Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Reagents and Instruments
2.3. Sample Preparation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wochner, K.F.; Becker-Algeri, T.A.; Colla, E.; Badiale-Furlong, E.; Drunkler, D.A. The Action of Probiotic Microorganisms on Chemical Contaminants in Milk. Crit. Rev. Microbiol. 2018, 44, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.A.; Al-Ameri, H.A. Aflatoxins. In Aflatoxins-Occurrence, Detoxification, Determination and Health Risks; IntechOpen: London, UK, 2022. [Google Scholar]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Fink-Gremmels, J.; Li, D.; Tong, X.; Tang, J.; Nan, X.; Yu, Z.; Chen, W.; Wang, G. An Overview of Aflatoxin B1 Biotransformation and Aflatoxin M1 Secretion in Lactating Dairy Cows. Anim. Nutr. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Gonçalves, J.L.; Rosim, R.E.; Cappato, L.P.; Cruz, A.G.; Oliveira, C.A.F.; Corassin, C.H. Effects of Different Sources of Saccharomyces Cerevisiae Biomass on Milk Production, Composition, and Aflatoxin M1 Excretion in Milk from Dairy Cows Fed Aflatoxin B1. J. Dairy Sci. 2017, 100, 5701–5708. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain. Related to Aflatoxin B1 as Undesirable Substance in Animal Feed. EFSA J. 2004, 2, 39. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Turna, N.S.; Wu, F. Aflatoxin M1 in Milk: A Global Occurrence, Intake, & Exposure Assessment. Trends Food Sci. Technol. 2021, 110, 183–192. [Google Scholar] [CrossRef]
- FDA CPG Sec 527.400 Whole Milk, Lowfat Milk, Skim Milk-Aflatoxin M1. FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1 (accessed on 26 September 2022).
- ANVISA Agência Nacional de Vigilância Sanitária. Resolução RDC No 7, de 18 de Fevereiro de 2011. Diário Oficial Da União—Seção 1, 37. 2011. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0007_18_02_2011_rep.html (accessed on 26 September 2022).
- Gürbay, A.; Aydın, S.; Girgin, G.; Engin, A.B.; Şahin, G. Assessment of Aflatoxin M1 Levels in Milk in Ankara, Turkey. Food Control 2006, 17, 1–4. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Ganev, K.C.; Khaneghah, A.M.; Portela, J.B.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The Occurrence and Effect of Unit Operations for Dairy Products Processing on the Fate of Aflatoxin M1: A Review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Var, I.; Kabak, B. Detection of Aflatoxin M1 in Milk and Dairy Products Consumed in Adana, Turkey. Int. J. Dairy Technol. 2009, 62, 15–18. [Google Scholar] [CrossRef]
- Kuharić, Ž.; Jakopović, Ž.; Čanak, I.; Frece, J.; Bošnir, J.; Pavlek, Ž.; Ivešić, M.; Markov, K. Removing Aflatoxin M1 from Milk with Native Lactic Acid Bacteria, Centrifugation, and Filtration. Arch. Ind. Hyg. Toxicol. 2018, 69, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; el Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef]
- Womack, E.D.; Sparks, D.L.; Brown, A.E. Aflatoxin M1 in Milk and Milk Products: A Short Review. World Mycotoxin J. 2016, 9, 305–315. [Google Scholar] [CrossRef]
- Hassan, H.F.; Kassaify, Z. The Risks Associated with Aflatoxins M1 Occurrence in Lebanese Dairy Products. Food Control 2014, 37, 68–72. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Corrêa, B.; Rosim, R.E.; Kobashigawa, E.; Oliveira, C.A.F. Distribution and Stability of Aflatoxin M1 during Processing and Storage of Minas Frescal Cheese. Food Control 2012, 24, 104–108. [Google Scholar] [CrossRef]
- Conteçotto, A.C.T.; Pante, G.C.; Castro, J.C.; Souza, A.A.; Lini, R.S.; Romoli, J.C.Z.; Abreu Filho, B.A.; Mikcha, J.M.G.; Mossini, S.A.G.; Machinski, M., Jr. Occurrence, Exposure Evaluation and Risk Assessment in Child Population for Aflatoxin M1 in Dairy Products in Brazil. Food Chem. Toxicol. 2021, 148, 111913. [Google Scholar] [CrossRef]
- Sumon, A.H.; Islam, F.; Mohanto, N.C.; Kathak, R.R.; Molla, N.H.; Rana, S.; Degen, G.H.; Ali, N. The Presence of Aflatoxin M1 in Milk and Milk Products in Bangladesh. Toxins 2021, 13, 440. [Google Scholar] [CrossRef]
- Bilandžić, N.; Varga, I.; Varenina, I.; Solomun Kolanović, B.; Božić Luburić, Đ.; Đokić, M.; Sedak, M.; Cvetnić, L.; Cvetnić, Ž. Seasonal Occurrence of Aflatoxin M1 in Raw Milk during a Five-Year Period in Croatia: Dietary Exposure and Risk Assessment. Foods 2022, 11, 1959. [Google Scholar] [CrossRef]
- Pires, R.C.; Portinari, M.R.P.; Moraes, G.Z.; Khaneghah, A.M.; Gonçalves, B.L.; Rosim, R.E.; Oliveira, C.A.F.; Corassin, C.H. Evaluation of Anti-Aflatoxin M1 Effects of Heat-Killed Cells of Saccharomyces Cerevisiae in Brazilian Commercial Yogurts. Qual. Assur. Saf. Crops Foods 2022, 14, 75–81. [Google Scholar] [CrossRef]
- Panara, A.; Katsa, M.; Kostakis, M.; Bizani, E.; Thomaidis, N.S. Monitoring of Aflatoxin M1 in Various Origins Greek Milk Samples Using Liquid Chromatography Tandem Mass Spectrometry. Separations 2022, 9, 58. [Google Scholar] [CrossRef]
- Xiong, J.; Wen, D.; Zhou, H.; Chen, R.; Wang, H.; Wang, C.; Wu, Z.; Qiu, Y.; Wu, L. Occurrence of Aflatoxin M1 in Yogurt and Milk in Central-Eastern China and the Risk of Exposure in Milk Consumers. Food Control 2022, 137, 108928. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, X.; Zhou, H.; Lei, M.; Liu, Y.; Ye, C.; Wu, W.; Wang, C.; Wu, L.; Qiu, Y. Aflatoxin M1 in Pasteurized, ESL and UHT Milk Products from Central China during Summer and Winter Seasons: Prevalence and Risk Assessment of Exposure in Different Age Groups. Food Control 2021, 125, 107908. [Google Scholar] [CrossRef]
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Occurrence of Aflatoxin M1 in Dairy Products in Brazil. Food Control 2011, 22, 1971–1974. [Google Scholar] [CrossRef]
- Shundo, L.; Navas, S.A.; Lamardo, L.C.A.; Ruvieri, V.; Sabino, M. Estimate of Aflatoxin M1 Exposure in Milk and Occurrence in Brazil. Food Control 2009, 20, 655–657. [Google Scholar] [CrossRef]
- Picinin, L.C.A.; Cerqueira, M.M.O.P.; Vargas, E.A.; Lana, Â.M.Q.; Toaldo, I.M.; Bordignon-Luiz, M.T. Influence of Climate Conditions on Aflatoxin M1 Contamination in Raw Milk from Minas Gerais State, Brazil. Food Control 2013, 31, 419–424. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Ulliana, R.D.; Ramos, G.L.P.A.; Cruz, A.G.; Oliveira, C.A.F.; Kamimura, E.S.; Corassin, C.H. Occurrence of Aflatoxin M1 in Milk and Minas Frescal Cheese Manufactured in Brazilian Dairy Plants. Int. J. Dairy Technol. 2021, 74, 431–434. [Google Scholar] [CrossRef]
- Jager, A.V.; Tedesco, M.P.; Souto, P.C.M.C.; Oliveira, C.A.F. Assessment of Aflatoxin Intake in São Paulo, Brazil. Food Control 2013, 33, 87–92. [Google Scholar] [CrossRef]
- Murshed, S. Evaluation and Assessment of Aflatoxin M1 in Milk and Milk Products in Yemen Using High-Performance Liquid Chromatography. J. Food Qual. 2020, 2020, 8839060. [Google Scholar] [CrossRef]
- Patyal, A.; Gill, J.P.S.; Bedi, J.S.; Aulakh, R.S. Occurrence of Aflatoxin M1 in Raw, Pasteurized and UHT Milk from Punjab, India. Curr. Sci. 2020, 118, 79–86. [Google Scholar] [CrossRef]
- Tadesse, S.; Berhanu, T.; Woldegiorgis, A.Z. Aflatoxin M1 in Milk and Milk Products Marketed by Local and Industrial Producers in Bishoftu Town of Ethiopia. Food Control 2020, 118, 107386. [Google Scholar] [CrossRef]
- Yunus, A.; Imtiaz, N.; Khan, H.; Ibrahim, M.; Zafar, Y. Aflatoxin Contamination of Milk Marketed in Pakistan: A Longitudinal Study. Toxins 2019, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Afif, C.; Joubrane, K.; Khabbaz, L.R.; Maroun, R.; Ismail, A.; Khoury, A. el Occurrence of Aflatoxin M1 in Raw, Pasteurized, UHT Cows’ Milk, and Dairy Products in Lebanon. Food Control 2020, 111, 107055. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Nikousefat, Z.; Karami, N. Occurrence, Seasonal Variation and Risk Assessment of Exposure to Aflatoxin M 1 in Iranian Traditional Cheeses. Food Control 2017, 79, 356–362. [Google Scholar] [CrossRef]
- Prado, G.; Oliveira, M.S.; Pereira, M.L.; Abrantes, F.M.; Santos, L.G.; Veloso, T. Aflatoxin M1 in Samples of “Minas” Cheese Commercialized in the City of Belo Horizonte-Minas Gerais/Brazil. Ciênc. Tecnol. Aliment. 2000, 20, 398–400. [Google Scholar] [CrossRef]
- Oliveira, C.A.F.; Franco, R.C.; Rosim, R.E.; Fernandes, A.M. Survey of Aflatoxin M₁ in Cheese from the North-East Region of São Paulo, Brazil. Food Addit. Contam. Part B Surveill. 2011, 4, 57–60. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef]
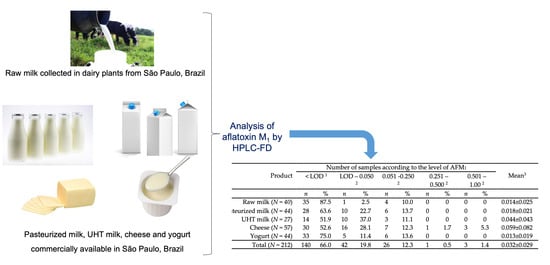
Product | Number of Samples According to the Level of AFM1 | Mean 3 | Range 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <LOD 1 | LOD–0.050 2 | 0.051–0.250 2 | 0.251–0.500 2 | 0.501–1.00 2 | ||||||||
| n | % | n | % | n | % | n | % | n | % | |||
| Raw milk (n = 40) | 35 | 87.5 | 1 | 2.5 | 4 | 10.0 | 0 | 0 | 0 | 0 | 0.114 ± 0.070 | 0.014–0.182 |
| Pasteurized milk (n = 44) | 28 | 63.6 | 10 | 22.7 | 6 | 13.7 | 0 | 0 | 0 | 0 | 0.032 ± 0.033 | 0.003–0.117 |
| UHT milk (n = 27) | 14 | 51.9 | 10 | 37.0 | 3 | 11.1 | 0 | 0 | 0 | 0 | 0.080 ± 0.038 | 0.020–0.148 |
| Minas cheese (n = 57) | 30 | 52.6 | 16 | 28.1 | 7 | 12.3 | 1 | 1.7 | 3 | 5.3 | 0.122 ± 0.190 | 0.017–0.695 |
| Yogurt (n = 44) | 33 | 75.0 | 5 | 11.4 | 6 | 13.6 | 0 | 0 | 0 | 0 | 0.050 ± 0.025 | 0.017–0.091 |
| Total (N = 212) | 140 | 66.0 | 42 | 19.8 | 26 | 12.3 | 1 | 0.5 | 3 | 1.4 | 0.080 ± 0.071 | 0.003–0.695 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corassin, C.H.; Borowsky, A.; Ali, S.; Rosim, R.E.; de Oliveira, C.A.F. Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update. Dairy 2022, 3, 842-848. https://doi.org/10.3390/dairy3040057
Corassin CH, Borowsky A, Ali S, Rosim RE, de Oliveira CAF. Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update. Dairy. 2022; 3(4):842-848. https://doi.org/10.3390/dairy3040057
Chicago/Turabian StyleCorassin, Carlos Humberto, Aline Borowsky, Sher Ali, Roice Eliana Rosim, and Carlos Augusto Fernandes de Oliveira. 2022. "Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update" Dairy 3, no. 4: 842-848. https://doi.org/10.3390/dairy3040057
APA StyleCorassin, C. H., Borowsky, A., Ali, S., Rosim, R. E., & de Oliveira, C. A. F. (2022). Occurrence of Aflatoxin M1 in Milk and Dairy Products Traded in São Paulo, Brazil: An Update. Dairy, 3(4), 842-848. https://doi.org/10.3390/dairy3040057