1. Introduction
New Zealand dairy systems have focused on increases in milk solid production [
1], calving ease and early puberty attainment when selecting replacement heifers in order to maximise farm profitability. Selecting for increased milk solids has resulted in a greater partitioning of nutrients to milk production. By increasing milk production, cows have greater nutritional demands that, if not met, result in decreased body condition scores, as well as reduced fertility and survival [
2]. Calving ease is an important breeding trait for many dairy farmers in order to reduce the incidence of dystocia that requires labour for assisted calving and risks the lives of the calf and cow. Calving ease can be improved by altering the weight and shape of the calf and the pelvic area of the cow. Earlier puberty attainment results in more heifers cycling at the planned start of mating, improving the first service conception rate. By having heifers pregnant earlier in the season, calving occurs at an earlier age, giving a longer lactation period and allowing for more time to recover from postpartum anoestrous prior to the start of the following mating season [
3]. To achieve early puberty attainment, heifers must reach 48–51% of their mature weight at a younger age [
4].
The result of these objectives is an altered growth trajectory with rapid growth required prior to puberty. Industry data have demonstrated this with changes in average 2-year and 4-year-old weights over a 10-year period. In the 2009/2010 milking season, the average live weight for a two-year-old Holstein–Friesian was 400 kg and the average live weight for a four-year-old was 512 kg [
5]. Compared with 2009/2010 cows, in the 2020/2021 milking season Holstein–Friesian cows were heavier at two years of age at 440 kg, but lighter at four years of age (503 kg) [
6]. A similar trend was observed in Holstein–Friesian–Jersey crossbreeds, indicating that the growth trajectory of dairy heifers has altered in the past 10 years, resulting in a greater live weight gain prior to two years of age. With changes in the growth trajectory, it is likely that the pattern of stature growth will have also changed. Therefore, previously reported patterns of stature growth may not reflect the current growth trajectory of New Zealand dairy heifers. Energy requirements for a heifer are dependent on breed, such that larger breeds need higher dietary requirements than smaller breeds to allow for a faster growth rate [
7]. However, if these energy requirements are not met, a faster growing animal is more susceptible to disruptions in growth as a result of feed shortages [
8].
The New Zealand dairy industry has a heavy reliance on pasture, rendering heifer growth susceptible to seasonal variation in pasture quality and availability. A compact calving period is required to ensure that calves are born just prior to spring, and pasture quality and quantity are maximised to meet the demand of the herd for lactation [
9]. Times of poor pasture availability due to low rainfall in mid-summer or low temperatures in midwinter result in slow pasture growth rates and poor-quality pasture [
10]. If deficits in pasture are not corrected with the use of supplementary feed, a heifer is likely to face a growth check. The importance of growth rate management has been emphasised due to its effect on future lactation performance [
11]. Seasonal growth checks have been shown to affect stature growth, such that heifers affected by seasonal pasture growth in the first winter of life were 2 cm taller at a given live weight at 15 months of age than heifers that grew in a linear manner, possibly as a result of compensatory growth [
4]. Differences in heifer growth emphasise how periods of poor-quality feed can alter the longitudinal bone growth trajectory. The differences in height may also be explained by a delay in puberty acquisition in heifers grown seasonally, resulting in a later physeal closure [
12].
It is recommended that farmers target a linear growth pattern, as it is associated with an earlier pubertal age compared to heifers affected by seasonal growth checks [
4]. A linear growth pattern also promotes continuous longitudinal and appositional bone growth and mineralisation to allow peak bone mass to be achieved. Heifers that fail to reach peak bone mass are likely to be at a greater risk of osteoporosis, in which the bone is unable to provide adequate resistance to bending (increased risk of fractures) [
13]. A condition that affects approximately 4% of all dairy farms in New Zealand is the spontaneous humeral fracture. The location of the humerus and the severity of this type of fracture means that fixation of the fracture is not practically or economically viable, resulting in the euthanasia of affected heifers. This poses not only a welfare issue, but a significant economic loss to the New Zealand dairy industry.
Humeral fractures tend to occur just prior to calving or in the 3 months after calving, and present as a spiral fracture down the length of the diaphysis. Microscopically, the presence of growth arrest lines can be detected. These growth arrest lines and a reduced cortical bone density indicate that the heifer has undergone a period of inadequate nutrition prior to calving [
13,
14]. With compromised bone strength and a sudden mobilisation of calcium stores as a result of lactation, the risk of spontaneous fractures is increased [
13]. These findings highlight the need to be able to describe normal growth and development in New Zealand dairy heifers, in order to identify deviations that may be associated with impaired growth and development. The location of the humerus greatly limits methodologies that can be used to quantify humerus growth and development in heifers, and thus the quantification of normal patterns of stature growth and development may provide a pragmatic early indicator of the effect of any nutritional challenge.
It is known that breed affects bone morphology and live weight at a given age [
15]; however, the effect it has on the pattern of stature growth has not been explored. Previous studies have described changes in height and girth [
4,
7], providing useful information on the distribution of live weight gain. However, these studies do not describe the relative timing or contribution of proximal (humerus and scapula) or distal (metacarpus and radius) growth in the forelimbs. Therefore, the aim of this study was to examine how the proportional contributions of stature change with increases in live weight in Holstein–Friesian, Jersey and Holstein–Friesian–Jersey crossbred heifers from birth to 23 months of age (just prior to calving).
2. Materials and Methods
This experiment was conducted with the approval of the Massey University Animal Ethics Committee (MUAEC 19/81).
The cohort consisted of 57 Holstein–Friesian, Jersey and Holstein–Friesian–Jersey crossbred singleton heifer calves born during the 2019 spring calving season at Massey University’s Dairy One farm. Calves were removed from their dam within 24 h of birth and dam identification was confirmed by DNA parentage. Dam parity was recorded for each calf as heifer (primiparous, n = 11) or cow (multiparous, n = 46). Calves were housed indoors for the first three weeks of life and moved on to pasture thereafter. Calves were reared under commercial management involving ad libitum colostrum for the first three days of life, followed by 7 L/day of whole cow milk split over two feeds for approximately six weeks. Calves were then reduced to 5 L per day and fed once daily until weaning.
Calves were weaned at approximately 100 kg (71–95 days of age) live weight. Ad libitum access to proprietary calf meal was offered until five months of age (SealesWinslow 20% muesli (20% crude protein, 3% fat, 13 MJ/kg ME) followed by SealesWinslow 18% pellets (18% crude protein, 3% fat, 12.5 MJ/kg ME); SealesWinslow, Whanganui, New Zealand). After being removed from the calf shed, calves were managed as one mob, except around weaning when calves on milk were managed separately to weaned calves. After weaning, the biggest 52 calves were grazed separately to the 5 smaller calves. At 185–209 days of age (April 2020), calves were moved to a grazier in Kimbolton, Manawatu, New Zealand. Calves were grazed under commercial management which involved separating calves into three mobs based on breed (Holstein–Friesian, Crossbred and Jersey) to minimise competition between different sized heifers and allow each mob to be managed to meet breed-specific live weight targets.
At approximately 549–573 days old, heifers were moved back to Massey University’s Dairy One Farm where they were grazed as one mob.
For analysis, heifers were classified into one of five breed groups as defined by Handcock et al. [
16], based on herd (pedigree) records for each calf (
Table 1).
2.1. Sampling and Measurements
Calves were measured fortnightly from birth (within first 48 h) until weaning. After weaning, calves were measured monthly until 23 months of age, except for at 7–8 months of age when a nationwide COVID-19 lockdown prevented the collection of two measurements. On each measurement day, the calves were weighted using a Tru-test weigh bridge (Tru-Test, Auckland, New Zealand) to the nearest kilogram. When contained within the weigh cage, single linear measurements to the nearest 0.5 cm were obtained using a flexible tape measure (Korband, Lincolnshire, UK). Linear measurements were obtained of wither height, girth, and wither-rump length (wither to tuber ischii). Leg measures of the left forelimb consisted of leg length (from ground to point of olecranon), toe length (from ground to the lateral aspect of the metacarpophalangeal joint), knee height (from ground to the lateral aspect of the antebrachiocarpal joint) and the cannon circumference (circumference of the metacarpus bone mid-length). Thoracic height was equal to wither height minus leg length, radial/ulna length was equal to leg length minus knee height, and metacarpus length (including the carpal bones) was equal to knee height minus toe height.
2.2. Statistical Analysis
Statistical analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Second-, third- and fourth-order Legendre polynomials were considered to predict live weight at a given age. The third order was selected based on R2, AIC and problems with convergences using the fourth-order polynomials. A third-order Legendre polynomial was fitted to age using random regression to obtain an average curve for live weight for the population and for each heifer with the random effect for animals. For this, live weight at day 0 and every month until 700 days of age was estimated for each heifer. Models included the fixed effect of the breed group. The effect of dam parity (primiparous or multiparous) was tested but was not significant, so was removed. Growth rates were determined using a general linear model with the fixed effect of breed and covariate of age.
Stature and live weight graphs were plotted using the raw data and with a third-order polynomial curve fitted. Plots included 95% confidence intervals.
Beta coefficients and the points of inflection for the relationship of length and height measures with live weight were calculated using a piecewise regression analysis with Number Cruncher Statistical Systems (NCSS 2020, LLC, Kaysville, UT, USA). The piecewise regression was fitted as a linear–linear regression model to fit a curve of best fit through all breed data for each stature measure. For all tests, a significance level of p < 0.05 was used.
3. Results
The third-order polynomial to predict live weight based on age was a good predictor of actual live weight (R
2 = 0.992) and live weight increased with age. Within the model, purebred Jersey calves had a significantly lighter live weight (
p < 0.05) at all ages than crossbred calves and purebred Holstein–Friesians. Purebred Holstein–Friesians and FX had the greatest overall growth rates (0.73 kg/d and 0.72 kg/d), followed by FJ (0.71 kg/d), JX (0.68 kg/d), and lastly J (0.63 kg/d) (
Figure 1).
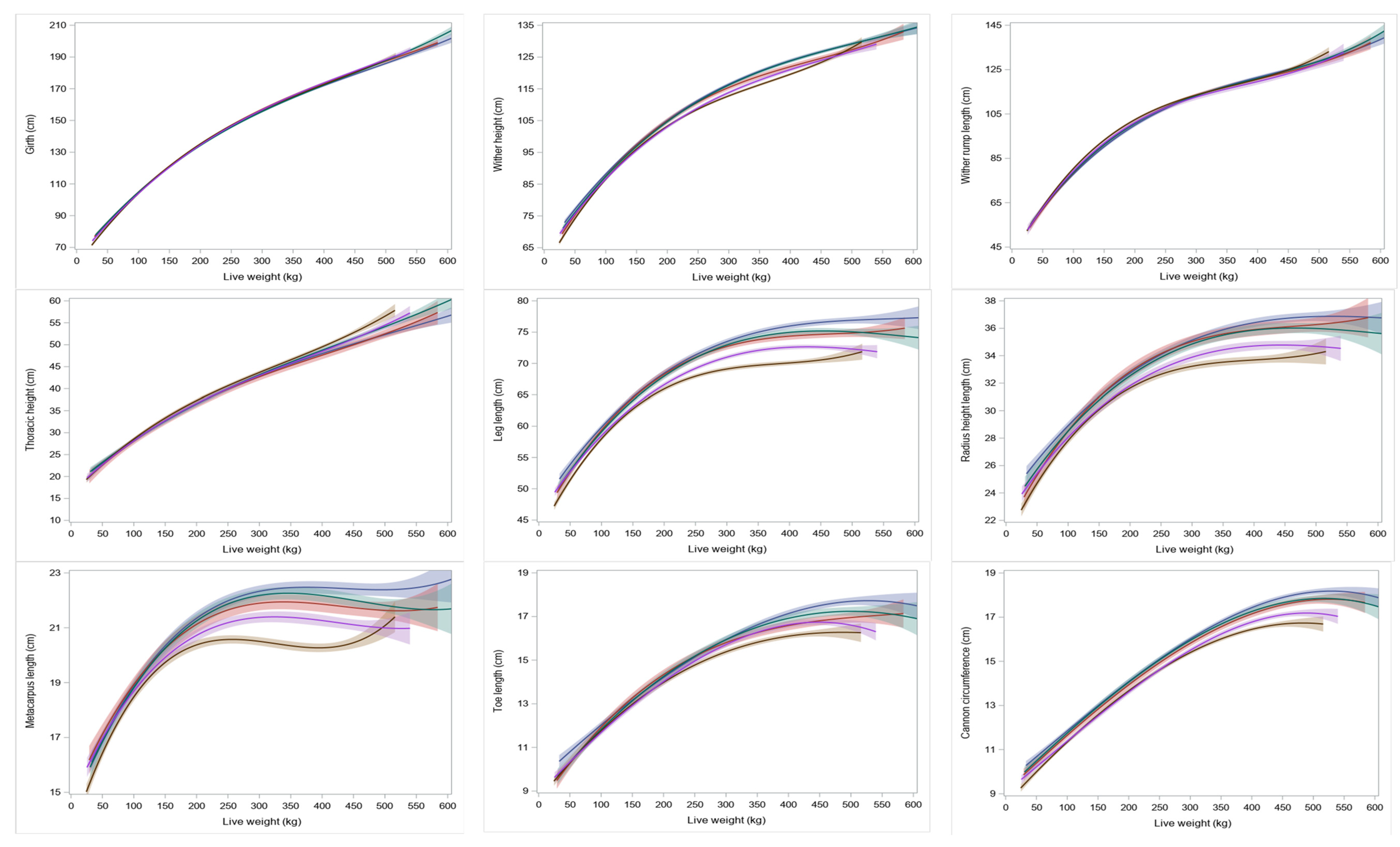
Breed had no effect on girth or wither-rump length at any given weight (
Figure 2). Once the heifers were yearlings (>260 kgs), the Holstein–Friesian and Holstein–Friesian cross calves had a greater wither height, leg length, radius length, metacarpus length and toe length at all weights than the Jersey calves (
Figure 2).
Girth had the greatest rate of increase per kg of liveweight both before and after the inflection point (
Table 2). Measurements of the forelimb (leg length, radius height, metacarpus length, toe length and cannon circumference) had the lowest rates of increase both before and after the corresponding inflection points. Measurement sites with limited growth potential (cannon circumference and toe length) appeared to have later inflection points, which may reflect difficulty in model fit rather than the underlying biology of growth at these sites. Prior to the inflection point there were similar rates of increases in height for both the thorax and the limb. Post inflection there were only moderate increases in leg length.
Relative proportions of limb segments to height are presented for the entire dataset rather than separate breeds due to negligible differences between the breeds. Data are presented as a relative proportion of limb segment length. At 50 kg, radius length (at the level of the ulna olecranon) contributed most to wither height (
Figure 3). However, at approximately 100 kg, the proportion of thoracic height to wither height had increased to be equal to that of the radius. Post 100 kg, thoracic height provided the largest proportion of wither height.
4. Discussion
Although overseas dairy systems have similar selection pressures, the heavy reliance on pasture in the New Zealand dairy system and differences in the mature size of the same breeds [
7] mean that research on stature growth may not be directly comparable. The mature weight of a Holstein–Friesian cow in New Zealand is reported as approximately 529 kg [
17], which is considerably lighter than the mature weights reported for Holstein–Friesian cattle from Brazil (681 kg) and the United Kingdom (636 kg) [
7,
18]. It is common under the New Zealand dairy system for heifers to undergo a growth check during winter (June to August) due to poor pasture quality and availability [
19]. However, in the current study, at 15 months of age heifers were approximately 78% of their mature live weight. With the linear growth pattern observed and a live weight well above the 60% mature weight industry target, it is unlikely that these heifers underwent a significant seasonal growth check, making them a good model for describing uninhibited growth. In addition, the high percentage of mature weight at 15 months of age may be due to a greater herd mature weight than the industry standard used to make this calculation.
The deviation of live weight at approximately 200 days of age between Jersey heifers from the Holstein–Friesians and crossbreeds reflects differences in mature live weight. Holstein–Friesians had the greatest absolute growth rate, the crossbreeds had an intermediate growth rate, and purebred Jerseys had the slowest growth rate. This is in agreement with Handcock et al. [
10], who demonstrated that the relative growth rate of Holstein–Friesians and Jersey breeds is similar. The relative growth rate at 20–22 months of age differed, with Jersey breed heifers having a greater relative growth rate compared to crossbreds and Holstein–Friesians, indicating an earlier maturity for Jersey breeds. However, a limitation of both Handcock et al. [
10] and the current study is that live weight was not corrected for fetal weight in the pregnant heifers. Without a specific conception date and calf birth weight for each heifer, the contributions of fetal weight to total live weight could not be estimated. Therefore, differences in the relative growth rate between breeds could not be confirmed during late pregnancy.
Linear measurements were obtained by a single operator familiar with the linear measurement of livestock throughout the trial. On each occasion, a single measurement was made of each anatomical variable of interest. The use of repeated measurements over measurement days and the fitting of polynomial equations to describe the pattern of growth would have minimised any source of error with the use of single measurements on each measurement day. Increases in distal forelimb measurements (metacarpal length) had slowed by 250 kg, reflecting the early maturity in distal limbs compared to the proximal limb. The heavier inflection points in radius height, cannon circumference and toe length were likely an artefact from the minimal increases in measures over time. Growth in the metacarpus after birth was limited, as shown by the small increases in length after 126 kg. Reports have suggested that the metacarpus is 85–90% of its mature length at birth [
20]. However, in the current study, the metacarpus length was approximately 75% of the mature length at birth. Due to the difficulties of palpating the joints of live cattle in a weigh crate, measures of the leg were carried out using visual landmarks. The carpometacarpal joint can only be located by palpation, so the antebrachiocarpal joint was used as it is easily visually identified. Therefore, the continued growth in the metacarpus length measure after a year was possibly a result of increases in carpal bone size rather than the metacarpus. Differences in bone maturity are the result of typical mammalian patterns of growth. In addition, differences in the function of proximal and distal limb bones and the resulting forces placed on them affect the requirement for increases in bone strength. For example, the metacarpus undergoes compressional forces as a result of stance with minimal effects from muscular torsion, thus requiring a lower bone strength [
21]. A proximal limb bone such as the humerus, on the other hand, is surrounded by the
biceps brachii,
brachiocephalicus and
brachialis muscles, so undergoes torsional forces as the animal moves. The humerus is subjected to increasing loads as this muscle mass increases with the increase in capacity (as measured in thorax growth in the current study) [
22,
23]. Therefore, distal limb bones such as the metacarpus, which undergo immediate loading and only moderate increases in relative bending strain with increasing live weight in non-cursorial animals, tend to mature earlier than proximal bones such as the humerus [
24,
25].
This pattern of growth is in agreement with Guilbert and Gregory [
20], where increases in live weight early in a heifers life were found to be the result of long bone growth (linear skeletal growth). However, the onset of puberty and increased oestrogen secretion ceases longitudinal bone growth by promoting physeal closure [
26]. A live weight of 250 kg occurred at 9 months of age in Holstein–Friesians and 10 months in Jersey calves. With 80% of mature height achieved in the first 7 months of age but only 35–45% of mature weight, increases in live weight after 7 months must be primarily from increases in capacity (girth) [
27]. The finding that increases in capacity continue after a year [
27] is in agreement with observations of the current study, where increases in girth remained constant up to 23 months of age. Both thoracic height and girth had the lowest ratio of Beta coefficients, demonstrating the continuation of growth after the point of inflection compared to other measures that had a sharp decrease in the rate of increase after the point of inflection (high beta ratio).
Calves born to primiparous cows tend to be lighter than calves born to multiparous cows [
28]. A lighter calf from a primiparous cow is likely the result of the dam still growing, and thus having a lighter live weight than multiparous cows. As such, dietary energy will be shared between fetal and dam growth. However, in the current study, the small number of calves born to primiparous cows did not allow for a true measure of differences in stature growth between calves born to primiparous and multiparous cows. In order to observe the true effect of dam parity on stature growth, more calves born to primiparous cows in each breed group would be needed.
Jersey heifers were consistently lighter at all ages than Holstein–Friesians, reflecting the lighter birth weight and lower absolute average daily gain through to maturity [
29]. At a given live weight, heifers with a greater proportion of Holstein–Friesian breed were heavier than heifers with a larger proportion of Jersey breed. Larger breed cows such as Holstein–Friesians tend to mature at a heavier weight and older age than lighter breed cows such as Jersey cows [
7,
30]. In the current study, the divergence of measures of leg length, radius length, metacarpus, and toe length in Jersey cows reflected the lighter mature weight of the Jersey cows compared to the heavier maturing Holstein–Friesian cows [
7]. However, measures of capacity such as girth, wither rump length, and thoracic height remained the same between all breed compositions at a given live weight. By having similar selection pressures for growth and milk production for both Holstein–Friesians and Jersey cows, the phenotype for body composition is likely to be similar, indicating an optimal body configuration at a given live weight. However, this trend was altered in leg measures at 200 kg due to the onset of puberty at a lighter liveweight for Jerseys and crossbreeds with a high proportion of Jersey breed, resulting in the earlier cessation of growth in the distal limb but continued increases in capacity. It is likely that this trend will occur in measures of capacity, as Jersey heifers near their mature weight earlier than Holstein–Friesian heifers.
The continued growth in girth and thoracic height measures in all breeds after the cessation of growth in the forelimb reflects the pQCT measures of bone morphology and strength in the metacarpus and humerus [
24], and contributes to an increasing body of evidence that the sensitive period for impaired humeral bone growth and development coincides with a heifer’s second winter. Physeal closure in the distal humerus of cattle occurs at approximately 15–20 months of age, corresponding with 80% of mature weight, whilst closure in the proximal humerus occurs between 3.5 and 4 years, when over 90% of mature weight has been attained [
17,
25]. Increases in capacity will result in increases in muscularity and loading on the humerus, requiring an increase in humeral bone strength to maintain strain within physiological limits [
31]. The effects of a seasonal growth check are compounded by the increasing demands for feed to support a fetus as a heifer reaches the latter stages of pregnancy, and the continuing growth of the heifer. At this time, nutrient partitioning to the fetus is maximised [
32]. If the nutritional requirements required for fetal and heifer growth are not reached, a growth check will occur. Although not observed in the current study, another possible cause for a growth check prior to calving is the movement of heifers from a grazier back to the home farm. During this time, heifers adjust to a new diet which may not have the same composition or availability as the grazier farm. In addition, heifers may be mixed in with other established grazing herds and shift down the social hierarchy, reducing their access to feed [
33].
The stature growth data here, and what is known about the New Zealand Dairy system, support the hypothesis that impaired nutrition during the second winter may be a primary risk factor for humerus fracture under New Zealand pastoral conditions [
13,
14]. The similarity of growth at the same live weight, but not age, indicates that all breeds may be susceptible to nutritional challenges during their second winter. Future research is required to examine how proximal and distal growth is affected by seasonal growth, with a particular focus on the second winter in order to understand the severity of the nutritional deficits required to alter the growth trajectory of a heifer.