Abstract
Thermoduric sporeformers survive heat treatment and can form biofilm on contact food surfaces that is difficult to clean and may cause cross contamination to milk products. It was hypothesized that cavitation would influence sporeformers’ ability to attach to contact surfaces and form biofilm. Common dairy sporeformers of Geobacillus stearothermophilus, Bacillus licheniformis, and Bacillus sporothermodurans were individually inoculated in sterile skim milk at the levels of 6.0 log CFU/mL. Inoculated samples were treated by cavitation at 80% amplitudes for 10 min each. Pre and post samples were used to develop biofilms on stainless steel coupons under static conditions. Scanning electron micrograph was used to observe the developed biofilms. All the experiments were conducted in triplicate and were statistically analyzed using a t test. The average counts of spiked milk samples were 7.2, 8.0, and 7.7 logs CFU/mL, respectively, for the three sporeformers. Post-cavitation counts were reduced significantly to 3.4, 4.2, and 3.7 logs CFU/mL, respectively. Pre-cavitation biofilm counts of the three sporeformers were 5.35, 6.42, and 6.5 logs CFU/ cm2, respectively in 72 h. The three sporeformers’ biofilm showed significantly (p < 0.05) lower counts after cavitation of 4.39, 5.44, and lower counts of 4.39 logs CFU/cm2, respectively, for the three organisms. The result showed that G. stearothermophilus formed the least biofilms among others after cavitation. Although the ultrasonication treatment reduced the number of sporeformer bacteria, the survivors still retained the ability to attach to the stainless-steel food contact surfaces.
1. Introduction
Biofilms are ubiquitous in the dairy industry; constituents of various spoilage microorganisms can slough off into milk or milk products, which leads to cross contamination [1]. Previous research has determined that the presence of biofilms in dairy products can significantly affect the propagation of associated sporeformers within the biofilms. It is mostly believed that biofilms adherence is more likely to sustain on hydrophilic surfaces as compared to hydrophobic [2]. On the other hand, some researchers have reported that biofilm may also attach to some hydrophobic surfaces due to several influencing factors [3]. The primary factors that help to form better biofilms are cell-surface hydrophobicity and secretion of exopolysaccharides [4]. These exopolysaccharides play an important role in binding the elements that help biofilm adhere to a biotic surface. Biofilm constituents, such as organic soils and organisms, and the residues of protein, carbohydrate, and fat, would help the colonization of biofilm formation, which serves as an important source of dairy food contamination, especially during prolonged processing runs [5]. Biofilms not only pose a threat to product safety but can also harm plant equipment. The presence of biofilms in the dairy industry may also induce intense problems, such as corrosion in piping systems and pasteurizers, and even cause post-pasteurization contamination [6]. Until recent developments in cavitation, controlling biofilms in the dairy industry has been mainly accomplished by determining the kind of contaminating residues, such as protein, carbs, or fat, and/or the type of organisms and applying effective methods to remove them from the contact surfaces [7]. Detergents and disinfectants have been selected based on characteristics such as efficacy, safety, and the relation between the chemical treatment and the post-treatment effects on the finished products [8]. Heat treatments such as pasteurization and ultra-high temperature treatment have been commonly used in the dairy industry for a long time to eliminate pathogens and reduce microbial counts; however, they may not affect all thermoduric sporeformers and their spores [9].
More recently, some novel techniques such as cavitation are being experimented with in dairy manufacturing to reduce thermoduric and sporeformers’ counts, and eventually reduce biofilms [10]. This technology induces sound waves above human hearing frequencies, and the primary effect of ultrasound power is called ‘cavitation’. It generates intense acoustic cavitation, leading to thousands of bubbles with strong vibration in the medium, which lead to bacterial inactivation [11]. These bubbles grow due to high acoustic intensities and then, speedily collapse, leading to the bubbles’ disintegration into small fractions and the generation of high temperatures [12]. Newer technology has been tried as a hurdle concept and this information provides useful information in the application of cavitation as one of the hurdle technologies that can be combined with other unit operations to accomplish the reduction of sporeformers. In this current study, it was hypothesized that the process of cavitation would influence the ability of dairy sporeformers to attach and aggregate on contact surfaces, and eventually form biofilms. This effect was studied using ultrasonication using three trials, and the data were statistically analyzed.
2. Material and Methods
2.1. Source of Sporeformers Bacteria and Propagation of Vegetative Cells
Three different aerobic sporeformers bacterial species were chosen to conduct the experiments, namely Geobacillus stearothermophilus (ATCCâ15952), Bacillus licheniformis (ATCCâ 6634), and Bacillus sporothermodurans (DSM 10599). These strains were purchased from the American Type Culture Collection (ATCC) and Deutsche Sammlung von Mikroorganisem and Zellkulturen (German Collection of Microorganisms and Cell Cultures—DSMZ). The above bacteria were sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA), and Deutsche Sammlung von Mikroorganisem (DSM, Braunschweig, Germany), respectively. All the selected bacteria were propagated using the protocol recommended previously [13] and were evaluated for the biofilm-forming ability after exposure to cavitation under static conditions. Sporeformers bacterial species were grown overnight in brain heart infusion (BHI) broth (Oxoid/Thermo Scientific, Basingstoke, UK) at their optimum temperature; and at the mid-exponential phase, the cultures were pelleted by centrifugation at 4500× g for 30 min. The pellets were suspended in phosphate buffer saline solution (PBS) (Thermo Scientific, Denver, CO, USA). Optimum density was measured for each organism to reach 0.3 OD. After this step, the cell suspensions were ready to be inoculated in skim-milk samples to conduct further experiments.
Design of Experiment
Three organisms were selected and exposed to cavitation, and the counts were taken pre- and post-treatment under the same set of conditions. Three individual trials were conducted for the overall compilation of data and statistical analysis.
2.2. Preparation of Sterilized Skim Milk Samples for Spiking Experiments
Nonfat dry milk (NFDM) was obtained from the SDSU dairy plant and was reconstituted by mixing 113 gm with 877 mL of sterilized water, followed by autoclaving at 121 °C for 15 min and cooling; and was subsequently stored in a refrigerator for future use. Sterile skim milk was inoculated at 6–7 log CFU/mL of the cell suspension prepared.
2.3. Enumeration of Vegetative Cells of Sporeformers
The aerobic plates count (APC) is a well-known standard procedure to count colonies [13] using a spread-plates technique on brain heart infusion agar plates used as the growth medium [14]. For enumeration purposes, the samples were serially diluted in a phosphate buffer saline (PBS) solution. Then, 100 μL of the dilution was spread on pre-poured agar plates using sterile plastic spreaders (Thermo Scientific, Denver, CO, USA). The plates were incubated for 24 h at the optimum temperatures, and the colonies were calculated using the formula suggested by Downes [15].
2.4. Standardization of Cavitation Parameters of the Spiked Samples
Cavitation was conducted using 500-watt ultrasonic waves, created by the 505 Vibra-Cell high-intensity processor (Sonicated and Materials, Inc., New Town, CT, USA) with a stainless steel 13 mm probe, which tends to resonate at 20 kHz. The probe was sanitized with 70% alcohol, followed by washing with distilled water before and after conducting each trial. Then, 3–4 cm of the probe height was vertically inserted in the sample [16].
About 20 mL of the spiked milk samples was sonicated in 40 mL glass beakers, and these beakers were kept in an ice bath at close to 0 °C to control any temperature rise during the process of cavitation. The ice bath containing the beaker with the milk sample was placed under the sonicator probe. The probe of cavitation was immersed into the milk samples. The parameters of this experiment were exposing the samples to 20 kHz frequency, 80% amplitudes, and 500 W power for 10 min each [17].
2.5. Biofilm Formation by Cavitated Spore Former Vegetative Cells on Stainless Steel (SS) Coupons under Lab Conditions
Petri dishes were filled with the cavitated spiked milk samples and then, SS coupons were immersed in the milk and incubated for 72 h at the optimum temperature for each organism. Milk was changed every 12 h to provide new nutrients for the bacteria to grow. After 72 h of incubation, the coupons were washed with PBS buffer and the biofilm-embedded bacteria were retrieved by swabbing each coupon. These swab samples were serially diluted and plated on brain heart infusion agar (BHI) using the spread-plates technique [18]. Samples without cavitation treatment were taken as a control and the means were compared for the overall log reduction. Both pre- and post-cavitation samples were studied to evaluate their ability to form biofilms on stainless steel coupons under laboratory conditions.
2.6. Scanning Electron Microscopy (SEM) of Biofilm to Create Visual Evidence
All the bacterial cells entrapped within biofilms on coupons were observed under the SEM as described by Bulla [19]. After forming biofilms, coupons were washed with 1 mL of PBS and then, air dried under ambient conditions. After drying, they were coated with a 5 nm layer of gold using a CRC-150 sputtering system (Plasma Science Inc., Lorton, VA, USA). The coupons were observed with a Hitachi scanning electron microscope (model S-3400N), Hitachi SCI Systems Ltd., Tokyo, Japan) at 10 kV accelerating voltage to observe the biofilm. The samples that were observed before the ultrasonication treatment were used as a control to compare the morphology of the vegetative cells pre and post treatment.
3. Statistical Analysis
Every trial was performed in triplicate with three observations each. The data obtained were statistically analyzed using SAS programming (SAS Institute Inc. Cary, NC, USA). Means were compared using the Tukey multiple comparison test using SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA) with a least significance difference level of 5%.
4. Results and Discussion
4.1. Effect of Cavitation on Survival of Sporeformers
Three types of sporeformers bacterial species—G. stearothermophilus (ATCC®15952), B. licheniformis (ATCC® 6634), and B. sporothermodurans (DSM 10599)—were tested to evaluate the effect of ultrasonication on the survival of vegetative cells and compared with the control treatment (Table 1).

Table 1.
Pre- and post-cavitation effect on vegetative cells counts (Log10 cfu/mL) at zero hours of the three sporeformers bacteria in skim milk.
Overall, the cavitation reduced the survivability substantially, which is comparable to our previous findings [20]. A previous experiment was conducted to evaluate the hydrodynamic cavitation, followed by thermal treatment to inactivate B. coagulans (ATCC 12245) at (4.67 ± 0.18 log) in skim-milk concentrate. Skim milk was spiked with B. coagulans and was treated by cavitation at 3600 RPM with a flow rate of 100 L h−1, followed by heat treatment at 75–85 °C for 14–106. The initial load of 4.67 ± 0.18 log of vegetative cells was reduced to 3.5 log CFU mL. These studies prove that hydrodynamic cavitation combined with a thermal process could reduce the sporeformers in milk and its products [21].
4.2. Effect of Ultrasonication on Biofilm Forming Ability of Sporeformers
Biofilm-Forming Ability of Sporeformers Pre–Post Cavitation
All three sporeformers bacteria were treated with ultrasonication, and the ability of both pre- and post-cavitated cells to form biofilms was evaluated to observe the influence of cavitation on their biofilm-forming ability (Table 2).

Table 2.
Mean values of the effect cavitation on biofilm-formation ability of the three organisms at 80% amplitudes for 10 min.
Without cavitation, G. stearothermophilus (ATCC®15952) formed biofilms at a viable counts level of log 5.35 cfu/cm2, whereas post-cavitation treatment log counts were 4.39 cfu/cm2 and a significant difference was observed for the pre and post count (p < 0.05). The mean value of the control trial of biofilm-forming ability of Bacillus licheniformis (ATCCâ 6634) was 6.42 logs; however, the log value obtained after treatment was 5.44. It is clearly seen that there is a significant difference between pre- and post-treatment biofilm counts (p < 0.05). Similarly, the effect of cavitation treatment on the biofilm-forming ability of Bacillus sporothermodurans (DSM 10599) was 6.5 logs, which was reduced to log 5.81 logs after cavitation; in addition, that is a significant difference between pre- and post-treatment biofilm counts (p < 0.05). A significant difference was observed for the pre- and post-cavitation count (p ≤ 0.05) for all three organisms.
However, in the control trials (pre-cavitation), B. sporothermodurans had the higher ability to form biofilms among the three sporeformers. Moreover, a previous study was conducted in our lab to demonstrate the effect of continuous ultrasonication and hydrodynamic cavitation on the reduction of sporeformers in skim milk: hydrodynamic cavitation treatment for 6 passes showed a 99.97% reduction in vegetative cells of Bacillus coagulans; however, continuous ultrasonication treatment with 12 passes resulted in a 92% reduction of vegetative cells of Bacillus coagulans. Moreover, this study demonstrated that hydrodynamic cavitation was found to be a more effective method to reduce microbial counts as compared to the ultrasonication process. In conclusion, each organism may behave differently when exposed to ultrasonication treatment [22]. A previous study in our lab was conducted to compare the attachment of the vegetative cells of Geobacillus stearothermophilus, Bacillus sporothermodurans, and Bacillus licheniformis on SS surfaces [23]. It was demonstrated that Bacillus sporothermodurans had the highest bacterial attachment, followed by G. stearothermophilus; however, B. licheniformis resulted in minimal adhesion among others. Similarly, Listeria monocytogenes has been evaluated for the biofilm-forming ability on SS at different temperatures [24]. After 5 days, Listeria monocytogenes could form substantial biofilms even at a low temperature on stainless-steel surfaces. Another study was conducted by (Pagán et al. 1999), which indicated that Listeria monocytogenes was resistant to ultrasonic waves under high pressure [25].
Although not directly from dairy processing, some of the previous studies have shown that cavitation could also be combined with antimicrobials to reduce biofilm formation. Pagán (1999) conducted a study to evaluate the ultrasonication effect in combination with gentamicin on the biofilm-growing ability of Pseudomonas aeruginosa in medical implants. After 24 h of biofilm growth on polycarbonate (PC), they were exposed to 12 μg/mL gentamicin sulphate, followed by ultrasonication treatment at different frequencies. The study showed that ultrasonication along with the antibiotic resulted in significantly reducing the biofilm formation of Pseudomonas aeruginosa. Moreover, lower frequency ultrasonication was found to not disturb the biofilm and this would be significant in the clinical application of this technology to the treatment of implant infection [25].
4.3. Effect of Cavitation on the Morphology of the Vegetative Cells
Several micrographs were taken under the scanning electron microscope (SEM) before and after cavitation at 80% for 10 min, to compare the physical changes in cell morphology. A comparison between cell morphology of the thermoduric organisms is shown in (Figure 1, Figure 2 and Figure 3).
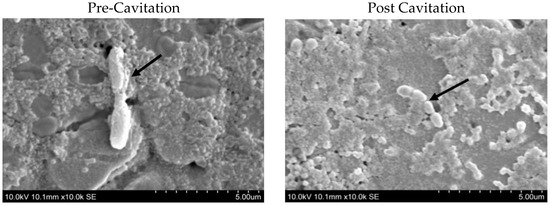
Figure 1.
Scanning electron microscopic images of Geobacillus stearothermophilus (ATCC®15952). Vegetative cells before and after cavitation. The arrows in the figures indicate the morphology of the vegetative cells pre and post cavitation.
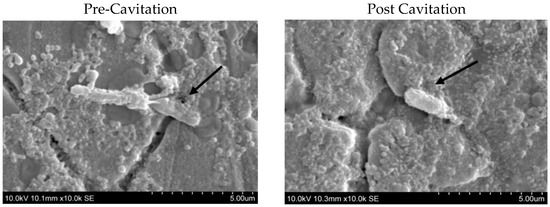
Figure 2.
Scanning electron microscopic images of Bacillus licheniformis (ATCC® 6634) vegetative. before and after Cavitation. The arrows in the figures indicate the morphology of the vegetative cells pre and post cavitation.
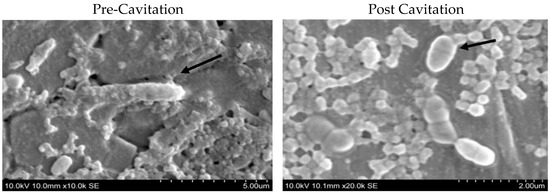
Figure 3.
Scanning electron microscopic images of Bacillus sporothermodurans (ATCC10599) vegetative cells before and after cavitation. The arrows in the figures indicate the morphology of the vegetative cells pre and post cavitation.
It can be clearly seen that the cell morphology of pretreatment vegetative cells was rod shaped, long, and with round-ended cells. Post-treatment cells, under the same parameters as pre-treatment cells, were clearly observed to be distorted. Most of the vegetative cells disintegrated into several pieces and became shorter with irregular dimensions. In addition, there were fewer unaffected cells appearing under the scanning electron microscope. This proves that cavitation treatment could cause structural alterations to the vegetative cells in all types of sporeformers. In addition, there are some investigations that indicated similar structural changes to cells after ultrasonication in other organisms, such as Escherichia coli and Streptococcus mutants [26].
5. Conclusions
The main objective of this study was to evaluate the effect of cavitation on the biofilm-forming ability of three different types of sporeformers. It was concluded that ultrasonication technology resulted in the overall reduction of all three sporeformer counts in skim milk; additionally, it affected the morphology of the vegetative cells, although the surviving cells still had the ability to form biofilms, though to a lower extent. This means that ultrasonication would result in a reduction of the bacterial count that may still retain the ability to form biofilms. Among the three organisms tested, Geobacillus stearothermophilus formed the least biofilms in both pre and post cavitation, 5.35 and 4.39 logs, respectively. The results obtained in the study thus support the hypothesis that the cavitated sporeformers would form lower biofilms. Scanning micrographs of the sporeformers exposed to cavitation showed cell morphology changes after the ultrasonication process. The outcome of this study provides a possible application such as ultrasonication to inactivate bacterial sporeformers in skim milk. The obtained result may be useful for future research to understand the effect of ultrasonication on other thermoduric bacteria, and to evaluate its economic feasibility in large-scale processing.
Author Contributions
Conceptualization, T.A. and S.A.; methodology, T.A. and S.A.; validation, T.A. and S.A.; investigation, T.A. and S.A.; writing—original draft preparation, T.A.; writing—review and editing, T.A. and S.A.; supervision, S.A.; project administration, S.A.; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Dairy Council (NDC) and administered by the Dairy Research Institute.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within.
Acknowledgments
Authors acknowledge the department of Dairy and Food Science and Agricultural Station of South Dakota State University. The Agricultural and Biosystems Engineering department is acknowledged for providing the cavitation device and the Electrical Engineering department for supporting SEM work. Special thanks to the government of Saudi Arabia for providing a scholarship to the first author to complete her higher education. This research was funded by the National Dairy Council (NDC) and administered by the Dairy Research Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alonso, P.V.P.; de Campos Ferreira, R.C.; Cotta, M.A.; Kabuki, D.Y. Influence of milk proteins on the adhesion and formation of Bacillus sporothermodurans biofilms: Implications for dairy industrial processing. Food Control 2022, 134, 108743. [Google Scholar]
- Habimana, O.; Semião, A.; Casey, E. The role of cell-surface interactions in bacterial initial adhesion and consequent biofilm formation on nanofiltration/reverse osmosis membranes. J. Membr. Sci. 2014, 454, 82–96. [Google Scholar]
- Courtney, H.S.; Ofek, I.; Penfound, T.; Nizet, V.; Pence, M.; Kreikemeyer, B.; Podbielbski, A.; Hasty, D.; Dale, J.B. Relationship between expression of the family of M proteins and lipoteichoic acid to hydrophobicity and biofilm formation in Streptococcus pyogenes. PLoS ONE 2009, 4, e4166. [Google Scholar] [CrossRef]
- Simoes, M.; Simoes, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Vishwakarma, V. Impact of environmental biofilms: Industrial components and its remediation. J. Basic Microbiol. 2020, 60, 198–206. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Erickson, D.J.; Sims, S.; Wolff, P. Characteristics of food contact surface materials: Stainless steel. Food Prot. Trends 2012, 32, 574–584. [Google Scholar]
- The, H.K.; Flint, S.; Brooks, J.; Knight, G. Biofilms in the Dairy Industry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mosteller, T.; Bishop, J. Sanitizer efficacy against attached bacteria in a milk biofilm. J. Food Prot. 1993, 56, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.E.; Smythe, B.; Crawford, R.; Oakley, E.; Hathaway, S.; Shepherd, J.M. Pasteurization of milk: The heat inactivation kinetics of milk-borne dairy pathogens under commercial-type conditions of turbulent flow. J. Dairy Sci. 2012, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Almalki, T.; Anand, S. 0543 Evaluation of the effect of cavitation on biofilm forming ability of sporeformers. J. Anim. Sci. 2016, 94 (Suppl. 5), 259. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Mason, T.J. Ultrasound processing of fluid foods. In Novel Thermal and Non-Thermal Technologies for Fluid Foods; Academic Press: Cambridge, MA, USA, 2012; pp. 135–165. [Google Scholar]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Power ultrasound to process dairy products. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 445–465. [Google Scholar]
- Novak, J.S.; Call, J.; Tomasula, P.; Luchansky, J.B. An assessment of pasteurization treatment of water, media, and milk with respect to Bacillus spores. J. Food Prot. 2005, 68, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Maturin, L.; Peeler, J.T. BAM: Aerobic Plate Count; US Food and Drug Administration: Silver Spring, MD, USA, 2001.
- Downes, J.; Munson, M.; Spratt, D.; Kononen, E.; Tarkka, E.; Jousimies-somer, H.; Wade, W.G. Characterisation of Eubacterium-like strains isolated from oral infections. J. Med. Microbiol. 2001, 50, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, B.; Lembke, F.; Hammer, P.; Stackebrandt, E.; Priest, F.G. Bacillus sporothermodurans, a new species producing highly heat-resistant endospores. Int. J. Syst. Evol. Microbiol. 1996, 46, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Downes, P.; Ito, F. Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Parkar, S.G.; Flint, S.; Palmer, J.; Brooks, J. Factors influencing attachment of thermophilic bacilli to stainless steel. J. Appl. Microbiol. 2001, 90, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bulla, L.; Julian, G.; Rhodes, R.; Hesseltine, C. Scanning electron and phase-contrast microscopy of bacterial spores. Appl. Microbiol. 1969, 18, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.N.; Anand, S.; Muthukumarappan, K.; Huegli, M. Inactivation of thermoduric aerobic sporeformers in milk by ultrasonication. Food Control 2014, 37, 232–239. [Google Scholar] [CrossRef]
- Sim, J.Y.; Beckman, S.L.; Anand, S.; Sergio; Martínez-Monteagudo, I. Hydrodynamic cavitation coupled with thermal treatment for reducing counts of B. coagulans in skim milk concentrate. J. Food Eng. 2021, 293, 110382. [Google Scholar] [CrossRef]
- Chaudhary, P.; Anand, S.; Monteagudo, S.M. Feasibility of hydrodynamic cavitation, in line with HTST pasteurization, for inactivating sporeformers and spores in skim milk. In Proceedings of the American Dairy Science association, Knoxville, TN, USA, 24 June 2017. [Google Scholar]
- Jindal, S.; Anand, S. Comparison of adhesion characteristics of common dairy sporeformers and their spores on unmodified and modified stainless steel contact surfaces. J. Dairy Sci. 2018, 101, 5799–5808. [Google Scholar] [CrossRef] [PubMed]
- Chavant, P.; Martinie, B.; Meylheuc, T.; Bellon-Fontaine, M.-N.; Hebraud, M. Listeria monocytogenes LO28: Surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 2002, 68, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Pagán, R.; Manas, P.; Alvarez, I.; Condón, S. Resistance of Listeria monocytogenesto ultrasonic waves under pressure at sublethal (manosonication) and lethal (manothermosonication) temperatures. Food Microbiol. 1999, 16, 139–148. [Google Scholar] [CrossRef]
- Qian, Z.; Stoodley, P.; Pitt, W.G. Effect of low-intensity ultrasound upon biofilm structure from confocal scanning laser microscopy observation. Biomaterials 1996, 17, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).