Design, Synthesis, and Molecular Docking of New Hydrazide–Hydrazone Derivatives with Imidazole Scaffold as Potential Antimicrobial Agents
Abstract
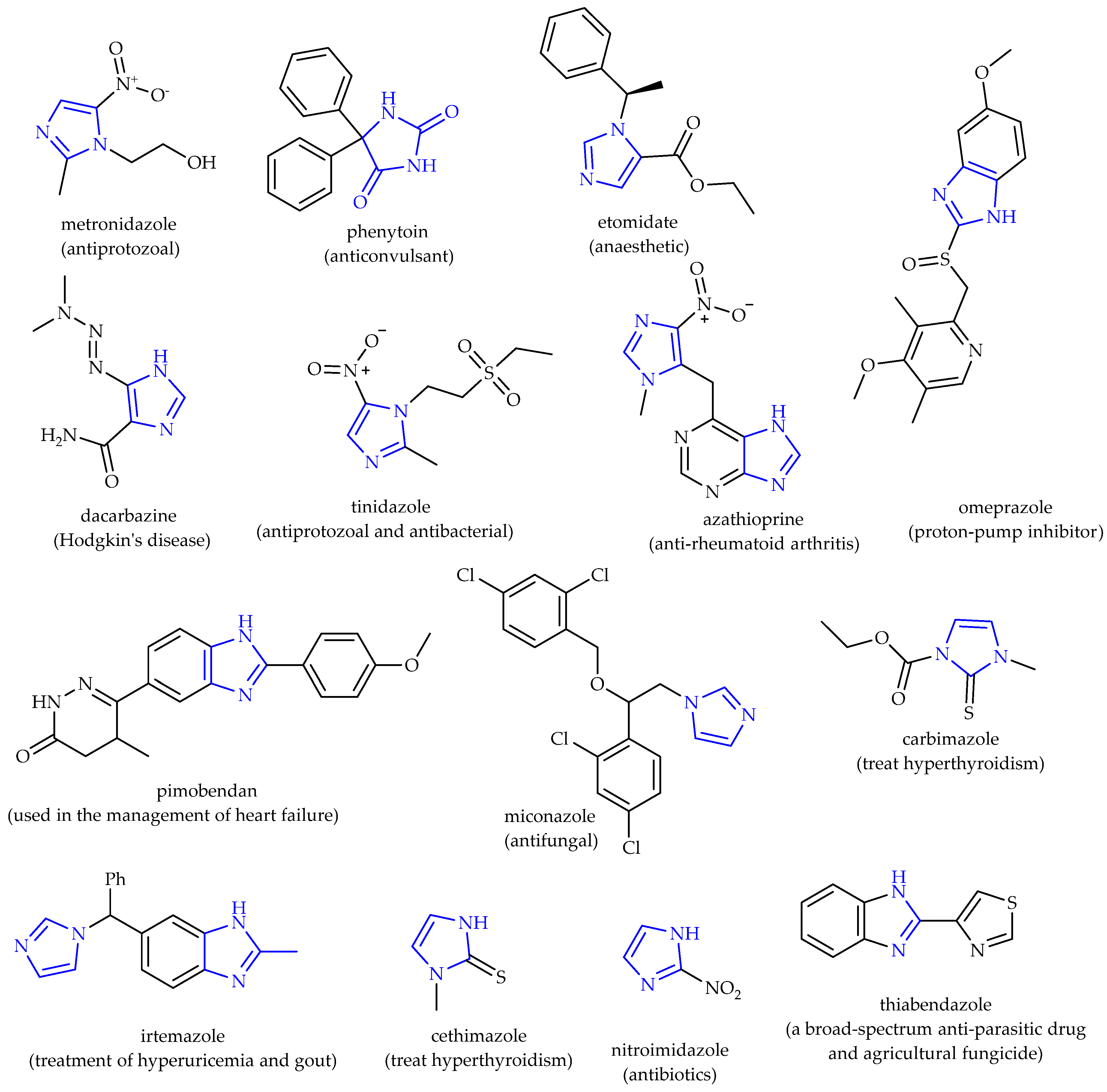
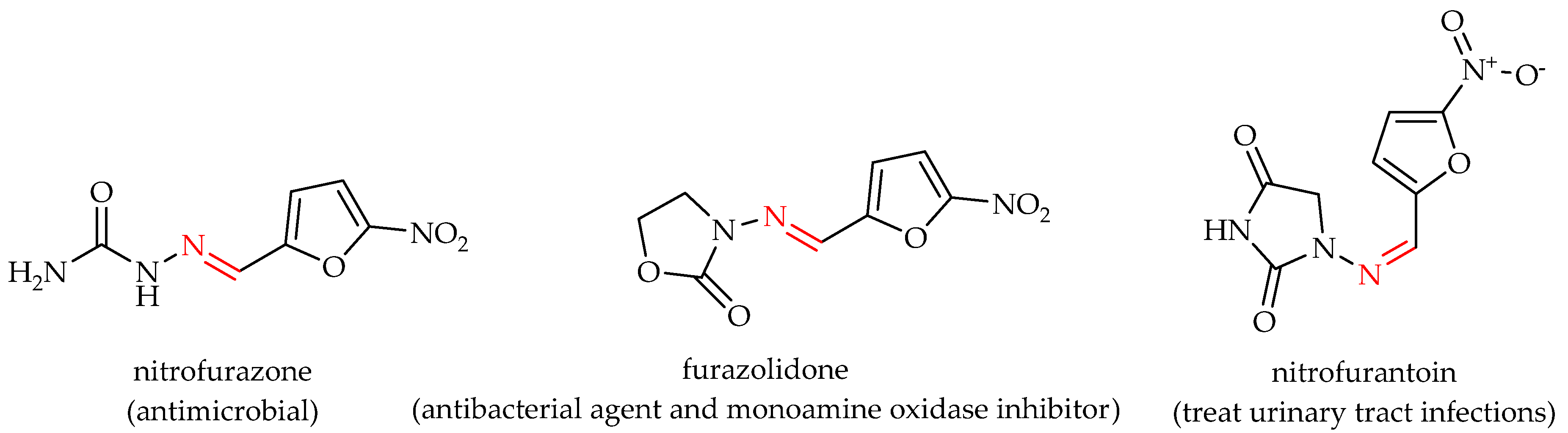
1. Introduction
2. Experimental Section
2.1. General Information
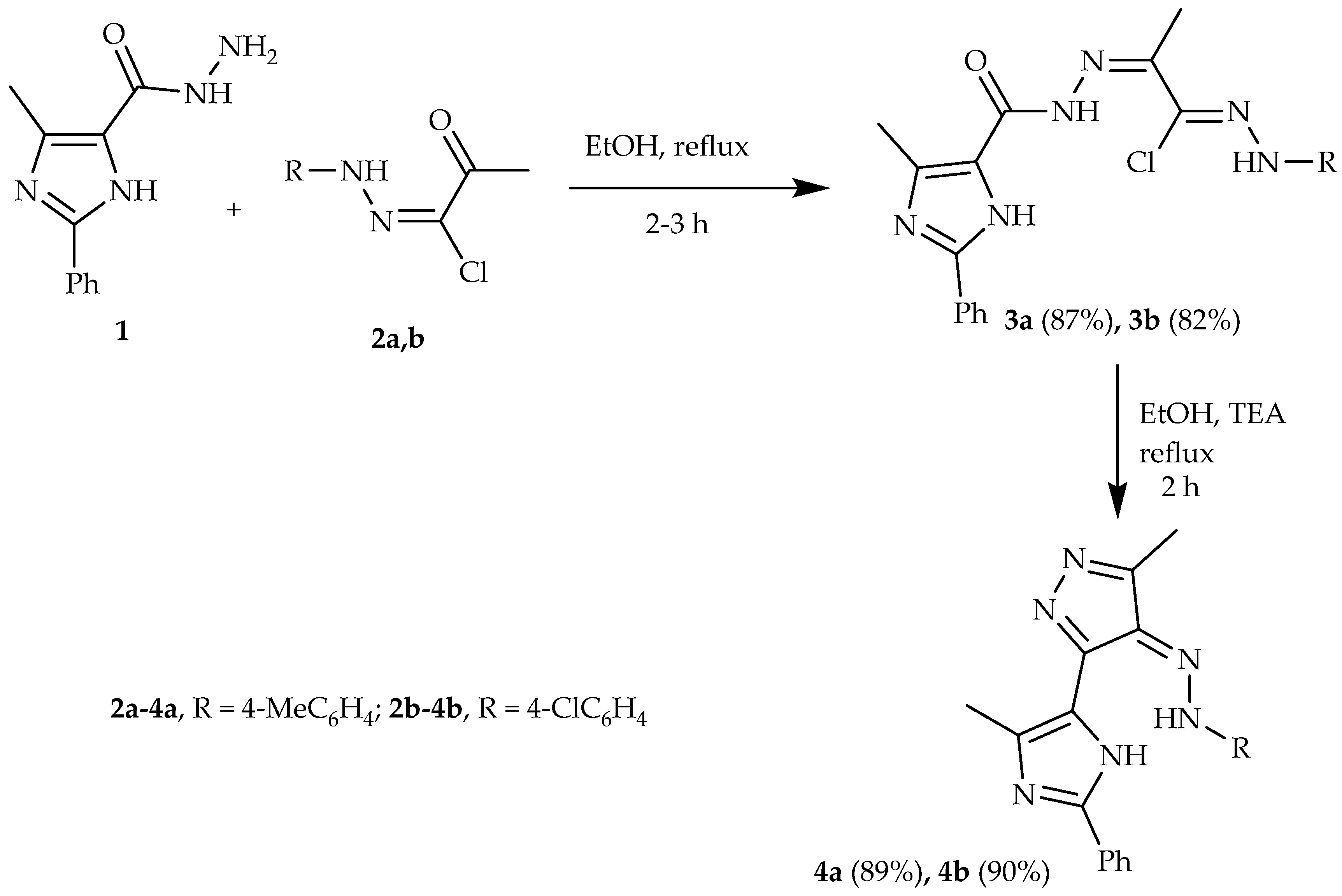
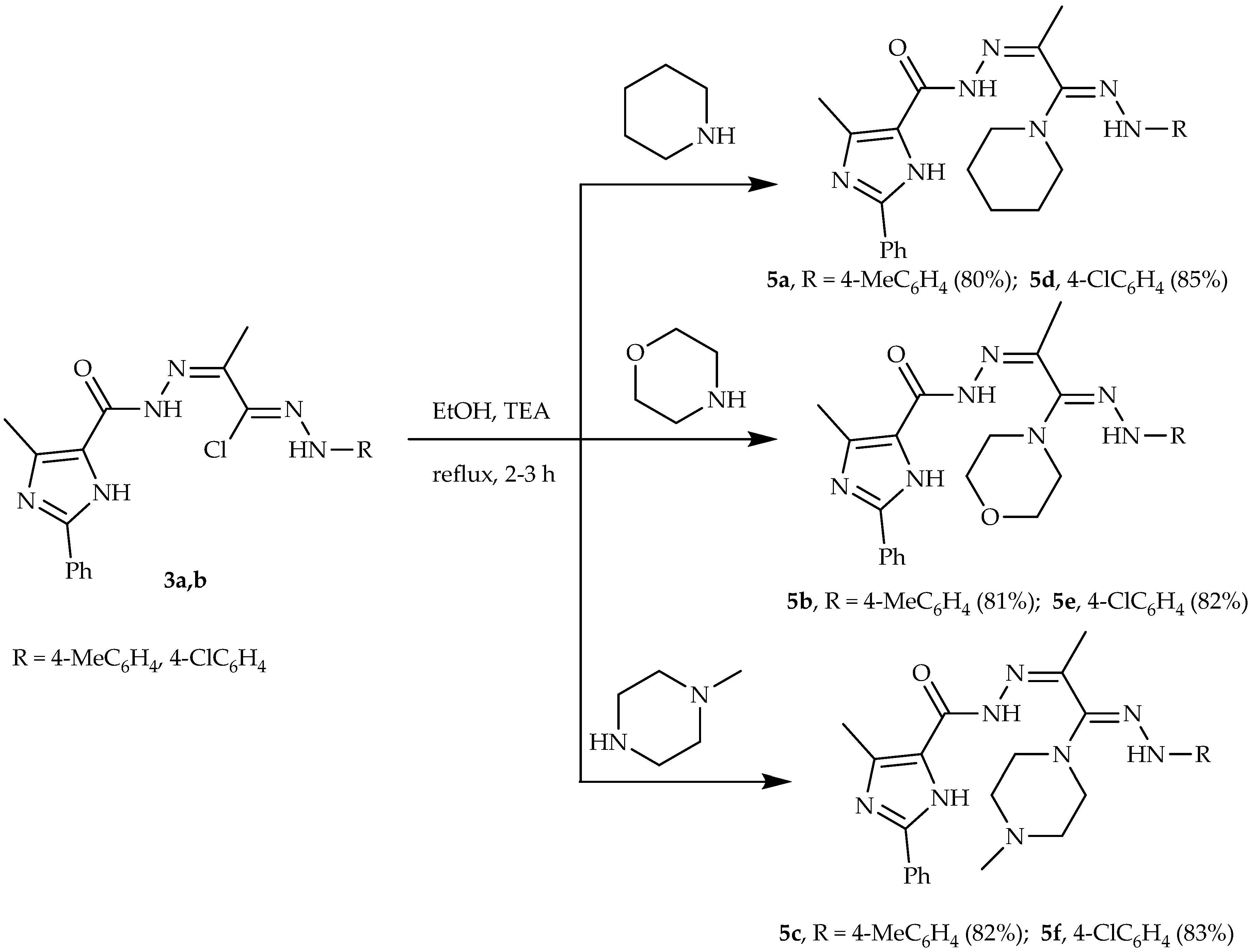
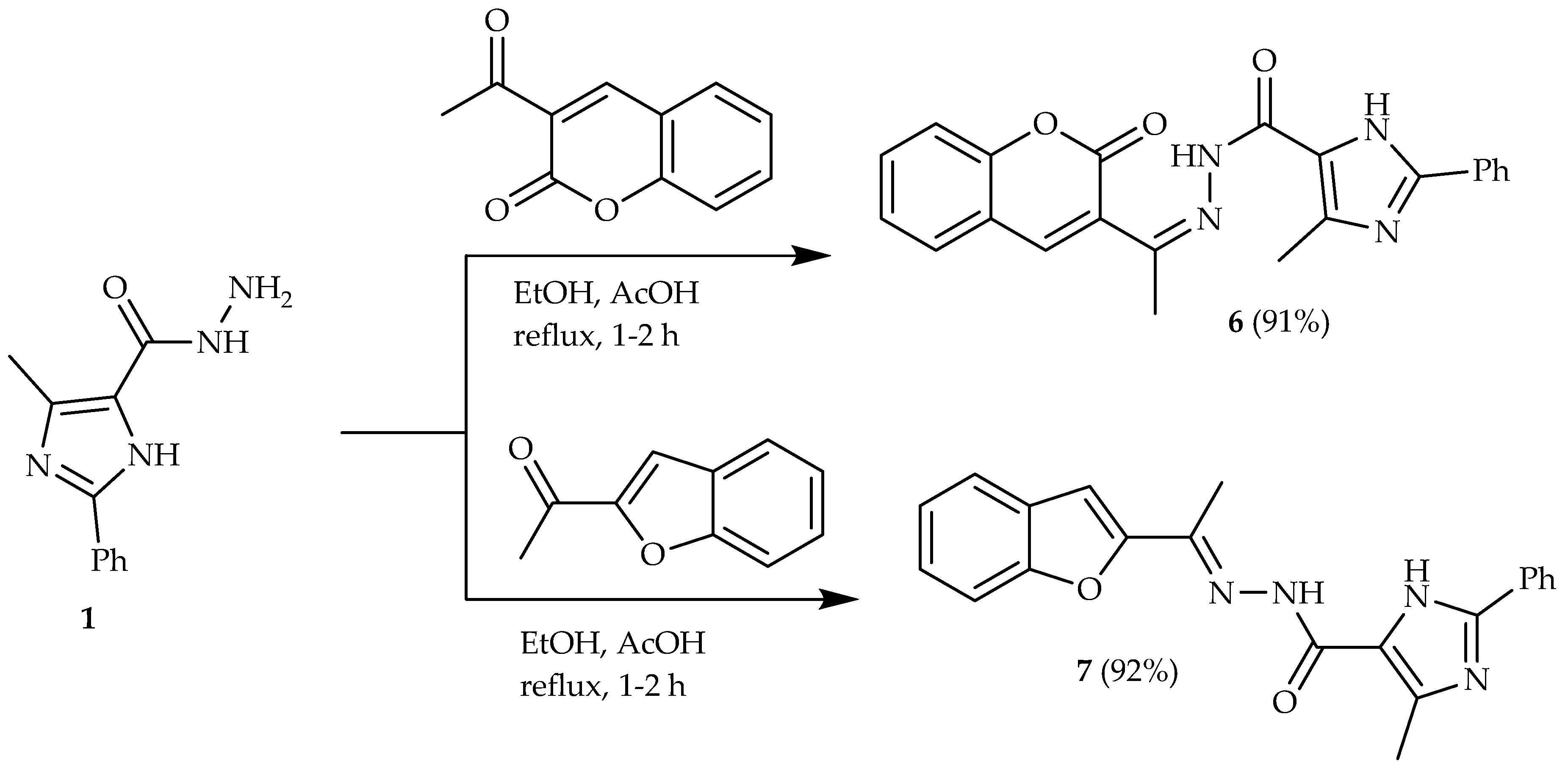
2.2. Synthesis
2.3. Antimicrobial Evaluation
2.4. Minimum Inhibitory Concentration
2.5. Statistical Analysis
2.6. Molecular Docking
3. Results
3.1. Chemistry
3.2. Antimicrobial Activities
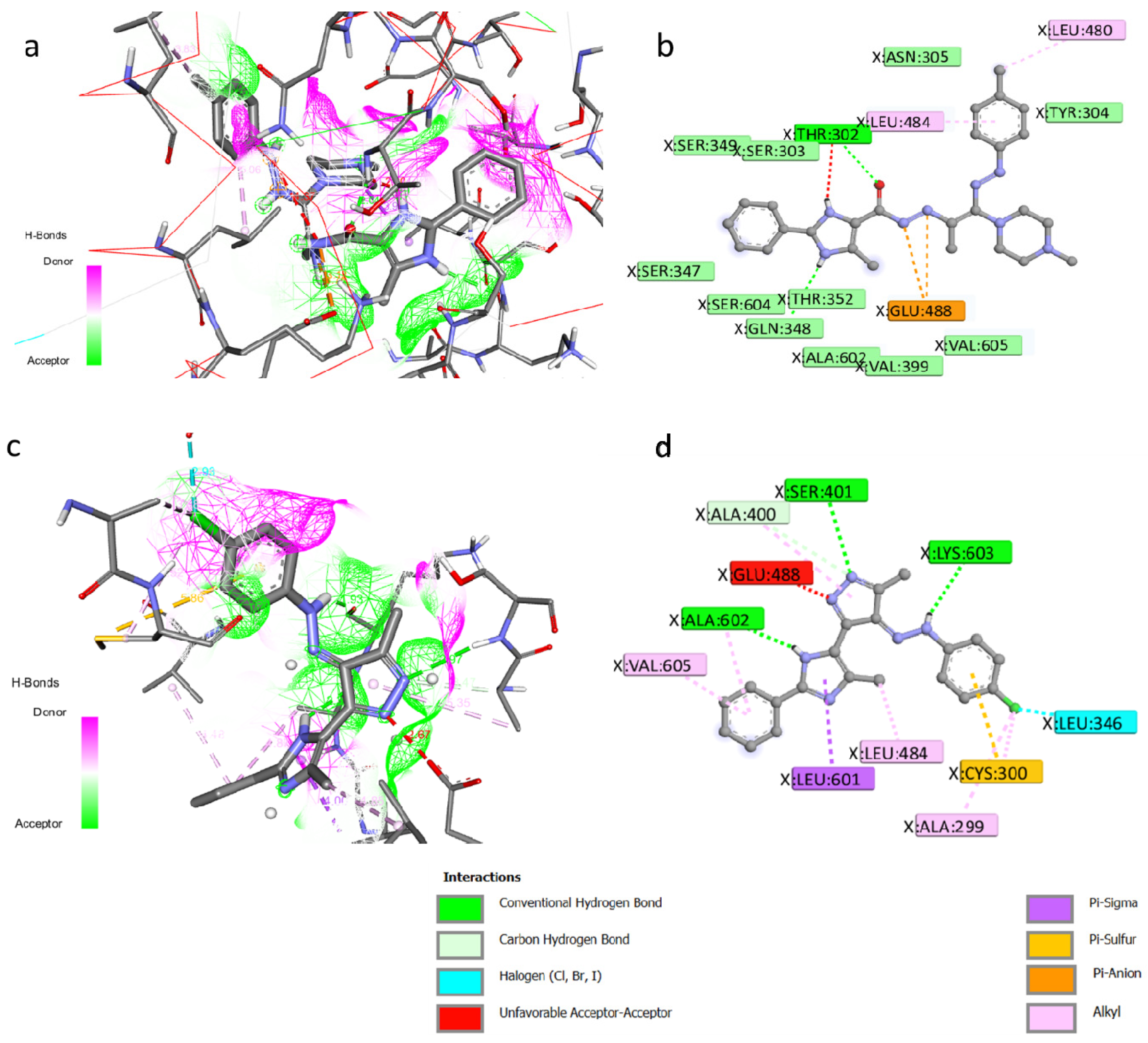
3.3. Molecular Docking
- 1.
- It replaces a highly polar substrate with a more drug-like, better-fitting ligand:
- ○
- The native ligand is highly polar and carries a phosphate group, which results in high enzyme binding but poor cell permeability and often rapid turnover.
- ○
- Compound 5c lacks a full phosphate but forms multiple non-polar and aromatic interactions (p-tolyl, imidazole, piperazine) plus targeted H-bonds—this yields strong complementary packing and van der Waals enthalpy without the liability of a charged phosphate. This can produce higher apparent affinity in cell assays (better uptake and enhanced enzyme binding).
- 2.
- There are additional enthalpic contacts beyond the substrate:
- ○
- Compound 5c formed H-bonds to SER401 and backbone atoms around ALA400/ALA602, mimicking some substrate H-bonding, and also formed an electrostatic/salt-bridge contact with LYS603. The combination of H-bonds and ionic contact often gives stronger binding energy than the native pattern alone.
- 3.
- The sulfur–π interaction with CYS300 is an extra interaction, while the sugar cannot make it. Sulfur–π interactions can contribute appreciably (0.5–2 kcal·mol−1 each) to binding and are selective if the cysteine position is unique.
- 4.
- There is access to both catalytic and auxiliary interaction sites:
- ○
- By using aromatic and ionic interactions, compound 5c can engage secondary pockets/side-chains that the sugar does not exploit. Those extra contact points increase specificity and the chance of outcompeting natural substrate at realistic concentrations.
- 5.
- There is lower susceptibility to catalytic turnover:
- ○
- Compound 5c appears to be a non-reactive binder (hydrazone/carbohydrazide moieties)—it is unlikely to be converted by the enzyme. This means it can stay bound longer (longer residence time), increasing inhibitory potency even if the equilibrium Kd is modest.
- 6.
- The compound exhibits better cellular activity because of its favorable physicochemical properties.
- ○
- Compared to a phosphate-bearing native ligand, the 5c compound’s pKa, lipophilicity, and overall neutral/partly basic character (piperazine) will usually give better membrane permeability and intracellular concentration.
3.4. Drug Likeness Properties and ADMET Prediction
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narasimhan, B.; Sharma, D.; Kumar, P.; Yogeeswari, P.; Sriram, D. Synthesis, antimicrobial and antimycobacterial evaluation of [2-(substituted phenyl)-imidazol-1-yl]-pyridin-3-yl-methanones. J. Enzyme Inhib. Med. Chem. 2011, 26, 720–727. [Google Scholar] [CrossRef]
- Brahmbhatt, H.; Molnar, M.; Pavić, V. Pyrazole nucleus fused trisubstituted imidazole derivatives as antioxidant and antibacterial agents. Karbala Int. J. Mod. Sci. 2018, 4, 200–206. [Google Scholar] [CrossRef]
- Reyes-Arellano, A.; Gómez-García, O.; Torres-Jaramillo, J. Synthesis of azolines and imidazoles and their use in drug design. Med. Chem. 2016, 6, 561–570. [Google Scholar] [CrossRef]
- Forman, S.A. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011, 114, 695–707. [Google Scholar] [CrossRef]
- de Oliveira, E.G.; Cardoso, A.M.; Paese, K.; Coradini, K.; de Oliveira, C.V.; Pohlmann, A.R.; Oliveira, M.S.; Guterres, S.S.; Beck, R.C. Reconstituted spray-dried phenytoin-loaded nanocapsules improve the in vivo phenytoin anticonvulsant effect and the survival time in mice. Int. J. Pharm. 2018, 551, 121–132. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; Lima, B.H.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Lin, Y.; Wang, C.; Kakiuchi, T.; Kikuchi, S. Metronidazole for Helicobacter pylori eradication therapy among children and adolescents in Japan: Overcoming controversies and concerns. Helicobacter 2019, 24, e12575. [Google Scholar] [CrossRef]
- Suarez-Almazor, M.E.; Spooner, C.; Belseck, E. Azathioprine for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef]
- Montoto, S.; Shaw, K.; Okosun, J.; Gandhi, S.; Fields, P.; Wilson, A.; Shanyinde, M.; Cwynarski, K.; Marcus, R.; de Vos, J.; et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J. Clin. Oncol. 2012, 30, 4111. [Google Scholar] [CrossRef]
- Fung, H.B.; Doan, T.L. Tinidazole: A nitroimidazole antiprotozoal agent. Clin. Ther. 2005, 27, 1859–1884. [Google Scholar] [CrossRef] [PubMed]
- Verdouw, P.D.; Hartog, J.M.; Duncker, D.J.; Roth, W.; Saxena, P.R. Cardiovascular profile of pimobendan, a benzimidazole-pyridazinone derivative with vasodilating and inotropic properties. Eur. J. Pharmacol. 1986, 126, 21–30. [Google Scholar] [CrossRef]
- Agili, F. Novel Thiazole Derivatives Containing Imidazole and Furan Scaffold: Design, Synthesis, Molecular Docking, Antibacterial, and Antioxidant Evaluation. Molecules 2024, 29, 1491. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Ko, P.-W.; Chang, Y.-J.; Kapoor, M.; Liang, Y.-C.; Chu, H.-L.; Lin, H.-H.; Horng, J.-C.; Hsu, M.-H. Design and Synthesis of Benzimidazole-Chalcone Derivatives as Potential Anticancer Agents. Molecules 2019, 24, 3259. [Google Scholar] [CrossRef]
- Sharma, P.; LaRosa, C.; Antwi, J.; Govindarajan, R.; Werbovetz, K.A. Imidazoles as Potential Anticancer Agents: An Update on Recent Studies. Molecules 2021, 26, 4213. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Torres-Piedra, M.; Vergara-Galicia, J.; Cesar Rivera-Leyva, J.; Estrada-Soto, S.; León-Rivera, I.; Aguilar-Guardarrama, B.; Rios-Gómez, Y.; Villalobos-Molina, R.; et al. Synthesis, vasorelaxant activity and antihypertensive effect of benzo[d]imidazole derivatives. Bioorg. Med. Chem. 2010, 18, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, U.; Biswal, S.; Sethy, S.; Kumar, H.K.; Banerjee, M. Imidazole and its biological activities: A. review. Asian J. Res. Chem. 2012, 5, 171–182. [Google Scholar]
- Rupčić, R.; Modrić, M.; Hutinec, A.; Čikoš, A.; Stanić, B.; Mesić, M.; Pešić, D.; Merćep, M. Novel tetracyclic imidazole derivatives: Synthesis, dynamic NMR study, and anti-inflammatory evaluation. J. Heterocycl. Chem. 2010, 47, 640–656. [Google Scholar] [CrossRef]
- Romero, D.H.; Heredia, V.E.; GarcíaBarradas, O.; López, M.E.; Pavón, E.S. Synthesis of imidazole derivatives and their biological activities. J. Chem. Biochem. 2014, 2, 45–83. [Google Scholar] [CrossRef]
- Abdelhamid, A.A.; Salah, H.A.; Marzouk, A.A. Synthesis of imidazole derivatives: Ester and hydrazide compounds with antioxidant activity using ionic liquid as an efficient catalyst. J. Heterocycl. Chem. 2020, 57, 676–685. [Google Scholar] [CrossRef]
- Ali, S.; Ali, M.; Khan, A.; Ullah, S.; Waqas, M.; Al-Harrasi, A.; Latif, A.; Ahmad, M.; Saadiq, M. Novel 5-(Arylideneamino)-1H-Benzo[d]imidazole-2-thiols as Potent Anti-Diabetic Agents: Synthesis, In Vitro α-Glucosidase Inhibition, and Molecular Docking Studies. ACS Omega 2022, 7, 43468–43479. [Google Scholar] [CrossRef]
- Marzouk, A.A.; Bass, A.K.A.; Ahmed, M.S.; Abdelhamid, A.A.; Elshaier, Y.A.M.M.; Salman, A.M.M.; Aly, O.M. Design, synthesis and anticonvulsant activity of new imidazolidindione and imidazole derivatives. Bioorg. Chem. 2020, 101, 104020. [Google Scholar] [CrossRef]
- Muheyuddeen, G.; Khan, M.Y.; Ahmad, T.; Srivastava, S.; Verma, S.; Ansari, M.S.; Sahu, N. Design, synthesis, and biological evaluation of novel imidazole derivatives as analgesic and anti-inflammatory agents: Experimental and molecular docking insights. Sci. Rep. 2024, 14, 23121. [Google Scholar] [CrossRef]
- Mobashar, A.; Najam, K.; Akbar, Z.; Barkat, K.; Hussain, K.; Nadeem, H.; Kharl, H.A.A.; Basri, M.A. Evaluation of anti-inflammatory and anti-arthritic activities of Benzimidazole derivative 2-((1H-benzo[d]imiadazol-2-yl) thio)-1-3, 5-diphenyl-1h-pyrazol-1-yl) ethanone. Atl. J. Lif. Sci. 2025, 2025. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Angelova, V.; Karabeliov, V.; Andreeva-Gateva, P.A.; Tchekalarova, J. Recent Developments of Hydrazide/Hydrazone Derivatives and Their Analogs as Anticonvulsant Agents in Animal Models. Drug. Dev. Res. 2016, 77, 379–392. [Google Scholar] [CrossRef]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone comprising compounds as promising anti-infective agents: Chemistry and structure-property relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Malm, A. Design, Synthesis, and in vitro Antimicrobial Activity of New Furan/Thiophene-1,3-Benzothiazin-4-one Hybrids. J. Heterocycl. Chem. 2016, 53, 479–486. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Piatkowska-Chmiel, I.; Gawrońska-Grzywacz, M.; Biernasiuk, A.; Izdebska, M.; Herbet, M.; Sysa, M.; Malm, A.; Dudka, J.; Wujec, M. New hydrazide-hydrazones and 1,3-thiazolidin-4-ones with 3-hydroxy-2-naphthoic moiety: Synthesis, in vitro and in vivo studies. Biomed. Pharmacother. 2018, 103, 1337–1347. [Google Scholar] [CrossRef]
- He, L.-Y.; Qiu, X.-Y.; Cheng, J.-Y.; Liu, S.-J.; Wu, S.-M. Synthesis, characterization and crystal structures of vanadium(V) complexes derived from halido-substituted tridentate hydrazone compounds with antimicrobial activity. Polyhedron 2018, 156, 105–110. [Google Scholar] [CrossRef]
- Rocha, C.S.; Bomfim Filho, L.F.O.; de Souza, A.E.; Diniz, R.; Denadai, Â.M.L.; Beraldo, H.; Teixeira, L.R. Structural studies and investigation on the antifungal activity of silver (I) complexes with 5-nitrofuran-derived hydrazones. Polyhedron 2019, 170, 723–730. [Google Scholar] [CrossRef]
- Kendel, A.; Miljanic, S.; Kontrec, D.; Soldin, Z.; Galic, N. Copper (II) complexes of aroylhydrazones: Preparation and structural characterization. J. Mol. Struct. 2020, 1207, 127783. [Google Scholar] [CrossRef]
- Liu, T.; Sun, C.; Xing, X.; Jing, L.; Tan, R.; Luo, Y.; Huang, W.; Song, H.; Li, Z.; Zhao, Y. Synthesis and evaluation of 2-[2-(phenylthiomethyl)-1H-benzo [D] imidazol-1-Yl) acetohydrazide derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2012, 22, 3122–3125. [Google Scholar] [CrossRef]
- Vogel, S.; Kaufmann, D.; Pojarová, M.; Müller, C.; Pfaller, T.; Kühne, S.; Bednarski, P.J.; von Angerer, E. Aroyl hydrazones of 2-phenylindole-3-carbaldehydes as novel antimitotic agents. Bioorg. Med. Chem. 2008, 16, 6436–6447. [Google Scholar] [CrossRef]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing powerhouse in colon cancer cells by hydrazide-hydrazone-based small molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, G. Hydrazone, Amide, carbamate, macromolecular and other prodrugs of doxorubicin. Open Drug Del. J. 2008, 2, 77–85. [Google Scholar] [CrossRef]
- Koç, H.C.; Atlihan, I.; Mega-Tiber, P.; Orun, O.; Güniz Küçükgüzel, Ş. Synthesis of some novel hydrazide-hydrazones derived from etodolac as potential antiprostate cancer agents. J. Res. Pharm. 2022, 26, 1018–1029. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Omar, M.M.; Hindy, A.M.M. Synthesis, characterization and biological activity of some transition metals with Schiff base derived from 2-thiophene carboxaldehyde and aminobenzoic acid. Spectrochim. Acta A 2005, 62, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Alkahtani, M.D.F.; Babar Taj, M.; Alnajeebi, A.M.; Alzahrani, S.O.; Babteen, N.A.; Alelwani, W.; Bannunah, A.M.; Noor, S.; Ayub, R.; et al. Synthesis of new naphthyl acetohydrazone-based metal complexes: Micellar interactions, DNA binding, antimicrobial, and cancer inhibition studies. Molecules 2021, 26, 1044. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, G.; Bellemin-Laponnaz, S.; Shah, M.A.; Uddin, A.; Shah, M.R.; Ali, I.; Ullah, R.; Hannan, A.; Hussain, H. Synthesis and characterization of novel hydrazone derivatives of isonicotinic hydrazide and their evaluation for antibacterial and cytotoxic potential. Molecules 2022, 27, 6770. [Google Scholar] [CrossRef]
- Alam, M.; Verma, G.; Shaquiquzzaman, M.; Marella, A.; Akhtar, M.; Ali, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Heidari, A.; Haghi, F.; Bikas, R.; Lis, T. Chiral lactic hydrazone derivatives as potential bioactive antibacterial agents: Synthesis, spectroscopic, structural and molecular docking studies. J. Mol. Struct. 2017, 1128, 391–399. [Google Scholar] [CrossRef]
- Heidari, A.; Haghi, F.; Noshiranzadeh, N.; Bikas, R. (S,E)-2-hydroxy-N-(2-hydroxy-5-nitrobenzylidene)propane hydrazide as a quorum sensing inhibitor of Pseudomonas aeruginosa. Med. Chem. Res. 2017, 26, 947–1955. [Google Scholar] [CrossRef]
- Ajani, O.O.; Iyaye, K.T.; Aderohunmu, D.V.; Olanrewaju, I.O.; Germann, M.W.; Olorunshola, S.J.; Bello, B.L. Microwaveassisted synthesis and antibacterial propensity of N0-s-benzylidene-2-propylquinoline-4-carbohydrazide and N0-((s-1H-pyrrol-2-yl)methylene)-2-propylquinoline-4-carbohydrazide motifs. Arab. J. Chem. 2020, 13, 1809–1820. [Google Scholar] [CrossRef]
- Krátký, M.; Bősze, S.; Baranyai, Z.; Stolaříková, J.; Vinšová, J. Synthesis and biological evolution of hydrazones derived from 4-(trifluoromethyl) benzohydrazide. Bioorg. Med. Chem. Lett. 2017, 27, 5185–5189. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.A.; Salama, I.; Gomaa, M.S.; Elaasser, M.M.; Abdel-Aziz, M.M.; Soliman, D.H. Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2017, 138, 698–714. [Google Scholar] [CrossRef]
- Manikandan, V.; Balaji, S.; Senbagam, R.; Vijayakumar, R.; Rajarajan, M.; Vanangamudi, G.; Arulkumaran, R.; Sundararajan, R.; Thirunarayanan, G. Synthesis and antimicrobial activities of some (E)-N0-1-(substituted benzylidene) benzohydrazides. Inter. J. Adv. Chem. 2017, 5, 17–24. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Moustafa, A.H.; El-Moneim, M.A.; Awad, H.M.; Esmat, A. Design and Synthesis of Hydrazide-Hydrazones Based 2-Oxonicotinonitrile Derivatives as Potential Antimicrobial Agents. J. Pharm. Appl. Chem. 2018, 4, 125–131. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Berecka, A.; Gumieniczek, A.; Malm, A.; Wujec, M. New hydrazide–hydrazones of isonicotinic acid: Synthesis, lipophilicity and in vitro antimicrobial screening. Chem. Biol. Drug. Des. 2018, 91, 915–923. [Google Scholar] [CrossRef]
- Haiba, N.S.; Khalil, H.H.; Moniem, M.A.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinical isolates. Bioorg. Chem. 2019, 89, 103013. [Google Scholar] [CrossRef]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl Carbonyl Moiety. Molecules 2019, 24, 4000. [Google Scholar] [CrossRef]
- Salem, M.A.; Ragab, A.; El-Khalafawy, A.; Makhlouf, A.H.; Askar, A.A.; Ammar, Y.A. Design, synthesis, in vitro antimicrobial evaluation and molecular docking studies of indol-2-one tagged with morpholinosulfonyl moiety as DNA gyrase inhibitors. Bioorg. Chem. 2020, 96, 103619. [Google Scholar] [CrossRef]
- Tiwari, S.; Kirar, S.; Banerjee, U.C.; Neerupudi, K.B.; Singh, S.; Wani, A.A.; Bharatam, P.V.; Singh, I.P. Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents. Bioorg. Chem. 2020, 99, 103787. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Rysz, B.; Biernasiuk, A.; Wujec, M. Synthesis of promising antimicrobial agents: Hydrazide-hydrazones of 5-nitrofuran-2-carboxylic acid. Chem. Biol. Drug. Des. 2020, 95, 260–269. [Google Scholar] [CrossRef]
- El-Etrawy, A.-A.; Sherbiny, F.F. Design, synthesis, biological evaluation and molecular modeling investigation of new N0-(2-Thiouracil-5-oyl) hydrazone derivatives as potential anti-breast cancer and anti-bacterial agents. J. Mol. Struct. 2021, 1232, 129993. [Google Scholar] [CrossRef]
- Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Berecka-Rycerz, A.; Malm, A.; Gumieniczek, A.; Wujec, M. Novel Derivatives of 4-Methyl-1,2,3-Thiadiazole-5-Carboxylic Acid Hydrazide: Synthesis, Lipophilicity, and In Vitro Antimicrobial Activity Screening. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Angelova, V.T.; Valcheva, V.; Vassilev, N.G.; Buyukliev, R.; Momekov, G.; Dimitrov, I.; Saso, L.; Djukic, M.; Shivachev, B. Antimycobacterial activity of novel hydrazide-hydrazone derivatives with 2H-chromene and coumarin scaffold. Bioorg. Med. Chem. Lett. 2017, 27, 223–227. [Google Scholar] [CrossRef]
- Angelova, V.T.; Valcheva, V.; Pencheva, T.; Voynikov, Y.; Vassilev, N.; Mihaylova, R.; Momekov, G.; Shivachev, B. Synthesis, antimycobacterial activity and docking study of 2-aroyl-[1] benzopyrano [4,3-c] pyrazol-4(1H)-one derivatives and related hydrazide-hydrazones. Bioorg. Med. Chem. Lett. 2017, 27, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.; Fahmy, S.; Rizk, O.; Sriram, D.; Mahran, M.A.; Labouta, I.M. Structure-based design of some isonicotinic acid hydrazide analogues as potential antitubercular agents. Bioorg. Chem. 2018, 80, 721–732. [Google Scholar] [CrossRef]
- Angelova, V.T.; Pencheva, T.; Vassilev, N.; Simeonova, R.; Momekov, G.; Valcheva, V. New indole and indazole derivatives as potential antimycobacterial agents. Med. Chem. Res. 2019, 28, 485–497. [Google Scholar] [CrossRef]
- Hassan, N.W.; Saudi, M.N.; Abdel-Ghany, Y.S.; Ismail, A.; Elzahhar, P.A.; Sriram, D.; Nassra, R.; Abdel-Aziz, M.M.; El-Hawash, S.A. Novel pyrazine based anti-tubercular agents: Design, synthesis, biological evaluation and in silico studies. Bioorg. Chem. 2020, 96, 103610. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, E.; Dincel, E.D.; Naesens, L.; Güzeldemirci, N.U. Design and synthesis of novel imidazo [2,1-b] thiazole derivatives as potent antiviral and antimycobacterial agents. Bioorg. Chem. 2020, 95, 103496. [Google Scholar] [CrossRef]
- Shah, S.R.; Katariya, K.D. 1,3-Oxazole-isoniazid hybrids: Synthesis, antitubercular activity, and their docking studies. J. Heterocycl. Chem. 2020, 57, 1682–1691. [Google Scholar] [CrossRef]
- Guilherme, F.D.; Simonetti, J.É.; Folquitto, L.R.S.; Reis, A.C.C.; Oliver, J.C.; Dias, A.L.T.; Dias, D.F.; Carvalho, D.T.; Brandão, G.C.; de Souza, T.B. Synthesis, chemical characterization and antimicrobial activity of new acylhydrazones derived from carbohydrates. J. Mol. Struct. 2019, 1184, 349–356. [Google Scholar] [CrossRef]
- Dascalu, A.-E.; Ghinet, A.; Lipka, E.; Furman, C.; Rigo, B.; Fayeulle, A.; Billamboz, M. Design, synthesis and evaluation of hydrazine and acyl hydrazone derivatives of 5-pyrrolidin-2-one as antifungal agents. Bioorg. Med. Chem. Lett. 2020, 30, 127220. [Google Scholar] [CrossRef]
- Raczynska, J.; Olchowy, J.; Konariev, P.V.; Svergun, D.I.; Milewski, S.; Rypniewski, W. The Crystal and Solution Studies of Glucosamine-6-phosphate Synthase from Candida albicans. J. Mol. Biol. 2007, 372, 672–688. [Google Scholar] [CrossRef]
- Stefaniak, J.; Nowak, M.G.; Wojciechowski, M.; Milewski, S.; Skwarecki, A.S. Inhibitors of glucosamine-6-phosphate synthase as potential antimicrobials or antidiabetics—Synthesis and properties. J. Enzyme Inhib. Med. Chem. 2022, 37, 1928–1956. [Google Scholar] [CrossRef]
- Fei, X.; Holmes, T.; Diddle, J.; Hintz, L.; Delaney, D.; Stock, A.; Renner, D.; McDevitt, M.; Berkowitz, D.B.; Soukup, J.K. Phosphatase-Inert Glucosamine 6-Phosphate Mimics Serve as Actuators of the glmS Riboswitc. ACS Chem. Biol. 2014, 9, 2875–2882. [Google Scholar] [CrossRef]
- Borik, R.M.A.; Abd El-Wahab, A.H.F.; Alamri, A.A.; Mostafa, M.S.; Ibrahim, D.A.E.S.; El-Aassar, M.R.; Elhenawy, A.A. Design, synthesis, antitumor activities, molecular docking and ADMET of new benzochromenes, benzochromenopyrimidines, and benzochromeno-triazolopyrimidines. Results Chem. 2025, 17, 102557. [Google Scholar] [CrossRef]
- Alamri, A.A.; Borik, R.M.A.; El-Wahab, A.H.F.A.; Mohamed, H.M.; Ismail, K.S.; El-Aassar, M.R.; Al-Dies, A.-A.M.; El-Agrody, A.M. Synthesis of Schiff bases based on Chitosan, thermal stability and evaluation of antimicrobial and antitumor activities. Sci. Rep. 2025, 15, 892. [Google Scholar] [CrossRef]
- Al-Dies, A.-A.M.; Alblewi, F.F.; Okasha, R.M.; Alsehli, M.H.; Borik, R.M.A.; Ihmaid, S.; Amr, A.E.-G.E.; Ghabbour, H.A.; Elhenawy, A.A.; El-Agrody, A.M. Synthesis, crystal structure, hirshfeld study, DFT analysis, molecular docking study, antimicrobial activity of β-enaminonitrile bearing 1H-pyran. Discov. Appl. Sci. 2025, 7, 71. [Google Scholar] [CrossRef]
- Al-Dies, A.A.M.; Abd El-Wahab, A.H.F.; Alamri, A.A.; Borik, R.M.A.; Mohamed, H.M.; Assirey, E.A.; Alsehli, M.H.; Moussa, Z.; Alzamly, A.; Mehany, A.B.M.; et al. Synthesis, crystal structure, DFT studies, molecular docking, of 2-amino-6-methoxy-4-(4-nitrophenyl)-4H-benzo [h] chromene-3-carbonitrile as tyrosinase inhibitor. J. Mol. Struct. 2025, 1322, 140289. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mabied, A.F.; Fettinger, J.C.; Hassan, A.H.E.; Farahat, A.A. 2-((5-(5-Methyl-2-phenyl-1H-imidazol-4-yl)-1,3,4-oxadiazol-2-yl)thio)-1-phenylethan-1-one. Molbank 2023, 2023, M1666. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx, Methods. Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Sadeghi-Kaji, S.; Shareghi, B.; Saboury, A.A.; Farhadian, S. Spermine as a porcine pancreatic elastase activator: Spectroscopic and molecular simulation studies. J. Biomol. Struct. Dyn. 2022, 38, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Shareghi, B.; Farhadian, S.; Tirgir, F. Evaluation of maltose binding to proteinase K: Insights from spectroscopic and computational approach. J. Mol. Liq. 2019, 280, 1–10. [Google Scholar] [CrossRef]
- Yadollahi, E.; Shareghi, B.; Farhadian, S. Insight of the interaction of Naphthol yellow S with trypsin: Experimental and computational techniques. J. Iran. Chem. Soc. 2022, 19, 2871–2882. [Google Scholar] [CrossRef]
- Yadollahi, E.; Shareghi, B.; Farhadian, S.; Hashemi Shahraki, F. Conformational dynamics of trypsin in the presence of caffeic acid: A spectroscopic and computational investigation. J. Biomol. Struct. Dyn. 2024, 42, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Telkar, S.; Arulmoli, T.; Fun, H.-K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab. J. Chem. 2013, 6, 197–204. [Google Scholar] [CrossRef]
- Polović, S.; Bilić, V.L.; Budimir, A.; Kontrec, D.; Galić, N.; Kosalec, I. Antimicrobial assessment of aroylhydrazone derivatives in vitro. Acta Pharm. 2019, 69, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Quinoline hydrazide/hydrazone derivatives: Recent insights on antibacterial activity and mechanism of action. Chem. Med. Chem. 2023, 18, e202200571. [Google Scholar] [CrossRef]
- Berillo, D.A.; Dyusebaeva, M.A. Synthesis of hydrazides of heterocyclic amines and their antimicrobial and spasmolytic activity. Saudi Pharm. J. 2022, 30, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M. Scope and Limitations on the Potent Antimicrobial Activities of Hydrazone Derivatives. In Frontiers in Anti-Infective Drug Discovery; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; Volume 9, pp. 123–155. [Google Scholar] [CrossRef]
- Gaucher-Wieczorek, F.; Guérineau, V.; Touboul, D.; Thétiot-Laurent, S.; Pelissier, F.; Badet-Denisot, M.-A.; Badet, B.; Durand, P. Evaluation of synthase and hemisynthase activities of glucosamine-6-phosphate synthase by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Biochem. 2014, 458, 61–65. [Google Scholar] [CrossRef] [PubMed]
| Sample No. | Inhibition Zone (cm) | ||||
|---|---|---|---|---|---|
| Escherichia coli (ATCC 25922) | Pseudomonas aeruginosa (ATCC 25619) | Staphylococcus aureus (ATCC 25923) | Candida albicans | Rhizopus racemosus | |
| 3a | 1.5 ± 0.2 | 1.4 ± 0.01 | 1.3± 0.05 | 1.0 ± 0.02 | 1.2 ± 0.05 |
| 3b | 1.3 ± 0.02 | 1.3 ± 0.02 | 1.6 ± 0.02 | 1.0 ± 0.02 | 1.1 ± 0.02 |
| 4a | 0 | 1.0 ± 0.2 | 2.4 ± 0.01 | 0 | 0 |
| 4b | 2.0 ± 0.05 | 2.2 ± 0.02 | 2.7 ± 0.05 | 0 | 0 |
| 5a | 1.4 ± 0.1 | 1.5 ± 0.01 | 2.5 ± 0.01 | 0 | 0 |
| 5b | 1.7 ± 0.3 | 2 ± 0.02 | 2.0 ± 0.1 | 0 | 1.4 ± 0.02 |
| 5c | 1.7 ± 0.03 | 1.7 ± 0.01 | 2.7 ± 0.01 | 0 | 0 |
| 5d | 1.4 ± 0.02 | 1.5 ± 0.01 | 2.5 ± 0.02 | 0 | 0 |
| 5e | 1.6 ± 0.02 | 2.0 ± 0.1 | 0 | 0 | 1.1 ± 0.01 |
| 5f | 1.5 ± 0.1 | 1.7 ± 0.02 | 2.5 ± 0.01 | 0 | 1.2 ± 0.01 |
| 6 | 1.6 ± 0.02 | 2,0 ± 0.02 | 1.3 ± 0.02 | 0 | 0 |
| 7 | 1.0 ± 0.02 | 1.4 ± 0.01 | 1.0 ± 0.02 | 0 | 0 |
| Amikacin | 1.7 ± 0.02 | 1.7 ± 0.02 | 2.0 ± 0.01 | - | - |
| Neomycin | - | - | - | 1.8 ± 0.01 | 1.7 ± 0.01 |
| Sample No. | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Escherichia coli (ATCC 25922) | Pseudomonas aeruginosa (ATCC 25619) | Staphylococcus aureus (ATCC 25923) | Candida albicans | Rhizopus racemosus | |
| 3a | 200 ± 1.1 | 150 ± 0.02 | 300 ± 0.02 | 300 ± 0.02 | 300 ± 1.0 |
| 3b | 300 ± 0.02 | 200 ± 0.01 | 300 ± 0.1 | 300 ± 0.02 | 300 ± 1.0 |
| 4a | - | 300 ± 0.03 | 200 ± 1.0 | - | - |
| 4b | 200 ± 0.05 | 200 ± 1.0 | 50 ± 0.01 | - | - |
| 5a | 300 ± 0.02 | 200 ± 0.02 | 100 ± 0.02 | - | 300 ± 0.02 |
| 5b | 300 ± 1.o | 200 ± 0.01 | 100 ± 0.02 | - | - |
| 5c | 200 ± 0.02 | 150 ± 0.02 | 50 ± 0.01 | - | - |
| 5d | 300 ± 0.02 | 200 ± 0.05 | 200 ± 0.02 | - | - |
| 5e | 300 ± 0.03 | 150 ± 0.01 | - | - | 300 ± 0.02 |
| 5f | 300 ± 0.03 | 200 ± 0.05 | 200 ± 0.02 | - | 300 ±0.05 |
| 6 | 300 ± 0.01 | 200 ± 0.02 | - | - | - |
| 7 | 300 ± 0.02 | 200 ± 0.02 | - | - | - |
| Comp. No. | Score Kcal/mol | Moieties from the Compound | Amino Acid Residues | Type of Interaction, Distance Å of H-Bonds |
|---|---|---|---|---|
| The co-crystallized ligand (GLP) | −6.2 | Phosphate group | THR352, GLN348, SER303, SER349, CYS300, SER347, LYS603 | Conventional H-bond |
| 4b | −8.7 | Sugar moiety | THR302, VAL399, ALA602, ALA400, GLY301 | |
| CO | THR302 | Conventional H-bond Unfavorable Acceptor—Acceptor | ||
| NH of imidazole | GLN348 | C-H bond | ||
| C=C | GLU488 | Pi-Anion | ||
| CH3 | LEU480 | Alkyl | ||
| Phenyl | LEU484 | Alkyl | ||
| 5c | −8.7 | N-pyrazole | SER401 | Conventional H-bond |
| NH-imidazole | ALA602 | |||
| NH | LYS603 | |||
| N-pyrazol | GLU488 | Unfavorable Acceptor—Acceptor | ||
| N=N pyrazole | ALA400 | C-H bond | ||
| Phenyl | CYS300 | Pi-Sulfur | ||
| Pyrazole | LEU601 | Pi-Sigma | ||
| Cl | LEU346 | Halogen | ||
| CH3 | LEU484 | Alkyl | ||
| Cl | ALA299 CYS300 | |||
| Phenyl | VAL605 |
| Properties | 4b | 5c |
|---|---|---|
| Physicochemical Properties | ||
| Chemical Formula | C20H17ClN6 | C26H32N8O |
| MW (g/mol) | 376.84 | 472.597 |
| HBA | 4 | 5 |
| HBD | 2 | 3 |
| MR | 117.81 | 148.31 |
| TPSA (Å2) | 77.79 | 101.01 |
| No. Lipinski violation | 0 violation | 0 violation |
| Lipinski drug-like | Yes | Yes |
| Parameters * (Unite) | 4b | 5c |
|---|---|---|
| Absorption | ||
| Water solubility (log mol/L) | −2.903 | −2.962 |
| Caco2 permeability (log Papp in 10−6 cm/s) | 0.926 | 0.201 |
| Intestinal absorption (% Absorbed) | 85.03 | 70.567 |
| Skin Permeability (log Kp) | −2.735 | −2.735 |
| P-glycoprotein substrate | Yes | Yes |
| P-glycoprotein I inhibitor | Yes | Yes |
| P-glycoprotein II inhibitor | Yes | Yes |
| Distribution | ||
| BBB permeability (log BB) | −0.97 | −1.251 |
| CNS permeability (log PS) | −1.69 | −2.429 |
| Metabolism | ||
| CYP2D6 inhibitor | Yes | No |
| CYP3A4 inhibitor | Yes | Yes |
| CYP1A2 inhibitor | Yes | No |
| CYP2C19 inhibitor | Yes | No |
| CYP2C9 inhibitor | Yes | Yes |
| CYP2D6 inhibitor | Yes | Yes |
| CYP3A4 inhibitor | Yes | No |
| Excretion | ||
| Total Clearance (log ml/min/kg) | 0.419 | 0.052 |
| Renal OCT2 substrate | Yes | Yes |
| Toxicity | ||
| AMES toxicity | Yes | No |
| Max. tolerated dose (human) (log mg/kg/day) | 0.226 | 0.167 |
| hERG I inhibitor | No | No |
| hERG II inhibitor | Yes | Yes |
| Oral Rat Acute Toxicity (LD50) (mol/kg) | 2.665 | 2.465 |
| Hepatotoxicity | No | Yes |
| Skin Sensitisation | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borik, R.M. Design, Synthesis, and Molecular Docking of New Hydrazide–Hydrazone Derivatives with Imidazole Scaffold as Potential Antimicrobial Agents. Chemistry 2025, 7, 172. https://doi.org/10.3390/chemistry7060172
Borik RM. Design, Synthesis, and Molecular Docking of New Hydrazide–Hydrazone Derivatives with Imidazole Scaffold as Potential Antimicrobial Agents. Chemistry. 2025; 7(6):172. https://doi.org/10.3390/chemistry7060172
Chicago/Turabian StyleBorik, Rita M. 2025. "Design, Synthesis, and Molecular Docking of New Hydrazide–Hydrazone Derivatives with Imidazole Scaffold as Potential Antimicrobial Agents" Chemistry 7, no. 6: 172. https://doi.org/10.3390/chemistry7060172
APA StyleBorik, R. M. (2025). Design, Synthesis, and Molecular Docking of New Hydrazide–Hydrazone Derivatives with Imidazole Scaffold as Potential Antimicrobial Agents. Chemistry, 7(6), 172. https://doi.org/10.3390/chemistry7060172






