Fatty Acid, Carotenoid and Fat-Soluble Vitamin Composition of Multispecies Swards Grown in Ireland—Implications for a Sustainable Feed in Dairy Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Derivatisation of Fatty Acids to FAME
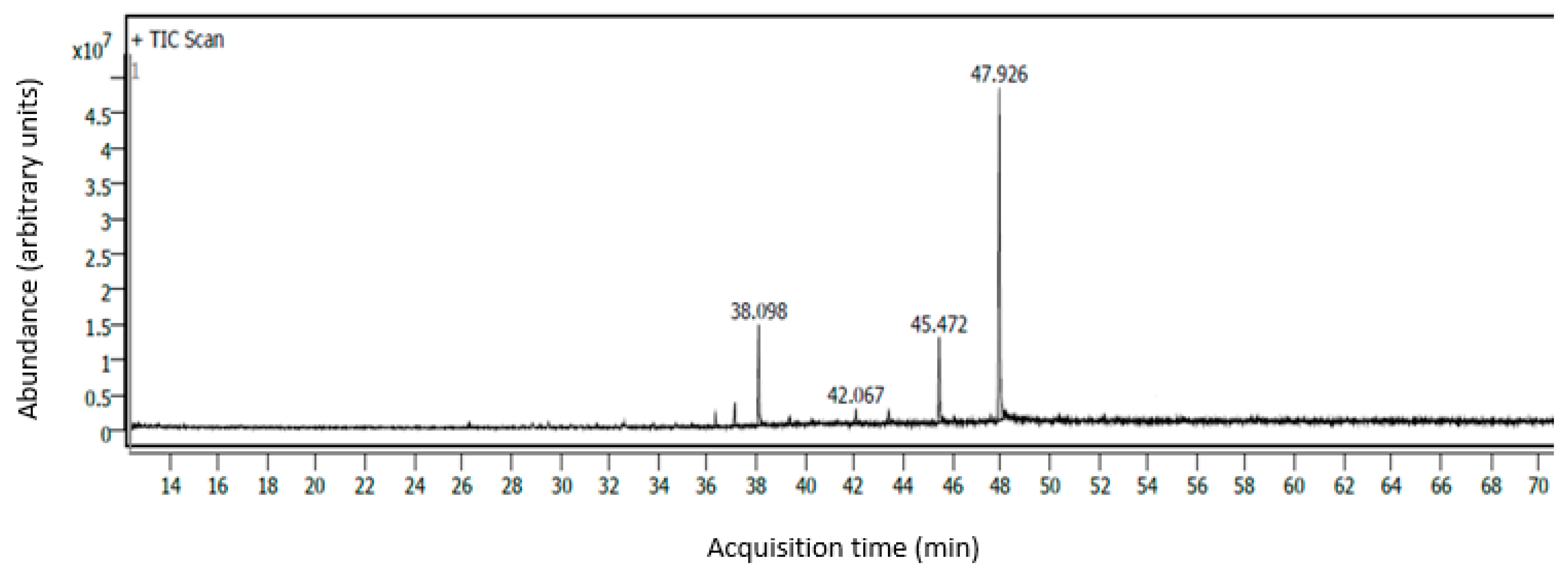
2.4. Identification and Quantification of Fatty Acids Using GC–MS
2.5. Identification and Quantification of Carotenoids and Fat-Soluble Vitamins Using LC–MS
2.6. Statistical Analysis
3. Results
3.1. Identification and Quantification of Fatty Acids of the Grazing Systems
3.2. Identification and Quantification of Carotenoids and Fat-Soluble Vitamins in Grazing Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef]
- Keenan, T.W.; Patton, S. The structure of milk. In Handbook of Milk Composition; Academic Press: Cambridge, MA, USA, 1995; pp. 5–50. [Google Scholar]
- Loften, J.R.; Linn, J.G.; Drackley, J.K.; Jenkins, T.C.; Soderholm, C.G.; Kertz, A.F. Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. J. Dairy Sci. 2014, 97, 4661–4674. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Teagasc. Grass Growth Curve. 2020. Available online: https://www.teagasc.ie/crops/grassland/pasturebase-ireland/grass-curve/ (accessed on 2 February 2023).
- Danielsson, H.; Nadeau, E.; Gustavsson, A.M.; Jensen, S.K.; Soegaard, K.; Nilsdotter-Linde, N. Contents of alpha-tocopherol and beta-carotene in grasses and legumes harvested at different maturities. Grassl. Sci. 2008, 13, 432–434. [Google Scholar]
- Piantoni, P.; Lock, A.L.; Allen, M.S. Palmitic acid increased yields of milk and milk fat and nutrient digestibility across production level of lactating cows. J. Dairy Sci. 2013, 96, 7143–7154. [Google Scholar] [CrossRef]
- Moallem, U. Invited review: Roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J. Dairy Sci. 2018, 101, 8641–8661. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Duttaroy, A. Conjugated Linoleic Acid and Its Beneficial Effects in Obesity, Cardiovascular Disease, and Cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. Int. Rev. J. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Strickland, J.M.; Wisnieski, L.; Herdt, T.H.; Sordillo, L.M. Serum retinol, β-carotene, and α-tocopherol as biomarkers for disease risk and milk production in periparturient dairy cows. J. Dairy Sci. 2021, 104, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, H.; Yang, J.; Wang, J.; Duan, Z.; Wang, C.; Liu, J.; Lao, Y. Effects of feeding lutein on production performance, antioxidative status, and milk quality of high-yielding dairy cows. J. Dairy Sci. 2014, 97, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Moyo, N.; Nielen, M.; Kruitwagen, C. Vitamin E supplementation and udder health: A metaanalysis. Mastitis in dairy production: Current knowledge and future solutions. In Proceedings of the 4th IDF International Mastitis Conference, Maastricht, The Netherlands, 12–15 June 2005; pp. 159–165. [Google Scholar]
- Palladino, R.A.; O’Donovan, M.; Kennedy, E.; Murphy, J.J.; Boland, T.M.; Kenny, D.A. Fatty acid composition and nutritive value of twelve cultivars of perennial ryegrass. Grass Forage Sci. 2009, 64, 219–226. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Didion, T.; Smedsgaard, J.; Jakobsen, J. Seasonal Variation of Provitamin D2 and Vitamin D2 in Perennial Ryegrass (Lolium perenne L.). J. Agric. Food Chem. 2011, 59, 10907–10912. [Google Scholar] [CrossRef]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.L.; Waghorn, G.C.; Brookes, I.M.; Kolver, E.S.; Attwood, G.T. New Zealand Society of Animal Production online archive. N. Z. Soc. Anim. Prod. 2000, 60, 9–14. [Google Scholar]
- Cranston, L.M.; Kenyon, P.R.; Morris, S.T.; Kemp, P.D. A review of the use of chicory, plantain, red clover and white clover in a sward mix for increased sheep and beef production. J. N. Z. Grassl. 2015, 77, 89–94. [Google Scholar] [CrossRef]
- Brunton, N.P.; Mason, C.; Collins, M.J. Rapid microwave assisted preparation of fatty acid methyl esters for the analysis of fatty acid profiles in foods. J. Anal. Chem. 2015, 70, 1218–1224. [Google Scholar] [CrossRef]
- Gentili, A.; Caretti, F.; Bellante, S.; Ventura, S.; Canepari, S.; Curini, R. Comprehensive Profiling of Carotenoids and Fat-Soluble Vitamins in Milk from Different Animal Species by LC-DAD-MS/MS Hyphenation. J. Agric. Food Chem. 2013, 61, 1628–1639. [Google Scholar] [CrossRef]
- Kusch, P. (Ed.) Gas Chromatography—Derivatization, Sample Preparation, Application Derivatization Methods in GC and GC/MS; BoD–Books on Demand: Nordestedt, Germany, 2019; pp. 118–119. [Google Scholar]
- Clapham, W.M.; Foster, J.G.; Neel, J.P.S.; Fedders, J.M. Fatty Acid Composition of Traditional and Novel Forages. J. Agric. Food Chem. 2005, 53, 10068–10073. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed. Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Kalač, P. Carotenoids, ergosterol and tocopherols in fresh and preserved herbage and their transfer to bovine milk fat and adipose tissues: A review. J. Agrobiol. 2013, 29, 1–13. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Moorby, J.M.; Scollan, N.D.; Tweed, J.K.S.; Humphrey, M.O. Effect of a stay-green trait on the concentrations and stability of fatty acids perennial ryegrass. Grass Forage Sci. 2002, 57, 360–366. [Google Scholar] [CrossRef]
- Mc Clements, D.J.; Decker, E.A. Lipids. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2008; pp. 155–216. [Google Scholar] [CrossRef]
- Van Ranst, G.; Lee, M.R.F.; Fievez, V. Red clover polyphenol oxidase and lipid metabolism. Animal 2011, 5, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Marei, W.F.A.; De Bie, J.; Mohey-Elsaeed, O.; Wydooghe, E.; Bols, P.E.J.; Leroy, J.L.M.R. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol. Reprod. 2017, 96, 1181–1196. [Google Scholar] [CrossRef]
- Van Hoeck, V.; Sturmey, R.G.; Bermejo-Alvarez, P.; Rizos, D.; Gutierrez-Adan, A.; Leese, H.J.; Bols, P.E.J.; Leroy, J.L.M.R.; Kim, S. Elevated Non-Esterified Fatty Acid Concentrations during Bovine Oocyte Maturation Compromise Early Embryo Physiology. PLoS ONE 2011, 6, e23183. [Google Scholar] [CrossRef]
- Senger, P. Pathways to Pregnancy and Parturition, 2nd ed.; Current Conceptions, Inc.: Redmond, OR, USA, 2003. [Google Scholar]
- Khiaosa-Ard, R.; Klevenhusen, F.; Soliva, C.R.; Kreuzer, M.; Leiber, F. Transfer of linoleic and linolenic acid from feed to milk in cows fed isoenergetic diets differing in proportion and origin of concentrates and roughages. J. Dairy Res. 2010, 77, 331–336. [Google Scholar] [CrossRef]
- Rapisarda, S.; Abu-Ghannam, N. Polyphenol Characterization and Antioxidant Capacity of Multi-Species Swards Grown in Ireland—Environmental Sustainability and Nutraceutical Potential. Sustainability 2023, 15, 634. [Google Scholar] [CrossRef]
- Lee, M.R.F. Forage polyphenol oxidase and ruminant livestock nutrition. Front. Plant Sci. 2014, 5, 694. [Google Scholar] [CrossRef]
- Cabiddu, A.; Salis, L.; Tweed, J.K.S.; Molle, G.; Decandia, M.; Lee, M.R.F. The influence of plant polyphenols on lipolysis and biohydrogenation in dried forages at different phenological stages: In vitro study. J. Sci. Food Agric. 2010, 90, 829–835. [Google Scholar] [CrossRef]
- Vasta, V.; Makkar, H.P.S.; Mele, M.; Priolo, A. Ruminal biohydrogenation as affected by tannins in vitro. Br. J. Nutr. 2009, 102, 82–92. [Google Scholar] [CrossRef]
- Lindqvist, H.; Nadeau, E.; Jensen, S.K. Alpha-tocopherol and β-carotene in legume-grass mixtures as influenced by wilting, ensiling and type of silage additive. Grass Forage Sci. 2012, 67, 119–128. [Google Scholar] [CrossRef]
- Chew, B.P. Role of carotenoids in the immune response. J. Dairy Sci. 1993, 76, 2804–2811. [Google Scholar] [CrossRef]
- Martin, K.R.; Failla, M.L.; Smith, J.C. β-Carotene and Lutein Protect HepG2 Human Liver Cells against Oxidant-Induced Damage. J. Nutr. 1996, 126, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Rodríguez, M. Optimum Vitamin Nutrition in Dairy cattle. In Optimum Vitamin Nutrition in the Production of Quality Animal Foods; Publishing Benchmark House: Sheffield, UK, 2012; pp. 335–373. [Google Scholar]
- Kavanagh, S. Dairy Manual. 2016; Section 6, 34. Available online: https://www.teagasc.ie/media/website/animals/dairy/FeedingDiaryCow.pdf (accessed on 2 February 2023).
- Beeckman, A.; Vicca, J.; Van Ranst, G.; Janssens, G.P.J.; Fievez, V. Monitoring of vitamin E status of dry, early and mid-late lactating organic dairy cows fed conserved roughages during the indoor period and factors influencing forage vitamin E levels. J. Anim. Physiol. Anim. Nutr. 2010, 94, 736–746. [Google Scholar] [CrossRef]
- Lynch, A.; Kerry, J.P.; Buckley, D.J.; Morrissey, P.A. Lopez-Bote, C. Use of high pressure liquid chromatography (HPLC) for the determination of cda-tocopherol levels in forage (silage/grass) samples collected from different regions in Ireland. Food Chem. 2001, 72, 521–524. [Google Scholar] [CrossRef]
- Sickel, H. High level of alpha-tocopherol in Norwegian alpine grazing plants. J. Agric. Food Chem. 2012, 60, 7573–7580. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of alpha-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Met Eireann. Monthly Data, The Irish Meteorological Service. 2019. Available online: https://www.met.ie/climate/available-data/monthly-data (accessed on 1 February 2023).
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Bernes, G.; Waller, K.P.; Jensen, S.K. Hay and silage as vitamin sources in organic sheep production. Grassl. Sci. Eur. 2008, 12, 251–255. [Google Scholar]
- Peña-Espinoza, M.; Thamsborg, S.M.; Desrues, O.; Hansen, T.V.A.; Enemark, H.L. Anthelmintic effects of forage chicory (Cichorium intybus) against gastrointestinal nematode parasites in experimentally infected cattle. Parasitology 2016, 143, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Nkomboni, D.; Bryant, R.H.; Grant, E.R. Effect of increasing dietary proportion of plantain on milk production and nitrogen use of grazing dairy cows in late lactation. Anim. Prod. Sci. 2021, 61, 770–779. [Google Scholar] [CrossRef]
- Stampa, K.; Schipmann-Schwarze, C.; Hamm, U. Consumer perceptions, preferences, and behaviour regarding pasture-raised livestock products: A review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Teagasc. Grassland Re-Seeding: How to Establish Multispecies Swards. 2020. Available online: https://www.teagasc.ie/publications/2020/grassland-re-seeding-how-to-establish-multispecies-swards.php (accessed on 2 February 2023).


| Forage Type | Grass | Legume | Herb | |||||
|---|---|---|---|---|---|---|---|---|
| Percentage (%) | Period | Perennial Ryegrass | Timothy | White Clover | Red Clover | Chicory | Plantain | Unsown |
| PRG | July | 90.6 | - | - | - | - | - | 9.4 |
| August | 99.2 | - | - | - | - | - | 0.8 | |
| September | 98.1 | - | - | - | - | - | 1.9 | |
| PRG+WC | July | 87.3 | - | 12.6 | - | - | - | 0.1 |
| August | 51.9 | - | 47.7 | - | - | - | 0.4 | |
| September | 53.6 | - | 46.4 | - | - | - | 0.00 | |
| MULTI | July | 38.1 | 23.3 | 5.3 | 16.8 | 11.9 | 3.6 | 1.0 |
| August | 6.2 | 5.1 | 2.1 | 65.7 | 14.2 | 6.7 | 0.0 | |
| September | 12.4 | 8.7 | 4.1 | 55.3 | 15.1 | 4.4 | 0.0 | |
| Fragmentor Voltage (V) | Collision Energy (V) | Precursor Ion (m/z) | Product Ion (m/z) | |
|---|---|---|---|---|
| Lutein | 160 | 15 | 551.0 | 429.0 |
| β-Carotene | 160 | 15 | 537.0 | 445.0 |
| Ergocalciferol | 120 | 20 | 397.0 | 159.0 |
| Cholecalciferol | 120 | 20 | 385.0 | 159.0 |
| α-tocopherol | 160 | 15 | 431.0 | 165.0 |
| γ-tocopherol | 140 | 15 | 416.0 | 151.0 |
| Phytomenadione | 160 | 20 | 451.0 | 187.0 |
| Internal Standard—Menaquinone | 160 | 15 | 445.0 | 187.0 |
| PRG | |||||||||||
| July | August | September | |||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | ||
| Butyric acid | C4:0 | 0.07 | 0.05 | 0.03 | 0.07 | 0.04 | 0.05 | 0.06 | 0.05 | 0.05 | 0.712 |
| Caproic acid | C6:0 | 0.16 | 0.16 | 0.17 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.17 | 0.822 |
| Caprylic acid | C8:0 | 0.12 | 0.13 | 0.15 | 0.13 | 0.14 | 0.13 | 0.14 | 0.13 | 0.13 | 0.113 |
| Capric acid | C10:0 | 0.15 | 0.16 | 0.15 | 0.15 | 0.17 | 0.15 | 0.15 | 0.15 | 0.15 | 0.580 |
| Undecylic acid | C11:0 | 0.21 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.364 |
| Lauric acid | C12:0 | 0.21 | 0.25 | 0.23 | 0.22 | 0.36 | 0.24 | 0.25 | 0.35 | 0.27 | 0.233 |
| Myristic acid | C14:0 | 0.34 | 0.28 | 0.27 | 0.32 | 0.31 | 0.31 | 0.34 | 0.37 | 0.34 | 0.097 |
| Myristoleic acid | C14:1 | 0.12 | 0.23 | 0.15 | 0.13 | 0.43 | 0.23 | 0.26 | 0.18 | 0.33 | 0.812 |
| Pentadecylic acid | C15:0 | 0.05 | 0.05 | 0.06 | 0.05 | 0.15 | 0.06 | 0.17 | 0.06 | 0.07 | 0.525 |
| Palmitic acid | C16:0 | 1.15 a | 1.46 a | 1.72 a | 1.71 a | 1.85 ab | 1.96 ab | 3.04 c | 2.52 bc | 2.69 c | <0.001 |
| Palmitoleic acid | C16:1 | 0.24 | 0.2 | 0.37 | 0.34 | 0.25 | 0.26 | 0.25 | 0.27 | 0.31 | 0.817 |
| Margaric acid | C17:0 | 0.08 | 0.06 | 0.06 | 0.08 | 0.07 | 0.06 | 0.06 | 0.07 | 0.11 | 0.658 |
| Stearic acid | C18:0 | 1.49 a | 1.53 a | 1.49 a | 1.63 ab | 1.49 a | 1.59 a | 1.67 a | 2.03 a | 1.5 a | 0.004 |
| Elaidic acid | C18:1 (t9) | 0.07 | 0.00 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | ND | 0.01 | 0.925 |
| Oleic acid | C18:1 (c9) | 1.17 | 0.48 | 0.59 | 0.5 | 0.39 | 0.61 | 0.76 | 0.55 | 0.61 | 0.122 |
| Vaccenic acid | C18:1 (t11) | 0.14 | 0.14 | 0.16 | 0.17 | 0.21 | 0.14 | 0.13 | 0.20 | 0.13 | 0.681 |
| Linoleic acid | C18:2 (c9,12) | 3.34 ab | 3.33 ab | 3.13 ab | 2.78 a | 3.44 ab | 4.79 ab | 4.96 ab | 3.59 ab | 5.21 b | 0.006 |
| γ-linolenic acid | C18:3 (c6,9,12) | 0.19 | 0.20 | 0.46 | 0.40 | 0.50 | 0.15 | 0.26 | 0.19 | 0.17 | 0.957 |
| α-linolenic acid | C18:3 (c6,9,15) | 9.61 a | 9.09 a | 12.82 ab | 13.46 ab | 21.89 bc | 16.75 ab | 30.12 cd | 30.36 cd | 35.94 d | <0.001 |
| Rumenic acid | C18:2 (c9,t11) | 0.16 | 0.15 | 0.16 | 0.15 | ND | ND | ND | ND | ND | - |
| PRG+WC | |||||||||||
| July | August | September | |||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | ||
| Butyric acid | C4:0 | 0.05 | 0.07 | 0.03 | 0.08 | 0.07 | 0.08 | 0.06 | 0.07 | 0.06 | 0.841 |
| Caproic acid | C6:0 | 0.16 | 0.18 | 0.16 | 0.17 | 0.18 | 0.16 | 0.16 | 0.16 | 0.16 | 0.451 |
| Caprylic acid | C8:0 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.14 | 0.13 | 0.13 | 0.13 | 0.279 |
| Capric acid | C10:0 | 0.15 | 0.16 | 0.15 | 0.15 | 0.16 | 0.14 | 0.15 | 0.16 | 0.16 | 0.942 |
| Undecylic acid | C11:0 | 0.19 | 0.20 | 0.20 | 0.21 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.285 |
| Lauric acid | C12:0 | 0.22 | 0.23 | 0.21 | 0.22 | 0.36 | 0.38 | 0.33 | 0.28 | 0.37 | 0.270 |
| Myristic acid | C14:0 | 0.3 | 0.31 | 0.31 | 0.33 | 0.35 | 0.3 | 0.31 | 0.39 | 0.32 | 0.825 |
| Myristoleic acid | C14:1 | 0.11 | 0.12 | 0.18 | 0.14 | 0.18 | 0.15 | 0.13 | 0.16 | 0.15 | 0.051 |
| Pentadecylic acid | C15:0 | 0.04 | 0.08 | 0.05 | 0.05 | 0.09 | 0.07 | 0.09 | 0.07 | 0.08 | 0.635 |
| Palmitic acid | C16:0 | 0.81 a | 1.23 ab | 1.27 ab | 1.61 abc | 3.00 e | 1.95 bcd | 2.51 b | 2.40 cde | 2.81 de | <0.001 |
| Palmitoleic acid | C16:1 | 0.26 | 0.19 | 0.20 | 0.32 | 0.26 | 0.43 | 0.26 | 0.26 | 0.24 | 0.851 |
| Margaric acid | C17:0 | 0.04 | 0.07 | 0.05 | 0.1 | 0.08 | 0.06 | 0.08 | 0.15 | 0.07 | 0.152 |
| Stearic acid | C18:0 | 1.55 a | 1.58 a | 1.7 a | 1.65 a | 1.50 a | 1.69 a | 1.51 a | 1.50 a | 1.48 a | 0.689 |
| Elaidic acid | C18:1 (t9) | ND | 0.02 | 0.02 | 0.02 | 0.01 | 0.05 | 0.02 | 0.02 | 0.01 | 0.975 |
| Oleic acid | C18:1 (c9) | 0.74 | 0.45 | 0.66 | 0.57 | 0.91 | 1.09 | 0.81 | 0.89 | 0.88 | 0.969 |
| Vaccenic acid | C18:1 (t11) | 0.15 | 0.16 | 0.13 | 0.15 | 0.17 | 0.16 | 0.16 | 0.13 | 0.15 | 0.643 |
| Linoleic acid | C18:2 (c9,12) | 3.57 a | 3.08 a | 2.86 a | 3.85 a | 5.93 b | 4.38 ab | 6.32 b | 4.77 ab | 2.70 a | <0.001 |
| γ-linolenic acid | C18:3 (c6,9,12) | 0.22 | 0.33 | 0.50 | 0.21 | 0.45 | 0.29 | 0.21 | 0.21 | 0.15 | 0.985 |
| α-linolenic acid | C18:3 (c6,9,15) | 8.73 a | 5.36 a | 6.66 a | 12.27 ab | 22.48 c | 21.37 bc | 23.8 c | 30.82 cd | 35.15 d | <0.001 |
| Rumenic acid | C18:2 (c9,t11) | 0.16 | 0.15 | 0.16 | ND | ND | ND | ND | ND | ND | - |
| MULTI | |||||||||||
| July | August | September | |||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | ||
| Butyric acid | C4:0 | 0.05 | 0.04 | 0.06 | 0.07 | 0.06 | 0.05 | 0.08 | 0.04 | 0.05 | 0.823 |
| Caproic acid | C6:0 | 0.16 | 0.16 | 0.16 | 0.15 | 0.16 | 0.16 | 0.17 | 0.17 | 0.16 | 0.115 |
| Caprylic acid | C8:0 | 0.14 | 0.14 | 0.13 | 0.14 | 0.15 | 0.14 | 0.13 | 0.13 | 0.14 | 0.558 |
| Capric acid | C10:0 | 0.16 | 0.16 | 0.15 | 0.15 | 0.16 | 0.15 | 0.15 | 0.14 | 0.15 | 0.794 |
| Undecylic acid | C11:0 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.21 | 0.20 | 0.21 | 0.23 | 0.090 |
| Lauric acid | C12:0 | 0.21 | 0.2 | 0.22 | 0.24 | 0.29 | 0.30 | 0.32 | 0.23 | 0.24 | 0.560 |
| Myristic acid | C14:0 | 0.31 | 0.3 | 0.29 | 0.29 | 0.32 | 0.34 | 0.38 | 0.40 | 0.35 | 0.293 |
| Myristoleic acid | C14:1 | 0.16 | 0.16 | 0.13 | 0.21 | 0.15 | 0.27 | 0.2 | 0.25 | 0.17 | 0.270 |
| Pentadecylic acid | C15:0 | 0.08 | 0.06 | 0.07 | 0.08 | 0.06 | 0.06 | 0.13 | 0.10 | 0.12 | 0.724 |
| Palmitic acid | C16:0 | 1.49 a | 1.50 a | 1.76 a | 1.82 a | 2.20 ab | 2.30 abc | 2.22 abc | 2.92 bc | 3.22 c | <0.001 |
| Palmitoleic acid | C16:1 | 0.21 | 0.22 | 0.23 | 0.36 | 0.30 | 0.40 | 0.33 | 0.35 | 0.3 | 0.935 |
| Margaric acid | C17:0 | 0.08 | 0.05 | 0.05 | 0.13 | 0.05 | 0.12 | 0.06 | 0.12 | 0.09 | 0.179 |
| Stearic acid | C18:0 | 1.59 ab | 1.53 a | 1.74 ab | 1.83 ab | 1.62 ab | 2.12 b | 1.88 ab | 1.51 a | 2.10 ab | 0.009 |
| Elaidic acid | C18:1 (t9) | 0.01 | 0.01 | ND | ND | 0.01 | 0.03 | 0.02 | 0.02 | 0.01 | 0.915 |
| Oleic acid | C18:1 (c9) | 0.68 | 0.48 | 0.47 | 0.56 | 0.17 | 0.86 | 0.91 | 0.95 | 0.81 | 0.984 |
| Vaccenic acid | C18:1 (t11) | 0.15 | 0.14 | 0.14 | 0.17 | 0.16 | 0.18 | 0.17 | 0.15 | 0.16 | 0.986 |
| Linoleic acid | C18:2 (c9,12) | 2.74 ab | 3.43 abc | 4.40 bcde | 3.92 abcd | 4.46 cde | 6.46 f | 5.50 def | 6.10 ef | 2.53 a | <0.001 |
| γ-linolenic acid | C18:3 (c6,9,12) | 0.17 | 0.23 | 0.25 | 0.16 | 0.81 | 0.18 | 0.16 | 0.48 | 0.2 | 0.860 |
| α-linolenic acid | C18:3 (c6,9,15) | 8.81 a | 9.97 ab | 12.11 ab | 13.25 ab | 16.5 ab | 19.53 bc | 19.85 bc | 26.88 cd | 34.25 d | <0.001 |
| Rumenic acid | C18:2 (c9,t11) | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| PRG | ||||||||||
| July | August | September | ||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | |
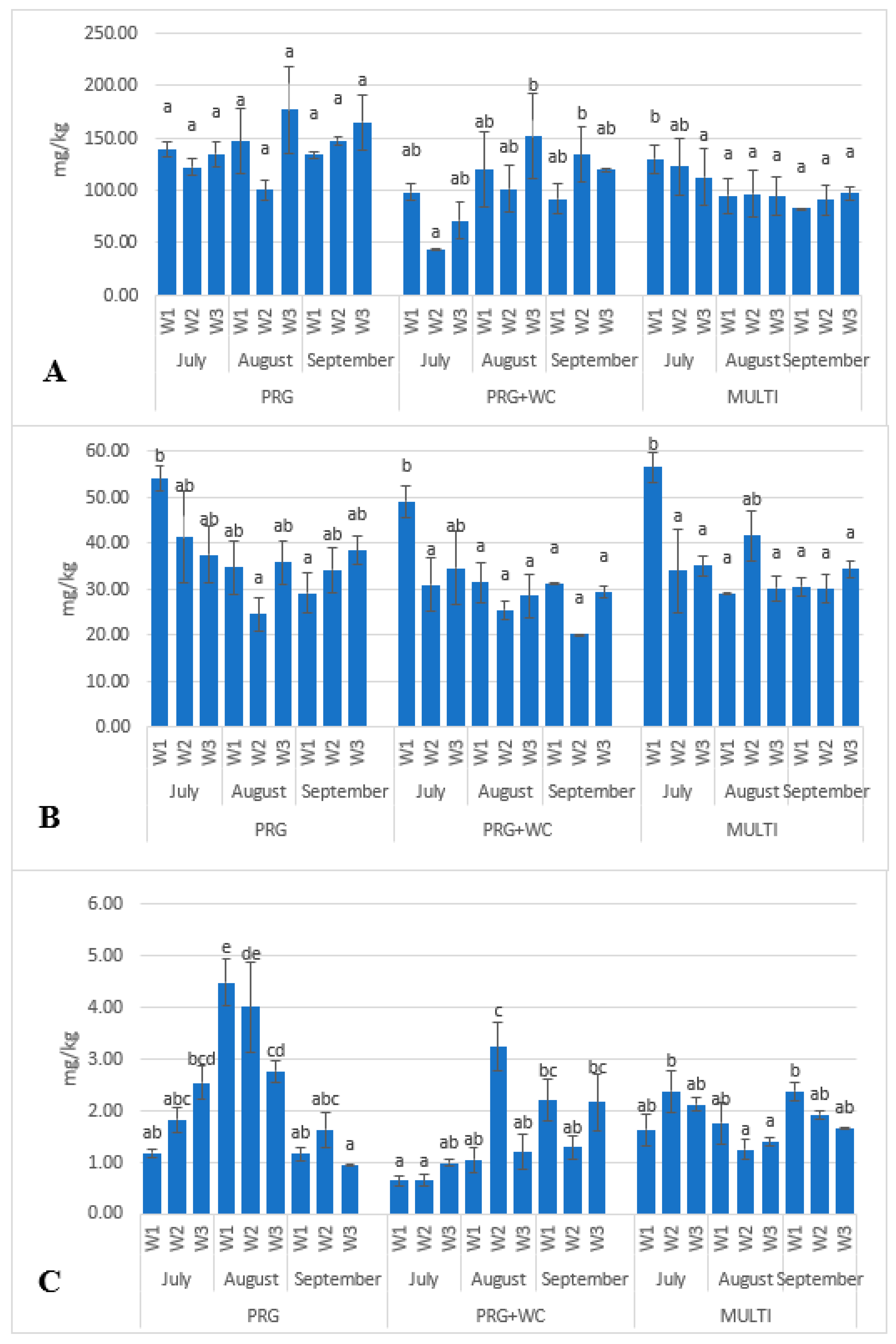
| Lutein | 54.15 b | 41.39 ab | 37.47 ab | 34.76 ab | 24.55 a | 35.9 ab | 29.16 a | 34.24 ab | 38.49 ab | 0.018 |
| β-Carotene | 139.19 a | 122.26 a | 135.03 a | 146.87 a | 100.23 a | 176.91 a | 133.97 a | 147.27 a | 164.91 a | 0.105 |
| Ergocalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Cholecalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| α-tocopherol | 1.17 ab | 1.82 abc | 2.54 bcd | 4.49 e | 4.01 de | 2.76 cd | 1.16 ab | 1.64 abc | 0.93 a | <0.001 |
| γ-tocopherol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Phytomenadione | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Menaquinone | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| PRG+WC | ||||||||||
| July | August | September | ||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | |
| Lutein | 49.18 b | 31.04 a | 34.69 ab | 31.46 a | 25.55 a | 28.53 a | 31.2 a | 20.29 a | 29.37 a | 0.004 |
| β-Carotene | 98.54 ab | 44.14 a | 71.33 ab | 120.05 ab | 101.34 ab | 151.83 b | 91.82 ab | 134.34 b | 119.74 ab | 0.025 |
| Ergocalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Cholecalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| α-tocopherol | 0.64 a | 0.64 a | 0.98 ab | 1.05 ab | 3.24 c | 1.2 ab | 2.2 bc | 1.29 ab | 2.16 bc | <0.001 |
| γ-tocopherol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Phytomenadione | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Menaquinone | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| MULTI | ||||||||||
| July | August | September | ||||||||
| W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | p-Value | |
| Lutein | 56.55 b | 34.02 a | 35.17 a | 29.20 a | 41.69 ab | 30.15 a | 30.58 a | 30.01 a | 34.38 a | 0.002 |
| β-Carotene | 130.03 s | 122.7 ab | 112.73 a | 95.15 a | 96.69 a | 94.3 a | 82.75 a | 90.66 a | 97.51 a | 0.282 |
| Ergocalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Cholecalciferol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| α-tocopherol | 1.63 ab | 2.38 b | 2.12 ab | 1.76 ab | 1.25 a | 1.39 a | 2.37 b | 1.92 ab | 1.66 ab | 0.009 |
| γ-tocopherol | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Phytomenadione | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Menaquinone | ND | ND | ND | ND | ND | ND | ND | ND | ND | - |
| Perennial Ryegrass | Timothy | White Clover | Red Clover | Chicory | Plantain | p-Value | |
|---|---|---|---|---|---|---|---|
| α-linolenic acid | 17.12 a | 13.48 a | 18.76 a | 37.85 b | 14.97 a | 22.04 a | <0.001 |
| Linoleic acid | 2.94 a | 3.39 a | 4.28 ab | 4.04 a | 3.56 a | 5.63 b | 0.001 |
| Palmitic acid | 2.15 a | 1.98 a | 3.98 b | 3.20 ab | 2.47 ab | 4.07 b | <0.001 |
| Stearic acid | 1.53 a | 1.59 a | 1.66 a | 1.60 a | 1.49 a | 1.55 a | 0.207 |
| Lutein | 62.56 c | 49.07 ab | 36.97 a | 48.09 ab | 48.49 ab | 59.36 c | 0.007 |
| β-carotene | 73.97 b | 59.82 ab | 46.59 ab | 55.53 ab | 21.06 a | 56.28 ab | 0.045 |
| α-tocopherol | 0.96 b | 0.88 b | 0.11 a | 0.03 a | 0.83 b | 0.40 ab | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapisarda, S.; O’Neill, G.; Abu-Ghannam, N. Fatty Acid, Carotenoid and Fat-Soluble Vitamin Composition of Multispecies Swards Grown in Ireland—Implications for a Sustainable Feed in Dairy Farming. Dairy 2023, 4, 300-315. https://doi.org/10.3390/dairy4020021
Rapisarda S, O’Neill G, Abu-Ghannam N. Fatty Acid, Carotenoid and Fat-Soluble Vitamin Composition of Multispecies Swards Grown in Ireland—Implications for a Sustainable Feed in Dairy Farming. Dairy. 2023; 4(2):300-315. https://doi.org/10.3390/dairy4020021
Chicago/Turabian StyleRapisarda, Samuel, Graham O’Neill, and Nissreen Abu-Ghannam. 2023. "Fatty Acid, Carotenoid and Fat-Soluble Vitamin Composition of Multispecies Swards Grown in Ireland—Implications for a Sustainable Feed in Dairy Farming" Dairy 4, no. 2: 300-315. https://doi.org/10.3390/dairy4020021
APA StyleRapisarda, S., O’Neill, G., & Abu-Ghannam, N. (2023). Fatty Acid, Carotenoid and Fat-Soluble Vitamin Composition of Multispecies Swards Grown in Ireland—Implications for a Sustainable Feed in Dairy Farming. Dairy, 4(2), 300-315. https://doi.org/10.3390/dairy4020021







