Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review
Abstract
:1. Introduction
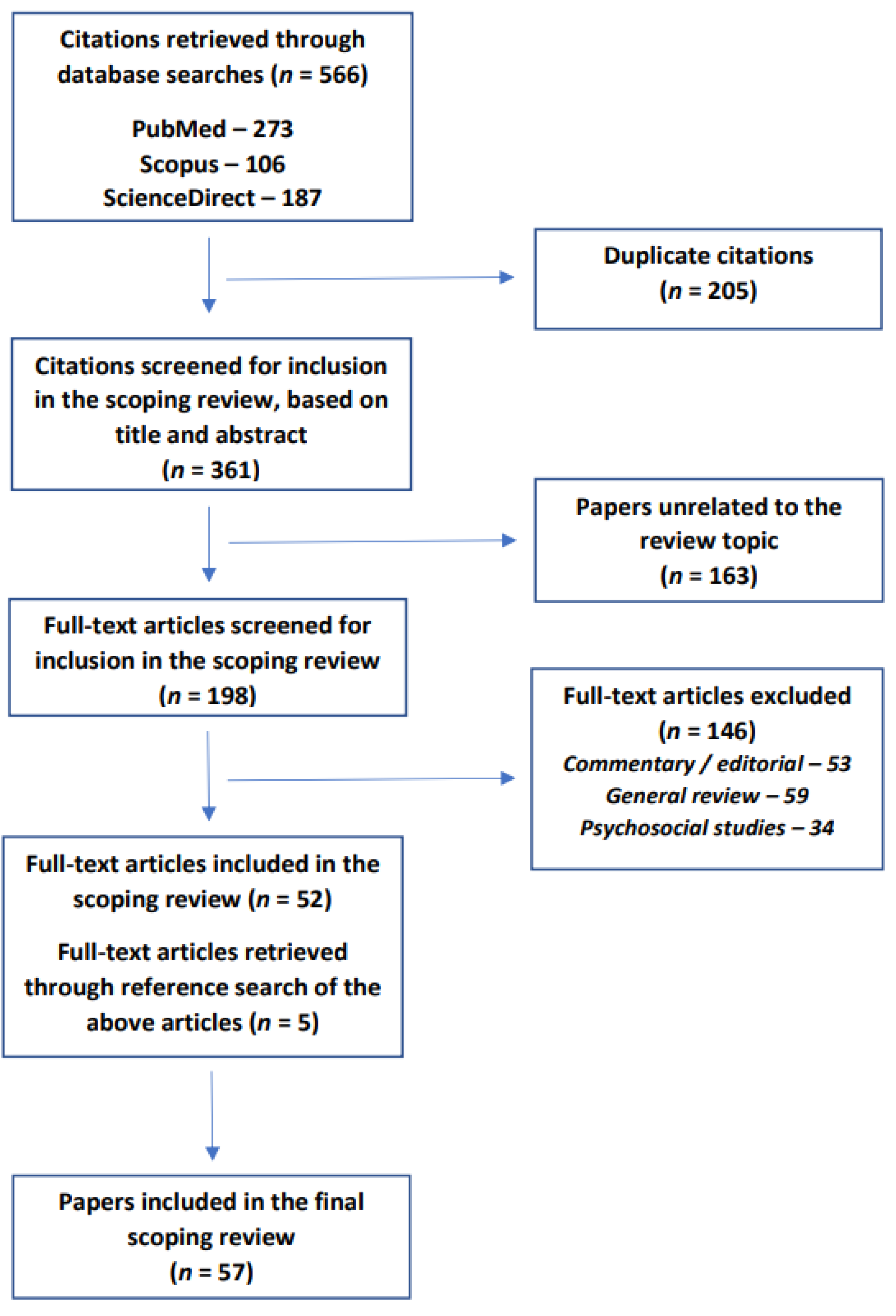
2. Materials and Methods
- (“social pain”, “social pain model”, “social pain hypothesis”, “social rejection” OR “social stress”) along with (“suicide”, “suicide attempt”, “suicide attempter”, “suicidal ideation”, “suicidal behaviour”, “suicide risk”, “suicide prevention” or “suicidality”). This search yielded a total of 287 citations (PubMed—140, Scopus—19, ScienceDirect—128)
- (“endogenous opioid”, “opioid receptor”, “serotonin”, “serotonergic”, “cannabinoid”, “endocannabinoid”, “oxytocin”, “cytokine” OR “interleukin”) along with (“suicide prevention”, “suicide attempt”, “suicide attempter”, “suicidal ideation”, “suicidal ideator”, “suicide risk” or “suicidality”) along with (“social pain”, “social stress” OR “social rejection”). The purpose of this second search was to identify studies examining the known neural substrates of social pain in relation to suicidality. This search yielded a total of 279 citations (PubMed—133, Scopus—87, ScienceDirect—59).
3. Results
- (a)
- Opioid receptor agonists and antagonists
- (b)
- Serotonin receptor agonists
- (c)
- Glutamate receptor antagonists
- (d)
- xytocin receptor agonists
- (e)
- Other therapeutic targets
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yip, P.; Zheng, Y.; Wong, C. Demographic and epidemiological decomposition analysis of global changes in suicide rates and numbers over the period 1990–2019. Inj. Prev. 2022, 28, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Global Burden of Disease Self-Harm Collaborators. Global, regional, and national burden of suicide mortality 1990 to 2016: Systematic analysis for the Global Burden of Disease Study 2016. BMJ 2019, 364, l94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, A.C.; Ohlsson, H.; Mościcki, E.; Crump, C.; Sundquist, J.; Lichtenstein, P.; Kendler, K.S.; Sundquist, K. On the Genetic and Environmental Relationship Between Suicide Attempt and Death by Suicide. Am. J. Psychiatry 2021, 178, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Armstrong, C.W.; Hudaib, A.-R.; Kulkarni, J.; Gurvich, C. A network meta-analysis of stress mediators in suicide behavior. Front. Neuroendocrinol. 2021, 63, 100946. [Google Scholar] [CrossRef]
- Angelakis, I.; Gillespie, E.L.; Panagioti, M. Childhood maltreatment and adult suicidality: A comprehensive systematic review with meta-analysis. Psychol. Med. 2019, 49, 1057–1078. [Google Scholar] [CrossRef] [Green Version]
- Lamontagne, S.J.; Ballard, E.D.; Zarate, C.A., Jr. Effect of stress on endophenotypes of suicide across species: A role for ketamine in risk mitigation. Neurobiol. Stress 2022, 18, 100450. [Google Scholar] [CrossRef]
- Aggarwal, S.; Patton, G.; Reavley, N.; Sreenivasan, S.A.; Berk, M. Youth self-harm in low- and middle-income countries: Systematic review of the risk and protective factors. Int. J. Soc. Psychiatry 2017, 63, 359–375. [Google Scholar] [CrossRef]
- Lovestad, S.; Love, J.; Vaez, M.; Waern, M.; Hensing, G.; Krantz, G. Suicidal ideation and attempts in population-based samples of women: Temporal changes between 1989 and 2015. BMC Public Health 2019, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.-L.; Zhong, B.-L.; Xiang, Y.-T.; Ungvari, G.S.; Lai, K.Y.C.; Chiu, H.F.K.; Caine, E.D. Prevalence of suicidal ideation and suicide attempts in the general population of China: A meta-analysis. Int. J. Psychiatry Med. 2015, 49, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Hubers, A.A.M.; Moaddine, S.; Peersmann, S.H.M.; Stijnen, T.; van Duijn, E.; van der Mast, R.C.; Dekkers, O.M.; Giltay, E.J. Suicidal ideation and completed suicide in both psychiatric and non-psychiatric populations: A meta-analysis. Epidemiol. Psychiatr. Sci. 2018, 27, 186–198. [Google Scholar] [CrossRef]
- Demesmaeker, A.; Chazard, E.; Hoang, A.; Vaiva, G.; Amad, A. Suicide mortality after nonfatal suicide attempt: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2022, 56, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Sobanski, T.; Josfeld, S.; Peikert, G.; Wagner, G. Psychotherapeutic interventions for the prevention of suicide re-attempts: A review. Psychol. Med. 2021, 15, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.R.; Huang, X.; Guzman, E.M.; Funsch, K.M.; Cha, C.B.; Ribeiro, J.D.; Franklin, J.C. Interventions for suicide and self-injury: A meta-analysis of randomized controlled trials across nearly 50 years of research. Psychol. Bull. 2020, 146, 1117–1145. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Trujillo Diaz, D.; Rupp, Z.W.; Kidambi, A.; Ramirez, K.L.; Flores, J.M.; Avila-Quintero, V.J.; Rhee, T.G.; Olfson, M.; Bloch, M.H. Pharmacological and somatic treatment effects on suicide in adults: A systematic review and meta-analysis. Depress. Anxiety 2022, 39, 100–112. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Ambrosi, E.; Giordano, G.; Girardi, P.; Tatarelli, R.; Lester, D. Antidepressants and Suicide Risk: A Comprehensive Overview. Pharmaceuticals 2010, 3, 2861–2883. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, S.; van Heeringen, C.; Audenaert, K. Structural and functional neuroimaging studies of the suicidal brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Bani-Fatemi, A.; Tasmim, S.; Graff-Guerrero, A.; Gerretsen, P.; Strauss, J.; Kolla, N.; Spalletta, G.; De Luca, V. Structural and functional alterations of the suicidal brain: An updated review of neuroimaging studies. Psychiatry Res. Neuroimaging 2018, 278, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hoertel, N.; Cipel, H.; Blanco, C.; Oquendo, M.A.; Ellul, P.; Leaune, E.; Limosin, F.; Peyre, H.; Costemale-Lacoste, J.F. Cerebrospinal fluid levels of monoamines among suicide attempters: A systematic review and random-effects meta-analysis. J. Psychiatr. Res. 2021, 136, 224–235. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Ferguson, E.; Green, J.A.; O’Carroll, R.E.; O’Connor, R.C. Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology 2016, 63, 370–379. [Google Scholar] [CrossRef]
- Black, C.; Miller, B.J. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry 2015, 78, 28–37. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Aguglia, A.; Amerio, A.; Orsolini, L.; Salvi, V.; Serafini, G.; Volpe, U.; Amore, M.; Aguglia, E. Peripheral BDNF levels in psychiatric patients with and without a history of suicide attempt: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021, 111, 110342. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, N.A.; Ashley-Koch, A.E.; Qin, X.J.; Lindquist, J.H.; Garrett, M.E.; Dennis, M.F.; Hair, L.P.; Huffman, J.E.; Jacobson, D.A.; Madduri, R.K.; et al. A genome-wide association study of suicide attempts in the million veterans program identifies evidence of pan-ancestry and ancestry-specific risk loci. Mol. Psychiatry 2022, 27, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Treviño, L.; Blasco-Fontecilla, H.; Braquehais, M.D.; Ceverino-Dominguez, A.; Baca-Garcia, E. Endophenotypes and suicide behaviour. Actas Esp. Psiquiatr. 2011, 39, 61–69. [Google Scholar]
- Shneidman, E.S. Some controversies in suicidology: Toward a mentalistic discipline. Suicide Life Threat. Behav. 1993, 23, 292–298. [Google Scholar] [PubMed]
- Rizvi, S.J.; Iskric, A.; Calati, R.; Courtet, P. Psychological and physical pain as predictors of suicide risk: Evidence from clinical and neuroimaging findings. Curr. Opin. Psychiatry 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Verrocchio, M.C.; Carrozzino, D.; Marchetti, D.; Andreasson, K.; Fulcheri, M.; Bech, P. Mental Pain and Suicide: A Systematic Review of the Literature. Front. Psychiatry 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberger, N.I. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 2012, 13, 421–434. [Google Scholar] [CrossRef]
- Bednarova, A.; Hlavacova, N.; Pecenak, J. Analysis of Motives and Factors Connected to Suicidal Behavior in Patients Hospitalized in a Psychiatric Department. Int. J. Environ. Res. Public Health 2022, 19, 6283. [Google Scholar] [CrossRef]
- Osafo, J.; Akotia, C.S.; Andoh-Arthur, J.; Quarshie, E.N. Attempted suicide in Ghana: Motivation, stigma, and coping. Death Stud. 2015, 39, 274–280. [Google Scholar] [CrossRef]
- Kinkel-Ram, S.S.; Kunstman, J.; Hunger, J.M.; Smith, A. Examining the relation between discrimination and suicide among Black Americans: The role of social pain minimization and decreased bodily trust. Stigma Health 2021. advance online publication. [Google Scholar] [CrossRef]
- Gunn, J.F.; Goldstein, S.E. Exploring the association between bullying victimization and suicidal thoughts through theoretical frameworks of suicide. Int. J. Bullying Prev. 2021, 3, 213–226. [Google Scholar] [CrossRef]
- Shiratori, Y.; Tachikawa, H.; Nemoto, K.; Endo, G.; Aiba, M.; Matsui, Y.; Asada, T. Network analysis for motives in suicide cases: A cross-sectional study. Psychiatry Clin. Neurosci. 2014, 68, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Courtet, P.; Olié, E. Social pain at the core of suicidal behavior. Encephale 2019, 45, S7–S12. [Google Scholar] [CrossRef]
- Rotge, J.Y.; Lemogne, C.; Hinfray, S.; Huguet, P.; Grynszpan, O.; Tartour, E.; George, N.; Fossati, P. A meta-analysis of the anterior cingulate contribution to social pain. Soc. Cogn. Affect. Neurosci. 2015, 10, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. Social support and pain: How does the brain feel the ache of a broken heart? J. Cancer Pain Symptom Palliation 2005, 1, 59–65. [Google Scholar] [CrossRef]
- Nobile, B.; Lutz, P.-E.; Olie, E.; Courtet, P. The role of opiates in social pain and suicidal behavior. Curr. Top. Behav. Neurosci. 2020, 46, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.R.; Hsu, D.T.; Taylor, S.F.; Ogden, R.T.; DeLorenzo, C.; Parsey, R.V. Rejection sensitivity and mu opioid receptor dynamics associated with mood alterations in response to social feedback. Psychiatry Res. Neuroimaging 2022, 324, 111505. [Google Scholar] [CrossRef]
- Sullivan, M.D.; Ballantyne, J.C. When Physical and Social Pain Coexist: Insights Into Opioid Therapy. Ann. Fam. Med. 2021, 19, 79–82. [Google Scholar] [CrossRef]
- Preller, K.H.; Pokorny, T.; Hock, A.; Kraehenmann, R.; Stämpfli, P.; Seifritz, E.; Scheidegger, M.; Vollenweider, F.X. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. USA 2016, 113, 5119–5124. [Google Scholar] [CrossRef] [Green Version]
- Tabak, B.A. The pandemic should catalyze research on social pain: Examining oxytocin administration studies. Biol. Psychol. 2022, 170, 108315. [Google Scholar] [CrossRef]
- Schneider, P.; Pätz, M.; Spanagel, R.; Schneider, M. Adolescent social rejection alters pain processing in a CB1 receptor dependent manner. Eur. Neuropsychopharmacol. 2016, 26, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, N.I.; Inagaki, T.K.; Rameson, L.T.; Mashal, N.M.; Irwin, M.R. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. Neuroimage 2009, 47, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, J.F. The Social Pain Model. Crisis 2017, 38, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Conejero, I.; Jaussent, I.; Cazals, A.; Thouvenot, E.; Mura, T.; Le Bars, E.; Guillaume, S.; Squalli, S.; Courtet, P.; Olié, E. Association between baseline pro-inflammatory cytokines and brain activation during social exclusion in patients with vulnerability to suicide and depressive disorder. Psychoneuroendocrinology 2019, 99, 236–242. [Google Scholar] [CrossRef]
- Cáceda, R.; James, G.A.; Stowe, Z.N.; Delgado, P.L.; Kordsmeier, N.; Kilts, C.D. The neural correlates of low social integration as a risk factor for suicide. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 619–631. [Google Scholar] [CrossRef]
- Olié, E.; Jollant, F.; Deverdun, J.; de Champfleur, N.M.; Cyprien, F.; Le Bars, E.; Mura, T.; Bonafé, A.; Courtet, P. The experience of social exclusion in women with a history of suicidal acts: A neuroimaging study. Sci. Rep. 2017, 7, 89. [Google Scholar] [CrossRef]
- Hsu, D.T.; Sanford, B.J.; Meyers, K.K.; Love, T.M.; Hazlett, K.E.; Wang, H.; Ni, L.; Walker, S.J.; Mickey, B.J.; Korycinski, S.T.; et al. Response of the μ-opioid system to social rejection and acceptance. Mol. Psychiatry 2013, 18, 1211–1217. [Google Scholar] [CrossRef]
- Way, B.M.; Taylor, S.E.; Eisenberger, N.I. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl. Acad. Sci. USA 2009, 106, 15079–15084. [Google Scholar] [CrossRef] [Green Version]
- Hishimoto, A.; Cui, H.; Mouri, K.; Nushida, H.; Ueno, Y.; Maeda, K.; Shirakawa, O. A functional polymorphism of the µ-opioid receptor gene is associated with completed suicides. J. Neural Transm. 2008, 115, 531–536. [Google Scholar] [CrossRef]
- Nobile, B.; Olie, E.; Ramoz, N.; Dubois, J.; Guillaume, S.; Gorwood, P.; Courtet, P. Association Between the A118G Polymorphism of the OPRM1 Gene and Suicidal Depression in a Large Cohort of Outpatients with Depression. Neuropsychiatr. Dis. Treat. 2021, 17, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.S.; Abrutyn, S.; Pescosolido, B.; Diefendorf, S. The Social Roots of Suicide: Theorizing How the External Social World Matters to Suicide and Suicide Prevention. Front. Psychol. 2021, 12, 621569. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, J.; Jahromi, M.S.; Ehsaei, Z. The effectiveness of different singly administered high doses of buprenorphine in reducing suicidal ideation in acutely depressed people with co-morbid opiate dependence: A randomized, double-blind, clinical trial. Trials 2018, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, O.M.; Lynch, S.; Klepacz, L. Case Report: Buprenorphine-A Treatment for Psychological Pain and Suicidal Ideation? Am. J. Addict. 2021, 30, 80–82. [Google Scholar] [CrossRef]
- Cameron, C.M.; Nieto, S.; Bosler, L.; Wong, M.; Bishop, I.; Mooney, L.; Cahill, C.M. Mechanisms Underlying the Anti-Suicidal Treatment Potential of Buprenorphine. Adv. Drug Alcohol Res. 2021, 1, 10009. [Google Scholar] [CrossRef]
- Coplan, P.M.; Sessler, N.E.; Harikrishnan, V.; Singh, R.; Perkel, C. Comparison of abuse, suspected suicidal intent, and fatalities related to the 7-day buprenorphine transdermal patch versus other opioid analgesics in the National Poison Data System. Postgrad. Med. 2017, 129, 55–61. [Google Scholar] [CrossRef]
- Gibbs, H.M.; Price, D.; Delgado, P.L.; Clothier, J.L.; Cáceda, R. Buprenorphine use for pain and suicidal ideation in severely suicidal patients. Int. J. Psychiatry Med. 2020, 55, 387–396. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, G.H.; Guo, X.; Hao, N.; Shi, Y.; Li, H.F.; Zhao, X.; Li, J.G.; Zhang, C.; Zhang, Y. Activation of δ-opioid Receptors in Anterior Cingulate Cortex Alleviates Affective Pain in Rats. Neuroscience 2022, 494, 152–166. [Google Scholar] [CrossRef]
- Striebel, J.M.; Kalapatapu, R.K. The anti-suicidal potential of buprenorphine: A case report. Int. J. Psychiatry Med. 2014, 47, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Thase, M.E.; Stanford, A.D.; Memisoglu, A.; Martin, W.; Claxton, A.; Bodkin, J.A.; Trivedi, M.H.; Fava, M.; Yu, M.; Pathak, S. Results from a long-term open-label extension study of adjunctive buprenorphine/samidorphan combination in patients with major depressive disorder. Neuropsychopharmacology 2019, 44, 2268–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yovell, Y.; Bar, G.; Mashiah, M.; Baruch, Y.; Briskman, I.; Asherov, J.; Lotan, A.; Rigbi, A.; Panksepp, J. Ultra-Low-Dose Buprenorphine as a Time-Limited Treatment for Severe Suicidal Ideation: A Randomized Controlled Trial. Am. J. Psychiatry 2016, 173, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argento, E.; Strathdee, S.A.; Tupper, K.; Braschel, M.; Wood, E.; Shannon, K. Does psychedelic drug use reduce risk of suicidality? Evidence from a longitudinal community-based cohort of marginalised women in a Canadian setting. BMJ Open 2017, 7, e016025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Ghavidel-Parsa, B.; Bidari, A.; Rahimi, A.; Gharibpoor, F.; Khosousi, M.J. No effect of approved fibromyalgia drugs on the social pain (invalidation) contrary to physical pain: An open-label short-term randomized clinical trial. Clin. Rheumatol. 2022, 41, 245–254. [Google Scholar] [CrossRef]
- Hendricks, P.S.; Thorne, C.B.; Clark, C.B.; Coombs, D.W.; Johnson, M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 2015, 29, 280–288. [Google Scholar] [CrossRef]
- Hendricks, P.S.; Johnson, M.W.; Griffiths, R.R. Psilocybin, psychological distress, and suicidality. J. Psychopharmacol. 2015, 29, 1041–1043. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.M.; Nock, M.K. MDMA/ecstasy use and psilocybin use are associated with lowered odds of psychological distress and suicidal thoughts in a sample of US adults. J. Psychopharmacol. 2022, 36, 46–56. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef]
- Ross, S.; Agin-Liebes, G.; Lo, S.; Zeifman, R.J.; Ghazal, L.; Benville, J.; Franco Corso, S.; Bjerre Real, C.; Guss, J.; Bossis, A.; et al. Acute and Sustained Reductions in Loss of Meaning and Suicidal Ideation Following Psilocybin-Assisted Psychotherapy for Psychiatric and Existential Distress in Life-Threatening Cancer. ACS Pharmacol. Transl. Sci. 2021, 4, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.H.; Han, B.H.; Palamar, J.J. Past-year hallucinogen use in relation to psychological distress, depression, and suicidality among US adults. Addict. Behav. 2022, 132, 107343. [Google Scholar] [CrossRef] [PubMed]
- Can, A.T.; Hermens, D.F.; Dutton, M.; Gallay, C.C.; Jensen, E.; Jones, M.; Scherman, J.; Beaudequin, D.A.; Yang, C.; Schwenn, P.E.; et al. Low dose oral ketamine treatment in chronic suicidality: An open-label pilot study. Transl. Psychiatry 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Canuso, C.M.; Singh, J.B.; Fedgchin, M.; Alphs, L.; Lane, R.; Lim, P.; Pinter, C.; Hough, D.; Sanacora, G.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Psychiatry 2018, 175, 620–630. [Google Scholar] [CrossRef]
- Chen, G.G.; Almeida, D.; Fiori, L.; Turecki, G. Evidence of Reduced Agmatine Concentrations in the Cerebral Cortex of Suicides. Int. J. Neuropsychopharmacol. 2018, 21, 895–900. [Google Scholar] [CrossRef]
- De Gioannis, A.; De Leo, D. Oral ketamine augmentation for chronic suicidality in treatment-resistant depression. Aust. N. Z. J. Psychiatry 2014, 48, 686. [Google Scholar] [CrossRef] [Green Version]
- Gallay, C.C.; Forsyth, G.; Can, A.T.; Dutton, M.; Jamieson, D.; Jensen, E.; Hermens, D.F.; Bennett, M.R.; Lagopoulos, J. Six-week oral ketamine treatment for chronic suicidality is associated with increased grey matter volume. Psychiatry Res. Neuroimaging 2021, 317, 111369. [Google Scholar] [CrossRef]
- Fu, D.J.; Ionescu, D.F.; Li, X.; Lane, R.; Lim, P.; Sanacora, G.; Hough, D.; Manji, H.; Drevets, W.C.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I). J. Clin. Psychiatry 2020, 81, 19m13191. [Google Scholar] [CrossRef]
- García-Pardo, M.P.; Miñarro, J.; Llansola, M.; Felipo, V.; Aguilar, M.A. Role of NMDA and AMPA glutamatergic receptors in the effects of social defeat on the rewarding properties of MDMA in mice. Eur. J. Neurosci. 2019, 50, 2623–2634. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Fu, D.J.; Qiu, X.; Lane, R.; Lim, P.; Kasper, S.; Hough, D.; Drevets, W.C.; Manji, H.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients With Major Depressive Disorder Who Have Active Suicide Ideation With Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II). Int. J. Neuropsychopharmacol. 2021, 24, 22–31. [Google Scholar] [CrossRef]
- Irwin, S.A.; Iglewicz, A.; Nelesen, R.A.; Lo, J.Y.; Carr, C.H.; Romero, S.D.; Lloyd, L.S. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: A 28-day open-label proof-of-concept trial. J. Palliat. Med. 2013, 16, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.; Choi, T.Y.; Chang, K.A.; Koo, J.W. Metabotropic Glutamate Receptor 5 in Amygdala Target Neurons Regulates Susceptibility to Chronic Social Stress. Biol. Psychiatry 2022, 92, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.P.; Port, J.D.; Blacker, C.J.; Sonmez, A.I.; Seewoo, B.J.; Leffler, J.M.; Frye, M.A.; Croarkin, P.E. Altered anterior cingulate glutamatergic metabolism in depressed adolescents with current suicidal ideation. Transl. Psychiatry 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Correia-Melo, F.S.; Santos-Lima, C.; Souza-Marques, B.; Leal, G.C.; Jesus-Nunes, A.P.; Mello, R.P.; Caliman-Fontes, A.T.; Bandeira, I.D.; Marback, R.F.; et al. Ketamine and Esketamine augmentation for suicidal ideation: A randomized, double-blinded clinical trial. Gen. Hosp. Psychiatry 2021, 68, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.R.; Heifets, B.D.; Bentzley, B.S.; Blasey, C.; Sudheimer, K.D.; Hawkins, J.; Lyons, D.M.; Schatzberg, A.F. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol. Psychiatry 2019, 24, 1779–1786. [Google Scholar] [CrossRef]
- Witt, K.; Potts, J.; Hubers, A.; Grunebaum, M.F.; Murrough, J.W.; Loo, C.; Cipriani, A.; Hawton, K. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials. Aust. N. Z. J. Psychiatry 2020, 54, 29–45. [Google Scholar] [CrossRef]
- Auer, B.J.; Byrd-Craven, J.; Grant, D.M.; Granger, D.A. Common oxytocin receptor gene variant interacts with rejection sensitivity to influence cortisol reactivity during negative evaluation. Horm. Behav. 2015, 75, 64–69. [Google Scholar] [CrossRef]
- Chu, C.; Hammock, E.; Joiner, T.E. Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J. Psychiatr. Res. 2020, 121, 173–181. [Google Scholar] [CrossRef]
- De Cagna, F.; Fusar-Poli, L.; Damiani, S.; Rocchetti, M.; Giovanna, G.; Mori, A.; Politi, P.; Brondino, N. The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Fang, A.; Hoge, E.A.; Heinrichs, M.; Hofmann, S.G. Attachment Style Moderates the Effects of Oxytocin on Social Behaviors and Cognitions During Social Rejection: Applying an RDoC Framework to Social Anxiety. Clin. Psychol. Sci. 2014, 2, 740–747. [Google Scholar] [CrossRef]
- Henningsson, S.; Leknes, S.; Asperholm, M.; Eikemo, M.; Westberg, L. A randomized placebo-controlled intranasal oxytocin study on first impressions and reactions to social rejection. Biol. Psychol. 2021, 164, 108164. [Google Scholar] [CrossRef]
- Jahangard, L.; Shayganfard, M.; Ghiasi, F.; Salehi, I.; Haghighi, M.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Brand, S. Serum oxytocin concentrations in current and recent suicide survivors are lower than in healthy controls. J. Psychiatr. Res. 2020, 128, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Chatzittofis, A.; Hellström, C.; Nordström, P.; Uvnäs-Moberg, K.; Asberg, M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 2012, 37, 482–490. [Google Scholar] [CrossRef]
- Krimberg, J.S.; Lumertz, F.S.; Orso, R.; Viola, T.W.; de Almeida, R. Impact of social isolation on the oxytocinergic system: A systematic review and meta-analysis of rodent data. Neurosci. Biobehav. Rev. 2022, 134, 104549. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, E.R.; Blumberg, H.P.; Silverman, W.K. Negative peer social interactions and oxytocin levels linked to suicidal ideation in anxious youth. J. Affect. Disord. 2019, 245, 806–811. [Google Scholar] [CrossRef]
- Okumura, T.; Nozu, T.; Kumei, S.; Ohhira, M. Central oxytocin signaling mediates the central orexin-induced visceral antinociception through the opioid system in conscious rats. Physiol. Behav. 2019, 198, 96–101. [Google Scholar] [CrossRef]
- Radke, S.; Jankowiak, K.; Tops, S.; Abel, T.; Habel, U.; Derntl, B. Neurobiobehavioral responses to virtual social rejection in females-exploring the influence of oxytocin. Soc. Cogn. Affect. Neurosci. 2021, 16, 326–333. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Otieno, S.; Li, H.; Leppänen, P. Oxytocin reduces romantic rejection-induced pain in online speed-dating as revealed by decreased frontal-midline theta oscillations. Psychoneuroendocrinology 2021, 133, 105411. [Google Scholar] [CrossRef] [PubMed]
- Chiurliza, B.; Joiner, T.E. The Influence of Acetaminophen and Observational Conditioning on the Acquired Capability for Suicide. Behav. Ther. 2018, 49, 681–690. [Google Scholar] [CrossRef]
- Dewall, C.N.; Macdonald, G.; Webster, G.D.; Masten, C.L.; Baumeister, R.F.; Powell, C.; Combs, D.; Schurtz, D.R.; Stillman, T.F.; Tice, D.M.; et al. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol. Sci. 2010, 21, 931–937. [Google Scholar] [CrossRef]
- Hungund, B.L.; Vinod, K.Y.; Kassir, S.A.; Basavarajappa, B.S.; Yalamanchili, R.; Cooper, T.B.; Mann, J.J.; Arango, V. Upregulation of CB1 receptors and agonist-stimulated [35S] GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol. Psychiatry 2004, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mato, S.; Pilar-Cuéllar, F.; Valdizán, E.M.; González-Maeso, J.; Rodríguez-Puertas, R.; Meana, J.; Sallés, J.; Crespo-Facorro, B.; Pazos, Á. Selective up-regulation of cannabinoid CB1 receptor coupling to Go-proteins in suicide victims with mood disorders. Biochem. Pharmacol. 2018, 157, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostadhadi, S.; Haj-Mirzaian, A.; Nikoui, V.; Kordjazy, N.; Dehpour, A.R. Involvement of opioid system in antidepressant-like effect of the cannabinoid CB1 receptor inverse agonist AM-251 after physical stress in mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef]
- Slavich, G.M.; Way, B.M.; Eisenberger, N.I.; Taylor, S.E. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. USA 2010, 107, 14817–14822. [Google Scholar] [CrossRef] [Green Version]
- Slavich, G.M.; Shields, G.S.; Deal, B.D.; Gregory, A.; Toussaint, L.L. Alleviating Social Pain: A Double-Blind, Randomized, Placebo-Controlled Trial of Forgiveness and Acetaminophen. Ann. Behav. Med. 2019, 53, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Clayden, R.C.; Zaruk, A.; Meyre, D.; Thabane, L.; Samaan, Z. The association of attempted suicide with genetic variants in the SLC6A4 and TPH genes depends on the definition of suicidal behavior: A systematic review and meta-analysis. Transl. Psychiatry 2012, 2, e166. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, M. Revisiting the Serotonin Hypothesis: Implications for Major Depressive Disorders. Mol. Neurobiol. 2016, 53, 2778–2786. [Google Scholar] [CrossRef]
- van Praag, H.M. Why has the antidepressant era not shown a significant drop in suicide rates? Crisis 2002, 23, 77–82. [Google Scholar] [CrossRef]
- Amendola, S.; Plöderl, M.; Hengartner, M.P. Did the introduction and increased prescribing of antidepressants lead to changes in long-term trends of suicide rates? Eur. J. Public Health 2021, 31, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Carver, C.S.; Hallmayer, J.F.; Zeitzer, J.M.; Palesh, O.; Neri, E.; Nouriani, B.; Spiegel, D. Serotonin transporter polymorphism, depressive symptoms, and emotional impulsivity among advanced breast cancer patients. Support. Care Cancer 2018, 26, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Van Hedger, K.; Bershad, A.K.; de Wit, H. Pharmacological challenge studies with acute psychosocial stress. Psychoneuroendocrinology 2017, 85, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ 2001, 165, 917–928. [Google Scholar]
- Kaye, S.; Darke, S.; Duflou, J. Methylenedioxymethamphetamine (MDMA)-related fatalities in Australia: Demographics, circumstances, toxicology and major organ pathology. Drug Alcohol Depend. 2009, 104, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Gołembiowska, K. Neurotoxicity of MDMA: Main effects and mechanisms. Exp. Neurol. 2022, 347, 113894. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Archibald, J.; MacMillan, E.L.; Enzler, A.; Jutzeler, C.R.; Schweinhardt, P.; Kramer, J. Excitatory and inhibitory responses in the brain to experimental pain: A systematic review of MR spectroscopy studies. Neuroimage 2020, 215, 116794. [Google Scholar] [CrossRef]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.; Watson, J.; Aguila, M.R.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020, 210, 116532. [Google Scholar] [CrossRef]
- Zhou, Q.; Sheng, M. NMDA receptors in nervous system diseases. Neuropharmacology 2013, 74, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, V.; Goudet, C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Garzón, J.; Rodríguez-Muñoz, M.; Sánchez-Blázquez, P. Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: Molecular implications for opioid tolerance. Curr. Drug Abus. Rev. 2012, 5, 199–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Roca, H.; Silva, J.A.; Arcaya, J.L.; Quintero, L.; Maixner, W.; Pinerua-Shuhaibar, L. Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav. Brain Res. 2006, 167, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Trevino, L.; Gonzalez-Blanco, L.; Alvarez-Vazquez, C.; Rodriguez-Revuelta, J.; Saiz Martinez, P.A. Glutamine and New Pharmacological Targets to Treat Suicidal Ideation. Curr. Top. Behav. Neurosci. 2020, 46, 179–196. [Google Scholar] [CrossRef]
- Le, T.T.; Cordero, I.P.; Jawad, M.Y.; Swainson, J.; Di Vincenzo, J.D.; Jaberi, S.; Phan, L.; Lui, L.; Ho, R.; Rosenblat, J.D.; et al. The abuse liability of ketamine: A scoping review of preclinical and clinical studies. J. Psychiatr. Res. 2022, 151, 476–496. [Google Scholar] [CrossRef]
- Valverde, A.P.; Camargo, A.; Rodrigues, A. Agmatine as a novel candidate for rapid-onset antidepressant response. World J. Psychiatry 2021, 11, 981–996. [Google Scholar] [CrossRef]
- Olescowicz, G.; Neis, V.B.; Fraga, D.B.; Rosa, P.B.; Azevedo, D.P.; Melleu, F.F.; Brocardo, P.S.; Gil-Mohapel, J.; Rodrigues, A. Antidepressant and pro-neurogenic effects of agmatine in a mouse model of stress induced by chronic exposure to corticosterone. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 395–407. [Google Scholar] [CrossRef]
- Macdonald, K.; Macdonald, T.M. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry 2010, 18, 1–21. [Google Scholar] [CrossRef]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Wang, Q.; Roy, B.; Turecki, G.; Shelton, R.C.; Dwivedi, Y. Role of Complex Epigenetic Switching in Tumor Necrosis Factor-α Upregulation in the Prefrontal Cortex of Suicide Subjects. Am. J. Psychiatry 2018, 175, 262–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Anderson, G.; Kubera, M.; Berk, M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin. Ther. Targets 2014, 18, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H.; Moore, P.B. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr. Scand. 2011, 124, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.; Gasparyan, A.; Navarrete, F.; Torregrosa, A.B.; Rubio, G.; Marín-Mayor, M.; Acosta, G.B.; Garcia-Gutiérrez, M.S.; Manzanares, J. Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4764. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Thomas, B.F.; Zhang, Y. Overcoming the Psychiatric Side Effects of the Cannabinoid CB1 Receptor Antagonists: Current Approaches for Therapeutics Development. Curr. Top. Med. Chem. 2019, 19, 1418–1435. [Google Scholar] [CrossRef]
- Casey, D.; Geulayov, G.; Bale, E.; Brand, F.; Clements, C.; Kapur, N.; Ness, J.; Patel, A.; Waters, K.; Hawton, K. Paracetamol self-poisoning: Epidemiological study of trends and patient characteristics from the multicentre study of self-harm in England. J. Affect. Disord. 2020, 276, 699–706. [Google Scholar] [CrossRef]
- Zalsman, G.; Hawton, K.; Wasserman, D.; van Heeringen, K.; Arensman, E.; Sarchiapone, M.; Carli, V.; Höschl, C.; Barzilay, R.; Balazs, J.; et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 2016, 3, 646–659. [Google Scholar] [CrossRef]
- Li, X.; Shorter, D.; Kosten, T.R. Buprenorphine Prescribing: To Expand or Not to Expand. J. Psychiatr. Pract. 2016, 22, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Mischkowski, D.; Crocker, J.; Way, B.M. From painkiller to empathy killer: Acetaminophen (paracetamol) reduces empathy for pain. Soc. Cogn. Affect. Neurosci. 2016, 11, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Meshkat, S.; Rodrigues, N.B.; Di Vincenzo, J.D.; Ceban, F.; Jaberi, S.; McIntyre, R.S.; Lui, L.; Rosenblat, J.D. Pharmacogenomics of ketamine: A systematic review. J. Psychiatr. Res. 2021, 145, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Yovell, Y.; Bar, G. Ultra-Low-Dose Buprenorphine for Mental Pain: Response to Ruan et al. Am. J. Psychiatry 2016, 173, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.C.; Portzky, G. Looking to the Future: A Synthesis of New Developments and Challenges in Suicide Research and Prevention. Front. Psychol. 2018, 9, 2139. [Google Scholar] [CrossRef] [Green Version]

| Publication | Publication Type | Study Population or Data Source | Key Findings |
|---|---|---|---|
| Agents acting at opioid receptors | |||
| Ahmadi et al., 2018 [54] | Randomized controlled trial | 51 male patients with comorbid major depression, opioid dependence and suicidal ideation | Single-dose buprenorphine (32, 64 or 96 mg) associated with a significant reduction in suicidal ideation; effect sustained at 2 weeks. |
| Benhamou et al., 2021 [55] | Case report | Male patient with bipolar disorder, opioid use disorder and recent suicide attempt | Buprenorphine therapy associated with rapid improvement in depression and suicidal ideation. |
| Cameron et al., 2021 [56] | Systematic review | Human and animal studies on the anti-suicidal effects of opioid receptor modulation | Based on analysis of literature, µ-receptor agonism unlikely to be the sole mechanism of anti-suicidal effects; κ-receptor antagonism probably of equal or greater importance. |
| Coplan et al., 2017 [57] | Retrospective cohort study | United States National Poison Data System | Buprenorphine associated with a significantly lower risk of abuse and suicide intent compared to all other opioid analgesics. |
| Gibbs et al., 2020 [58] | Retrospective chart review | Case records of 6 adult patients with depression, chronic pain and opioid use disorder with suicidal risk | 4 of 6 patients showed reduced suicidal ideation with off-label buprenorphine. |
| Ma et al., 2022 [59] | Animal study | Rat model of physical and affective pain | Activation of δ-opioid receptors by a synthetic opioid agonist led to a reduction in affective pain. |
| Striebel and Kalapatapu 2014 [60] | Case report | Female patient with treatment resistant depression, opioid use disorder, recent suicide attempt | Sublingual buprenorphine (16 mg/day) associated with rapid reduction in suicidal ideation and no further attempts; effect sustained at 3 month follow-up. |
| Thase et al., 2020 [61] | Open-label trial | 769 patients with depression | Combined buprenorphine and samidorphan (2 mg/2 mg/day) not associated with any significant change in suicidal ideation, despite sustained improvements in depression at 6 and 12 months. |
| Yovell et al., 2016 [62] | Randomized controlled trial | 88 patients with severe suicidal ideation and no history of substance abuse | Buprenorphine (mean dose 0.44 mg/day) associated with a significant decrease in suicidal ideation at 2 and 4 weeks compared to placebo. |
| Agents acting at serotonin receptors | |||
| Argento et al., 2017 [63] | Community-based cohort study | 766 female sex workers in an urban area | Lifetime psychedelic use associated with a 60% reduction in hazard ratio for suicidality. |
| Davis et al., 2021 [64] | Randomized controlled trial | 24 patients with depression | 2 doses of oral psilocybin (20–30 mg/70 kg) in combination with 11 h of psychotherapy did not lead to a significant decrease in suicidal ideation compared to waiting-list controls. |
| Ghavidel-Parsa et al., 2022 [65] | Randomized controlled trial | 44 patients with fibromyalgia | Duloxetine (30–60 mg/day) did not lead to significant decreases in social pain despite improvement in physical pain. |
| Hendricks et al., 2015a [66] | Secondary analysis of survey data | United States National Survey on Drug Use and Health, 2008–2012; data on 191,832 participants | Lifetime psychedelic use associated with significantly lower psychological distress, suicidal ideation, planning or attempts. |
| Hendricks et al., 2015b [67] | Secondary analysis of survey data | United States National Survey on Drug Use and Health, 2008–2012; data on 191,832 participants | Psilocybin use associated with significantly lower levels of psychological distress and suicidal ideation, planning or attempts. |
| Jones and Nock 2022 [68] | Secondary analysis of survey data | United States National Survey on Drug Use and Health, 2008–2019; data on 484,732 participants | Psilocybin and MDMA use both associated with significantly lower levels of psychological distress and suicidal ideation. |
| Mitchell et al., 2021 [69] | Randomized controlled trial | 90 patients with severe PTSD | No significant difference in suicidality between MDMA and placebo. |
| Mithoefer et al., 2018 [70] | Randomized controlled trial | 26 patients with PTSD | MDMA (30, 75 or 125 mg) plus psychotherapy associated with a significant decrease in suicidality post-treatment; however, transient initial increases in suicidality were observed with the 30 mg and 125 mg doses. |
| Preller et al., 2016 [39] | Randomized cross-over trial | 21 healthy participants subjected to a social exclusion task | Administration of the 5HT2A/1A agonist psilocybin (0.215 mg/kg) associated with reduced feelings of social pain. |
| Ross et al., 2021 [71] | Randomized controlled trial | 11 patients with advanced cancer and suicidal ideation | Single-dose psilocybin (0.3 mg/kg) in combination with psychotherapy resulted in significant decreases in suicidal ideation compared to placebo + psychotherapy; effect sustained at 6 months. |
| Yang et al., 2022 [72] | Secondary analysis of survey data | United States National Survey on Drug Use and Health, 2015–2020; data on 241,675 participants | MDMA use associated with significantly lower levels of psychological distress and suicidal ideation. |
| Agents acting at glutamate receptors | |||
| Can et al., 2021 [73] | Open-label trial | 32 patients with chronic suicidal ideation | Oral ketamine (0.5–3 mg/kg) associated with significant reduction in suicidal ideation over 6 weeks; effect sustained up to 4 weeks after stopping medication |
| Canuso et al., 2018 [74] | Randomized controlled trial | 68 patients with depression and imminent suicide risk | Intranasal esketamine (84 mg twice weekly) superior to placebo in reducing depressive symptoms and suicidal ideation, but not clinician-assessed suicide risk. |
| Chen et al., 2018 [75] | Post-mortem study | Brain tissue of 26 suicidal deaths and 14 controls | Agmatine levels significantly reduced in the cortex of suicides, independent of a diagnosis of depression. |
| De Gioannis and De Leo 2014 [76] | Case report | Male patient with treatment-resistant depression and chronic suicidality | Oral ketamine (0.5 mg/kg) associated with a rapid improvement in suicidal ideation. |
| Gallay et al., 2021 [77] | Secondary analysis of imaging data from open-label trial | 30 patients with chronic suicidal ideation | Oral ketamine (0.5–3 mg/kg) associated with increased grey matter volume in periaqueductal gray, bilateral basal ganglia and thalami. |
| Fu et al., 2020 [78] | Randomized controlled trial | 226 patients with depression and active suicidal ideation | Intranasal esketamine (84 mg twice weekly) superior to placebo for depressive symptoms, but not suicidality. |
| Garcia-Pardo et al., 2019 [79] | Animal study | Mouse model of social defeat | Blockade of NMDA and AMPA glutamate receptors attenuated the effects of social defeat. |
| Ghavidel-Parsa et al., 2022 [65] | Randomized controlled trial | 27 patients with fibromyalgia | Pregabalin (75–150 mg/day) did not lead to significant decreases in social pain despite improvement in physical pain. |
| Ionescu et al., 2021 [80] | Randomized controlled trial | 227 patients with depression and active suicidal ideation and intent | Intranasal esketamine (84 mg twice weekly) superior to placebo for depression, but not suicidality. |
| Irwin et al., 2013 [81] | Open-label trial | 14 patients in hospice care with depression | Oral ketamine (0.5 mg/kg) associated with significant improvements in depression over 4 weeks, but no change in suicidal ideation. |
| Kim et al., 2022 [82] | Animal study | Mouse model of chronic social stress | Expression of mGlu5 receptors in specific cortical areas associated with resilience to social stress. |
| Lewis et al., 2020 [83] | Observational study | 24 adolescents with depression, 11 with and 13 without suicidal ideation | Suicidal ideation associated with higher glutamate levels in anterior cingulate cortex; association between glutamate and suicidal ideation mediated by psychological pain. |
| Vieira et al., 2021 [84] | Randomized controlled trial | 59 patients with treatment-resistant depression and suicidal ideation | Single infusions of ketamine (0.5 mg/kg) and esketamine (0.25 mg/kg) equally effective in reducing suicidal ideation; effect sustained up to 7 days post-infusion. |
| Williams et al., 2019 [85] | Randomized cross-over trial | 12 patients with depression, 11 of whom had active suicidal ideation | Naltrexone 50 mg significantly attenuated the effect of ketamine 0.5 mg/kg on suicidal ideation. |
| Witt et al., 2020 [86] | Meta-analysis of randomized controlled trials | Data on 15 trials involving 572 patients with mood or anxiety disorders | Ketamine 0.5 mg/kg associated with significant reduction in suicidal ideation persisting up to 3 days; no data on suicide attempts. |
| Agents acting at oxytocin receptors | |||
| Auer et al., 2015 [87] | Observational study | 94 healthy volunteers exposed to a social stress task | Oxytocin receptor gene OXTR rs53576 polymorphism interacts with rejection sensitivity to influence cortisol response to social stress. |
| Chu et al., 2020 [88] | Observational study | 31 suicide attempters, 21 controls with depression but no suicide attempt, 48 healthy controls | Oxytocin levels decreased following exposure to an experimental task simulating social exclusion in suicide attempters, but not in the other groups. |
| De Cagna et al., 2019 [89] | Systematic review of randomized controlled trials | 15 clinical trials of intranasal oxytocin in depression or anxiety disorders | No significant effect of oxytocin on core symptoms of anxiety or depression. |
| Fang et al., 2014 [90] | Randomized controlled trials | 60 male volunteers with no history of psychiatric illness or active suicidal ideation | No significant direct effect of oxytocin on reactions to an experimental social exclusion task; possible interaction with attachment style, with a favourable effect observed only in those with low attachment avoidance. |
| Henningsson et al., 2021 [91] | Randomized controlled trial | 100 healthy volunteers (50 pairs of men and women) | No significant direct effect of oxytocin on reactions to an experimental social exclusion task; possible interaction between gender and oxytocin, with a favourable effect observed only in women. |
| Jahangard et al., 2020 [92] | Observational study | 16 suicide survivors; 16 recent (<12 weeks) suicide attempters; 16 healthy controls | Serum oxytocin significantly lower in suicide survivors than controls; oxytocin level negatively correlated with suicidal ideation. |
| Jokinen et al., 2012 [93] | Observational study | 28 suicide attempters and 19 healthy volunteers | Cerebrospinal fluid oxytocin lower in attempters than controls; oxytocin level negatively correlated with suicide intent in attempters. |
| Krimberg et al., 2022 [94] | Meta-analysis of animal studies | 12 studies examining the relationship between oxytocin and social isolation in rodents | Oxytocin administration reverses the behavioural problems (aggression, anxiety, overactivity, reduced social interaction) caused by social isolation. |
| Lebowitz et al., 2019 [95] | Observational study | 168 adolescents with anxiety disorders | Negative interactions with peers were associated with suicidal ideation only in adolescents with high oxytocin levels. |
| Okumura et al., 2019 [96] | Animal study | Rat model of visceral pain | Centrally administered oxytocin had analgesic effects which were blocked by centrally administered naloxone, indicating that this effect is mediated through the central opioid system. |
| Radke et al., 2021 [97] | Randomized controlled trial | 43 healthy women randomized to oxytocin (n = 22) or placebo (n = 21) | No significant effect of oxytocin on behavioural or neuroimaging responses to a simulation of online social rejection. |
| Zhang et al., 2021 [98] | Randomized controlled trial | 61 healthy volunteers randomized to oxytocin (n = 30) or placebo (n = 31) exposed to an experimental task simulating romantic rejection | Oxytocin associated with a reduction in social pain compared to placebo, based on self-report and analysis of EEG data. |
| Other molecular pathways of interest | |||
| Chiurliza and Joiner 2018 [99] | Randomized controlled trial | 43 healthy volunteers randomized to acetaminophen or no treatment | No significant effect of acetaminophen (1000 mg, single dose) on measures of capacity for suicide. |
| Dewall et al., 2010 [100] | Randomized controlled trial | 62 healthy volunteers randomized to acetaminophen (n = 30) or placebo (n = 32) | Acetaminophen 1000 mg/day associated with reduced social pain over 21 days; this effect was associated with reduced activation of the dorsal anterior cingulate cortex and anterior insula. |
| Eisenberger et al., 2009 [42] | Randomized controlled trial | 36 healthy volunteers randomized to endotoxin (n = 20) or placebo (n = 16) | Endotoxin associated with increased levels of IL-6, depression, and social pain compared to placebo. |
| Hungund et al., 2004 [101] | Post-mortem study | Brain tissue from 10 completed suicides and 10 normal controls | Increased CB1 receptor density and receptor-stimulated binding observed in the dorsolateral prefrontal cortex in completed suicides. |
| Mato et al., 2018 [102] | Post-mortem study | Brain tissue from 12 patients with depression and completed suicide and 12 matched controls | Increased functional coupling of CB1 receptors in the prefrontal cortex of cases, but not controls. |
| Muscatell et al., 2015 [103] | Observational study | 31 healthy female volunteers exposed to a social stress task | Social rejection associated with elevated IL-6 and increased amygdala activity; greater increase in IL-6 correlated with amygdala activation. |
| Ostadhadi et al., 2016 [104] | Animal study | Mouse model of depression | Administration of a CB1 receptor inverse agonist associated with antidepressant effects; this effect was potentiated by administration of naltrexone. |
| Raison et al., 2013 [105] | Randomized controlled trial | 60 patients with depression randomized to infliximab (n = 30) or placebo (n = 30) | In patients with elevated baseline hs-CRP, infliximab was superior to placebo in reducing depressive symptoms and suicidality. |
| Schneider et al., 2016 [41] | Animal study | Rat model of social rejection | Administration of rimonabant attenuated the effects of social rejection on behaviour and social pain. |
| Slavich et al., 2010 [106] | Observational study | 124 healthy volunteers exposed to a social stress task | Social rejection associated with elevations in IL-6 and sTNFalphaRII; sTNFalphaRII levels correlated with increased activity in dorsal anterior cingulate cortex and anterior insula. |
| Slavich et al., 2019 [107] | Randomized controlled trial | 42 healthy volunteers randomized to acetaminophen (n = 15), pill placebo (n = 14) or no treatment (n = 13) | Acetaminophen (1000 mg/day) superior to control groups in reducing social pain, but only in subjects with high levels of forgiveness. |
| Concern | Drug | Cause of Mechanism | Risk Reduction Strategy |
|---|---|---|---|
| Lack of evidence | All except ketamine and possibly psilocybin | Lack of controlled clinical trials; variations in definition of suicidal phenotype | Well-designed phase II and III trials with clear operational definitions of suicide-related outcomes |
| Abuse or dependence | Buprenorphine, ketamine, psilocybin, MDMA [114,115,116,117,126,139] | Activation of mesolimbic dopamine and opioid pathways; toxicity from accidental or intentional overdoses | Careful selection of subjects for treatment; limited dispensing; screening for misuse at follow-up; legislation. |
| Behavioural toxicity | MDMA, psilocybin, possibly acetaminophen [114,115,116,140] | Challenging experiences (“bad trips”) leading to negative emotional responses and suicidality; blunting of social pain pathway | Careful monitoring of mental status; use of alternate agents; education of patients and caregivers regarding potential adverse effects. |
| Variable efficacy | All | Pharmacogenomic variations in molecular targets or downstream mediators | Genetic analysis of data from controlled trials; pharmacogenomic testing and personalized medicine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkumar, R.P. Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review. Psych 2022, 4, 494-515. https://doi.org/10.3390/psych4030038
Rajkumar RP. Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review. Psych. 2022; 4(3):494-515. https://doi.org/10.3390/psych4030038
Chicago/Turabian StyleRajkumar, Ravi Philip. 2022. "Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review" Psych 4, no. 3: 494-515. https://doi.org/10.3390/psych4030038
APA StyleRajkumar, R. P. (2022). Pharmacological Strategies for Suicide Prevention Based on the Social Pain Model: A Scoping Review. Psych, 4(3), 494-515. https://doi.org/10.3390/psych4030038






