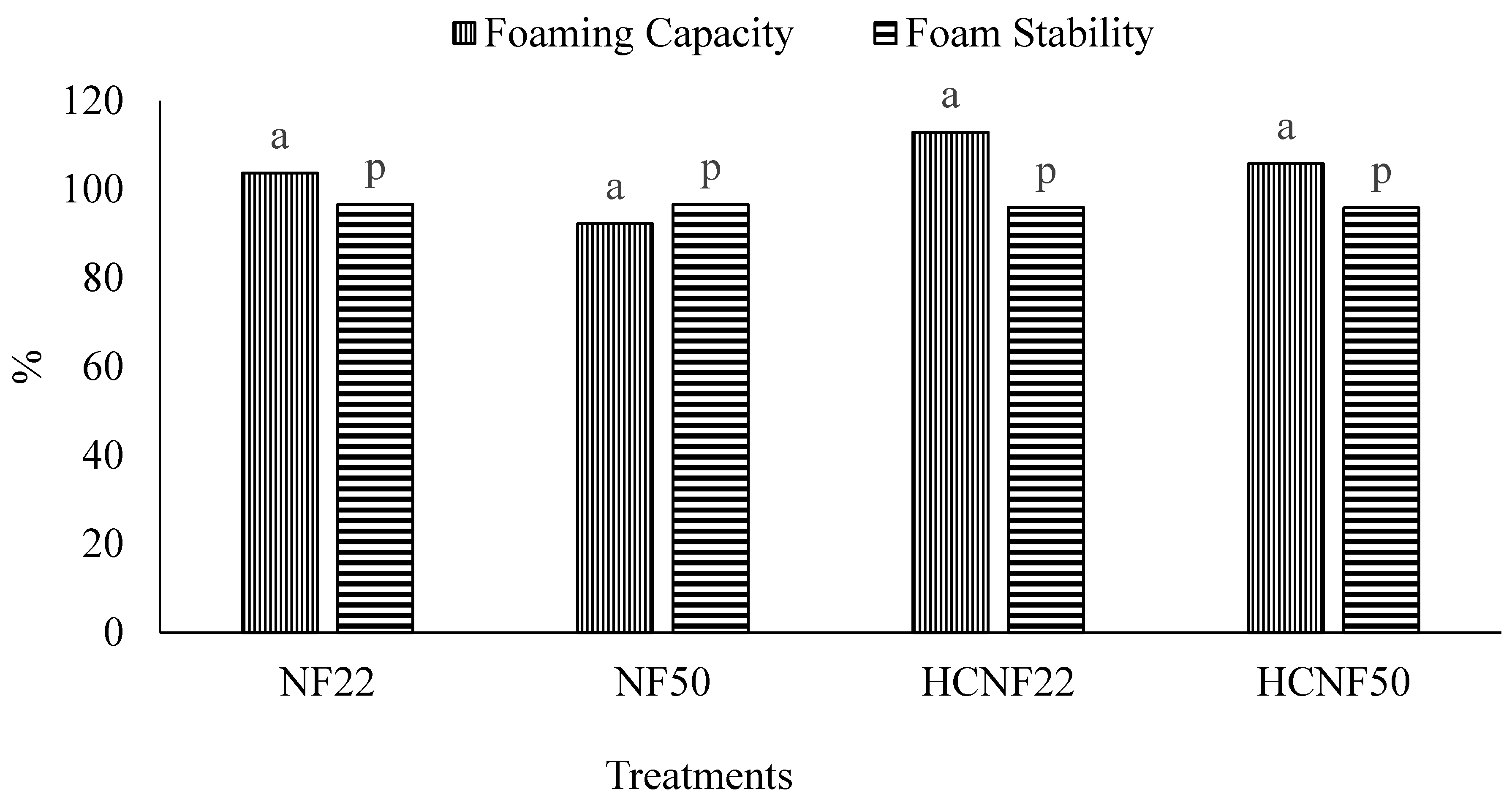
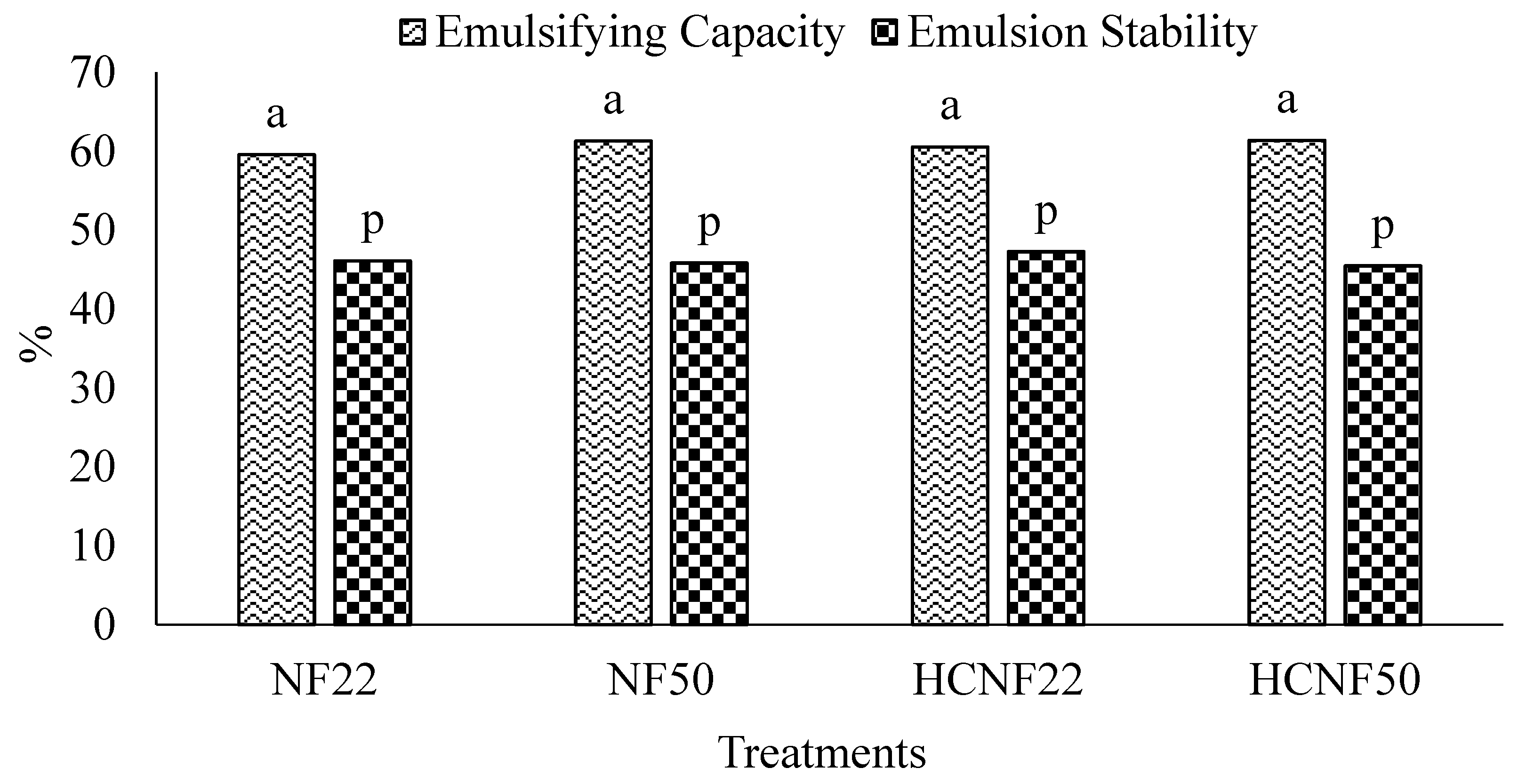
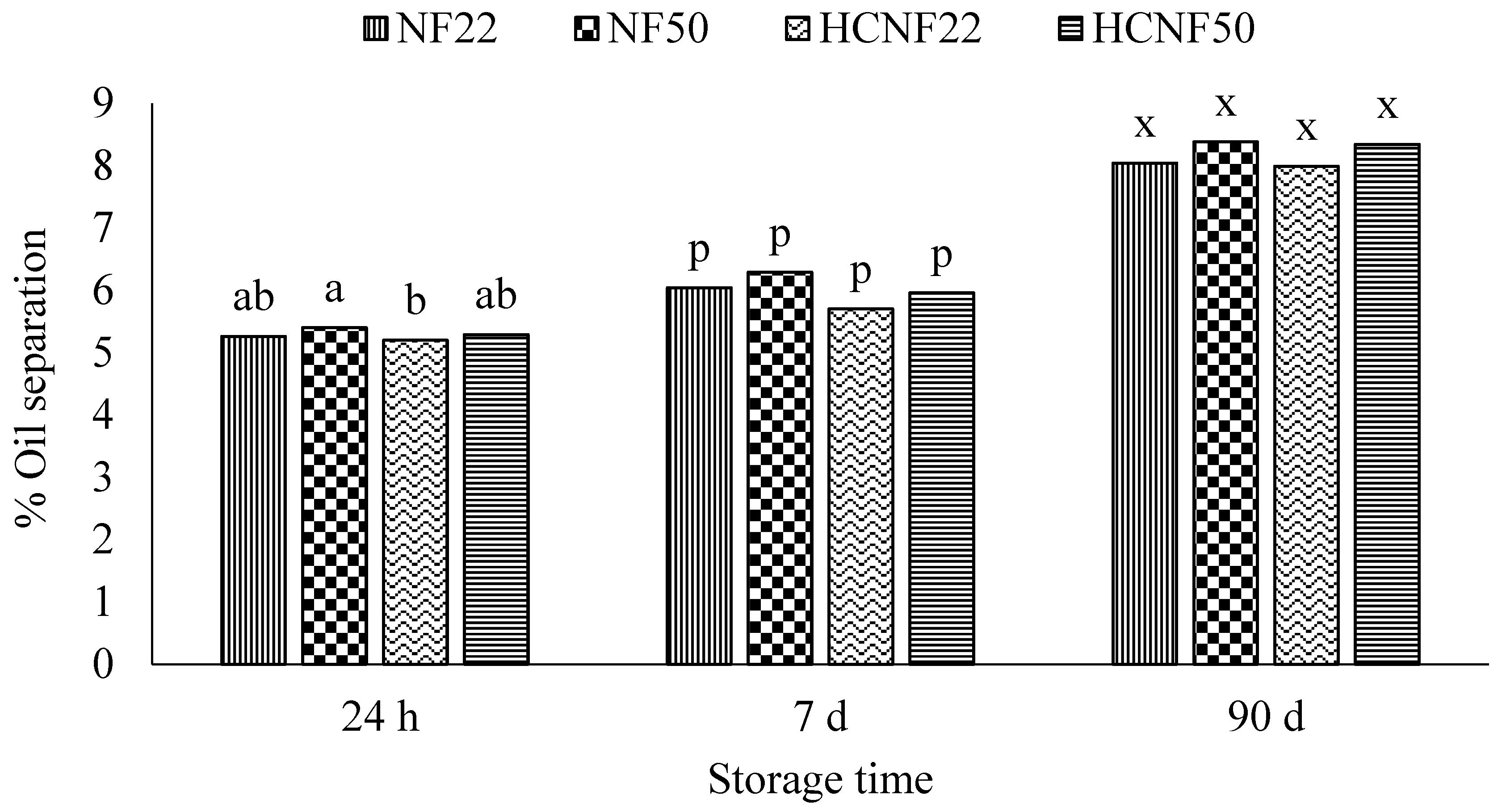
3.4. Functional Properties
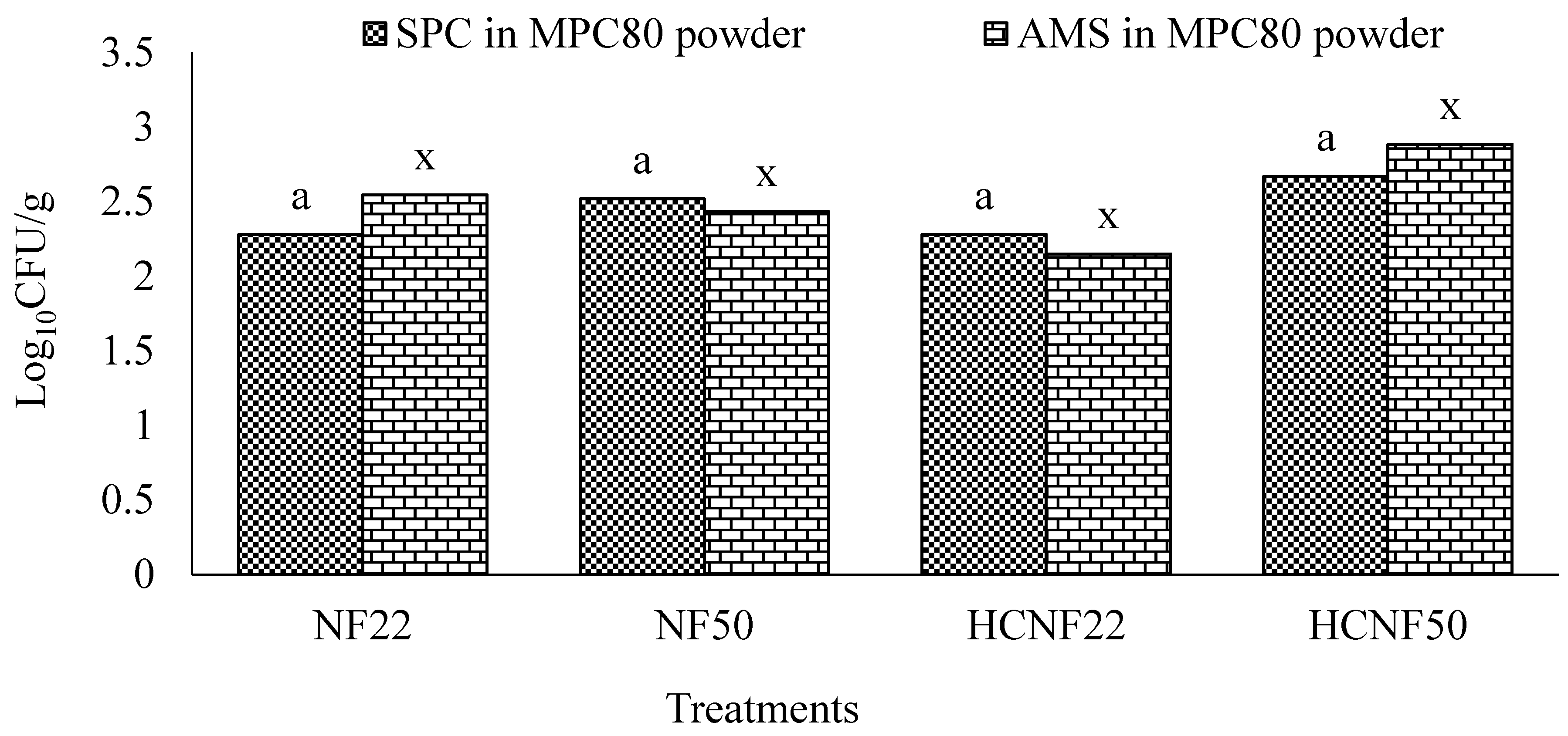
3.5. Microbial Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, H.; Sharma, P.; Patel, S. Milk Protein Concentrate (MPC) and Isolate (MPI). In Encyclopedia of Dairy Sciences, 3rd ed.; Paul, L., McSweeney, H., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 132–140. [Google Scholar]
- Cao, J.; Wang, G.; Wu, S.; Zhang, W.; Liu, C.; Li, H.; Li, Y.; Zhang, L. Comparison of nanofiltration and evaporation technologies on the storage stability of milk protein concentrates. Dairy Sci. Technol. 2016, 96, 107–121. [Google Scholar] [CrossRef]
- Mishra, A.; Panthi, R.R.; Beckman, S.L.; Vijayaragavan, K.S.; Anand, S.; Metzger, L.E. Effects of hydrodynamic cavitation and temperature on nanofiltration performance for concentrating ultrafiltered skim milk. Int. J. Dairy Technol. 2023, 76, 364–370. [Google Scholar] [CrossRef]
- Sutariya, S., Sunkesula, V., Kumar, R., Shah, K., Fatih, Y., Eds.; Emerging applications of ultrasonication and cavitation in dairy industry: A review. Cogent Food Agric. 2018, 4, 1. [Google Scholar]
- Arya, S.S.; Sawant, O.; Sonawane, S.K.; Show, P.L.; Waghamare, A.; Hilares, R.; Santos, J.C.D. Novel, Nonthermal, Energy Efficient, Industrially Scalable Hydrodynamic Cavitation—Applications in Food Processing. Food Rev. Int. 2019, 36, 668–691. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Arora, S.; Borad, S.; Sharma, R.; Gupta, V.K. Physico-chemical, functional, and rheological properties of milk protein concentrate 60 as affected by disodium phosphate addition, diafiltration and homogenization. J. Food Sci. Technol. 2017, 54, 1678–1688. [Google Scholar] [CrossRef]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- Li, K.; Woo, M.W.; Patel, H.; Metzger, L.; Selomulya, C. Improvement of rheological and functional properties of milk protein concentrate by hydrodynamic cavitation. J. Food Eng. 2018, 221, 106–113. [Google Scholar] [CrossRef]
- Udabage, P.; Puvanenthiran, A.; Yoo, J.A.; Versteeg, C.; Augustin, M.A. Modified water solubility of milk protein concentrate powders through the application of static high-pressure treatment. J. Dairy. Res. 2012, 79, 76–83. [Google Scholar] [CrossRef]
- Huppertz, T. Foaming properties of milk: A review of the influence of composition and processing. Int. J. Dairy Technol. 2010, 63, 477–488. [Google Scholar] [CrossRef]
- Sunkesula, V.; Kommineni, A.; Meletharayil, G.H.; Marella, C.; Metzger, L.E. Short communication: Effect of pH on the heat stability of reconstituted reduced calcium milk protein concentrate dispersions. J. Dairy. Sci. 2021, 104, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J. The measurement and significance of ionic calcium in milk—A review. Int. J. Dairy Technol. 2011, 64, 1–13. [Google Scholar] [CrossRef]
- Udabage, P.; McKinnon, I.R.; Augustin, M.A. Effects of mineral salts and calcium chelating agents on the gelation of renneted skim milk. J. Dairy. Sci. 2001, 84, 1569–1575. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Iqbal, T.; Delaney, C.; Twomey, T.; Keogh, M.K. Effect of powder properties and storage conditions on the flowability of milk powders with different fat contents. J. Food Eng. 2004, 64, 435–444. [Google Scholar] [CrossRef]
- Li, R.; Roos, Y.H.; Miao, S. The effect of water plasticization and lactose content on flow properties of dairy model solids. J. Food Eng. 2016, 170, 50–57. [Google Scholar] [CrossRef]
- Mediwaththe, A.; Chandrapala, J.; Huppertz, T.; Vasiljevic, T. Heat-induced changes of milk protein concentrate suspensions as affected by addition of calcium sequestering salts and shearing. Int. Dairy J. 2024, 149, 105829. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Amamcharla, J.K.; Metzger, L.E. Development of a rapid method for the measurement of lactose in milk using a blood glucose biosensor. J. Dairy Sci. 2011, 94, 4800–4809. [Google Scholar] [CrossRef]
- ISO 8967/IDF 134: 2005; International Dairy Federation Standard 134A: Dried Milk and Dried Milk Products—Determination of Bulk Density. International Dairy Federation: Brussels, Belgium, 2005.
- International Dairy Federation (IDF). Special Issue 9501-Heat Induced Changes in Milk, 2nd ed.; International Dairy Federation: Brussels, Belgium, 1995. [Google Scholar]
- Lucey, J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Heamar, Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- APHA. American Public Heath Association. Chapter 09 Microbiological Methods for Dairy Products. In Standard Methods for the Examination of Dairy Products; Duncan, S.E., Yaun, B.R., Bruhn, S.S.S.J., Eds.; APHA Press: Washington, DC, USA, 2012. [Google Scholar]
- Kent, D.J.; Chauhan, K.; Boor, K.J.; Wiedmann, M.; Martin, N.H. Spore test parameters matter: Mesophilic and thermophilic spore counts detected in raw milk and dairy powders differ significantly by test method. J. Dairy Sci. 2016, 99, 5180–5191. [Google Scholar] [CrossRef]
- Braun, K.; Hanewald, A.; Vilgis, T.A. Milk Emulsions: Structure and Stability. Foods 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Hill, A.; Corredig, M. Rheological properties of rennet gels containing milk protein concentrates. J. Dairy Sci. 2008, 91, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Lau, E. Preformulation studies. In Separation Science and Technology; Ahuja, S., Scypinski, S., Eds.; Academic Press: Cambridge, MA, USA, 2001; Volume 3, pp. 173–233. [Google Scholar]
- Fox, P.; Morrissey, P.A. The heat stability of milk. J. Dairy Res. 1977, 44, 627–646. [Google Scholar] [CrossRef]
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of milk powders and milk-based powders for end use applications: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528. [Google Scholar] [CrossRef]
- Renhe, I.; Zhao, Z.; Corredig, M. A comparison of the heat stability of fresh milk protein concentrates obtained by microfiltration, ultrafiltration and diafiltration. J. Dairy Res. 2019, 86, 347–353. [Google Scholar] [CrossRef]
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef]
- Singh, H.; Shalabi, S.I.; Fox, P.F.; Flynn, A.; Barry, A. Rennet coagulation of heated milk: Influence of pH adjustment and before or after heating. J. Dairy Res. 1988, 55, 205–215. [Google Scholar] [CrossRef]
- Rupp, L.S.; Molitor, M.S.; Lucey, J.A. Effect of processing methods and protein content of the concentrate on the properties of milk protein concentrate with 80% protein. J. Dairy Sci. 2018, 101, 7702–7713. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Singh, H. Influence of calcium chloride addition on the properties of emulsions stabilized by whey protein concentrate. Food Hydrocoll. 2000, 4, 337–346. [Google Scholar] [CrossRef]
- Pramularsih, I.; Kyere, E.O.; Md Zain, S.N.; Flint, S. Testing for total bacteria in dairy powder—Comparison of test incubation temperatures (a case study). Int. Dairy J. 2022, 134, 105452. [Google Scholar] [CrossRef]
| Properties | NF22 1 | NF50 1 | HCNF22 1 | HCNF50 1 |
|---|---|---|---|---|
| Moisture (% wt/wt) | 4.00 a | 3.32 a | 3.96 a | 3.18 a |
| Total Protein (% dry basis) | 79.10 a | 78.06 a | 78.62 a | 78.10 a |
| Lactose (% dry basis) | 9.58 a | 9.39 a | 9.34 a | 9.78 a |
| Total Ash (% dry basis) | 8.30 a | 7.65 b | 7.55 b | 7.66 b |
| Crude Fat (% dry basis) | 3.35 a | 3.80 a | 3.98 a | 4.01 a |
| Calcium (mg/100 g) | 2231 a | 2128 a | 2049 a | 2012 a |
| Properties | NF22 2 | NF50 2 | HCNF22 2 | HCNF50 2 |
|---|---|---|---|---|
| Particle Diameter (μm) | 9.30 a | 9.51 a | 8.93 a | 9.49 a |
| Circularity | 0.87 a | 0.86 a | 0.86 a | 0.88 a |
| Elongation | 0.16 a | 0.17 a | 0.17 a | 0.17 a |
| Convexity | 0.98 a | 0.98 a | 0.98 a | 0.98 a |
| Treatments 1 | Loose Density (kg/m3) | Tapped Density (kg/m3) | Flowability/Angle of Friction 3 | HCT 2 (min) |
|---|---|---|---|---|
| NF22 | 273 a | 344 bc | 41.69 a | 22.89 a |
| NF50 | 278 a | 348 b | 41.67 a | 20.22 b |
| HCNF22 | 272 a | 356 ab | 40.47 a | 19.34 b |
| HCNF50 | 286 a | 366 a | 39.89 a | 18.04 b |
| CaCl2 (% wt/wt) | SKM 2 | NF22 3 | NF50 3 | HCNF22 3 | HCNF50 3 |
|---|---|---|---|---|---|
| 0 | 20 | 105 a | 125 a | 100 a | 121 a |
| 0.05 | 7 | 39 c | 52 a | 40 c | 49 b |
| 0.1 | <1 | 14 c | 21 a | 13 c | 18 b |
| 0.25 | <1 | 4 a | 6 a | 4 a | 5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Sunkesula, V.; Hammam, A.R.A.; Metzger, L.E. Effect of Cavitation and High-Temperature Nanofiltration of Ultrafiltered Skim Milk on the Functionality of Milk Protein Concentrate Powder. Dairy 2024, 5, 610-624. https://doi.org/10.3390/dairy5040046
Mishra A, Sunkesula V, Hammam ARA, Metzger LE. Effect of Cavitation and High-Temperature Nanofiltration of Ultrafiltered Skim Milk on the Functionality of Milk Protein Concentrate Powder. Dairy. 2024; 5(4):610-624. https://doi.org/10.3390/dairy5040046
Chicago/Turabian StyleMishra, Achyut, Venkateswarlu Sunkesula, Ahmed R. A. Hammam, and Lloyd E. Metzger. 2024. "Effect of Cavitation and High-Temperature Nanofiltration of Ultrafiltered Skim Milk on the Functionality of Milk Protein Concentrate Powder" Dairy 5, no. 4: 610-624. https://doi.org/10.3390/dairy5040046
APA StyleMishra, A., Sunkesula, V., Hammam, A. R. A., & Metzger, L. E. (2024). Effect of Cavitation and High-Temperature Nanofiltration of Ultrafiltered Skim Milk on the Functionality of Milk Protein Concentrate Powder. Dairy, 5(4), 610-624. https://doi.org/10.3390/dairy5040046






